Electrochemical Performance of a PVDF-HFP-LiClO4-Li6.4La3.0Zr1.4Ta0.6O12 Composite Solid Electrolyte at Different Temperatures
Abstract
:1. Introduction
2. Experimental Section
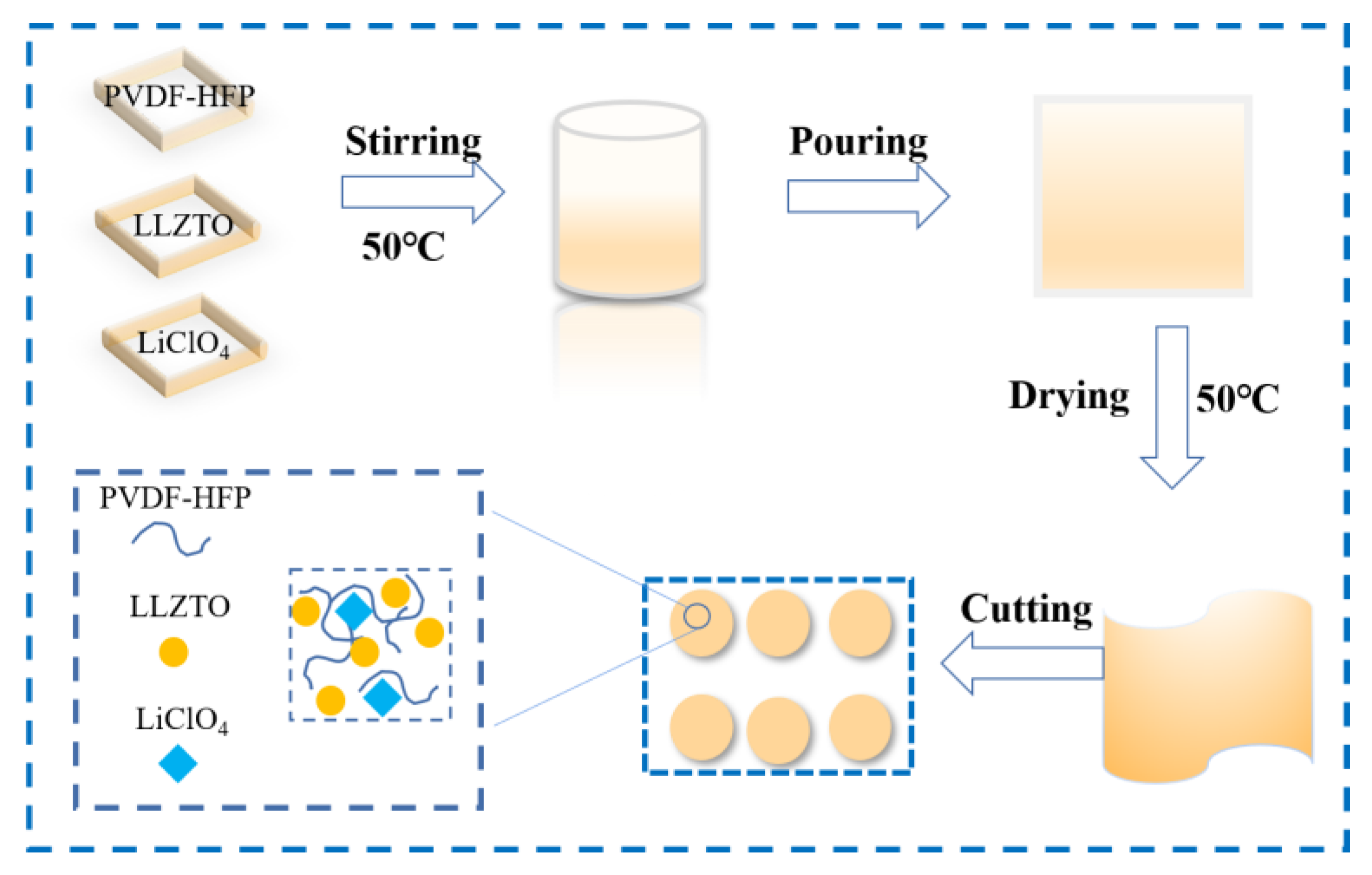
2.1. Preparation of PVDF-HFP-LiClO4-LLZTO Composite Solid Electrolyte
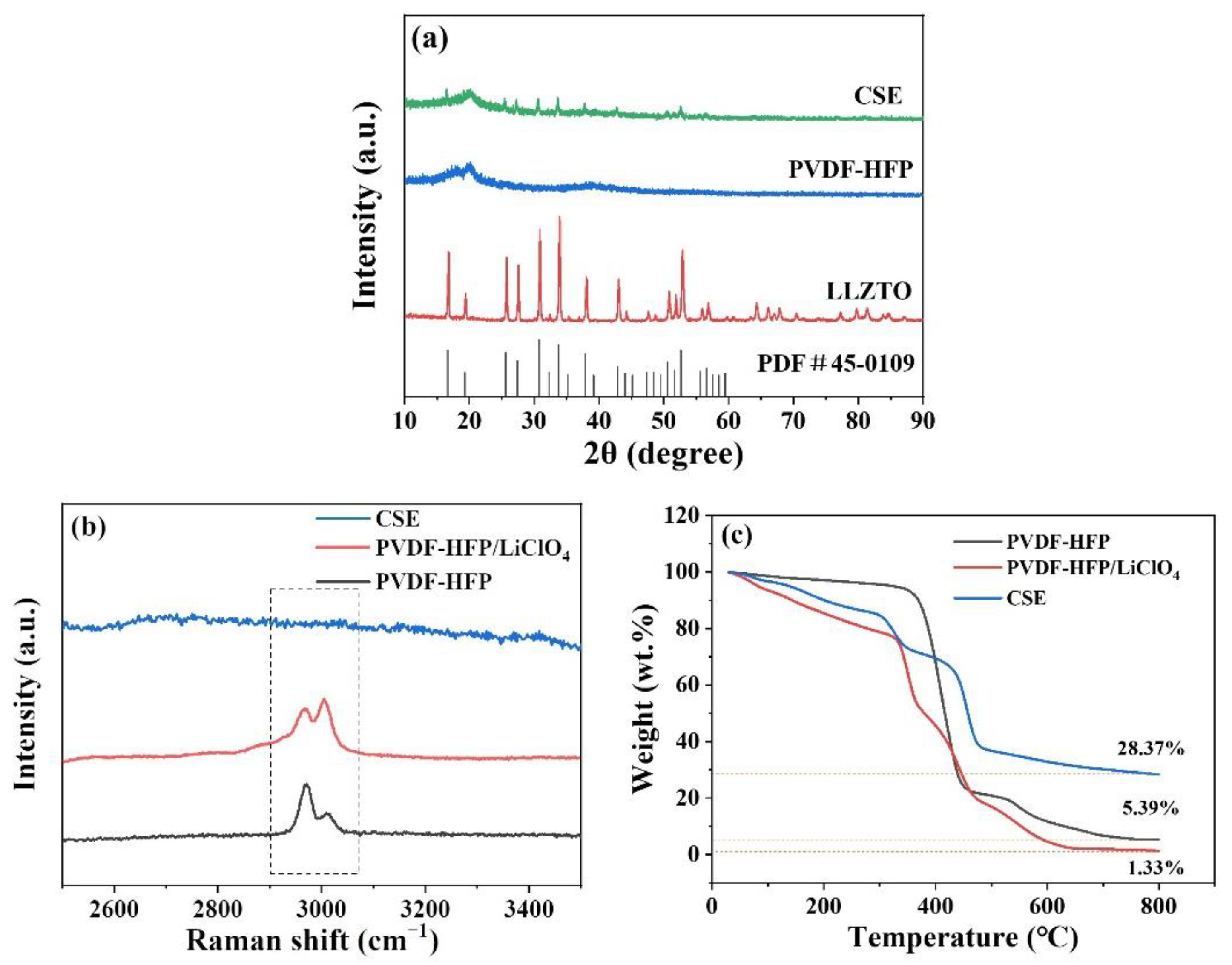
2.2. Physical Characterization
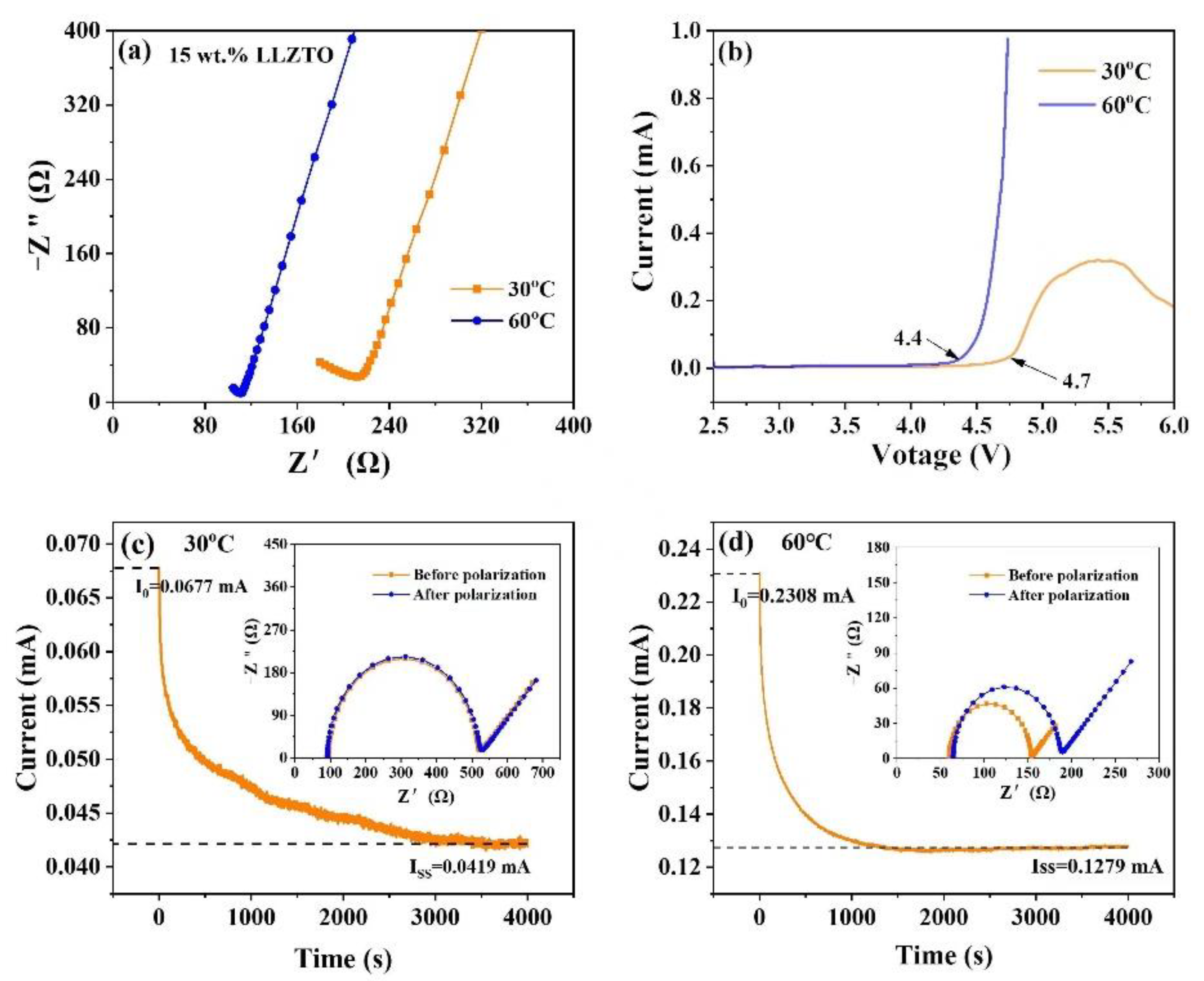
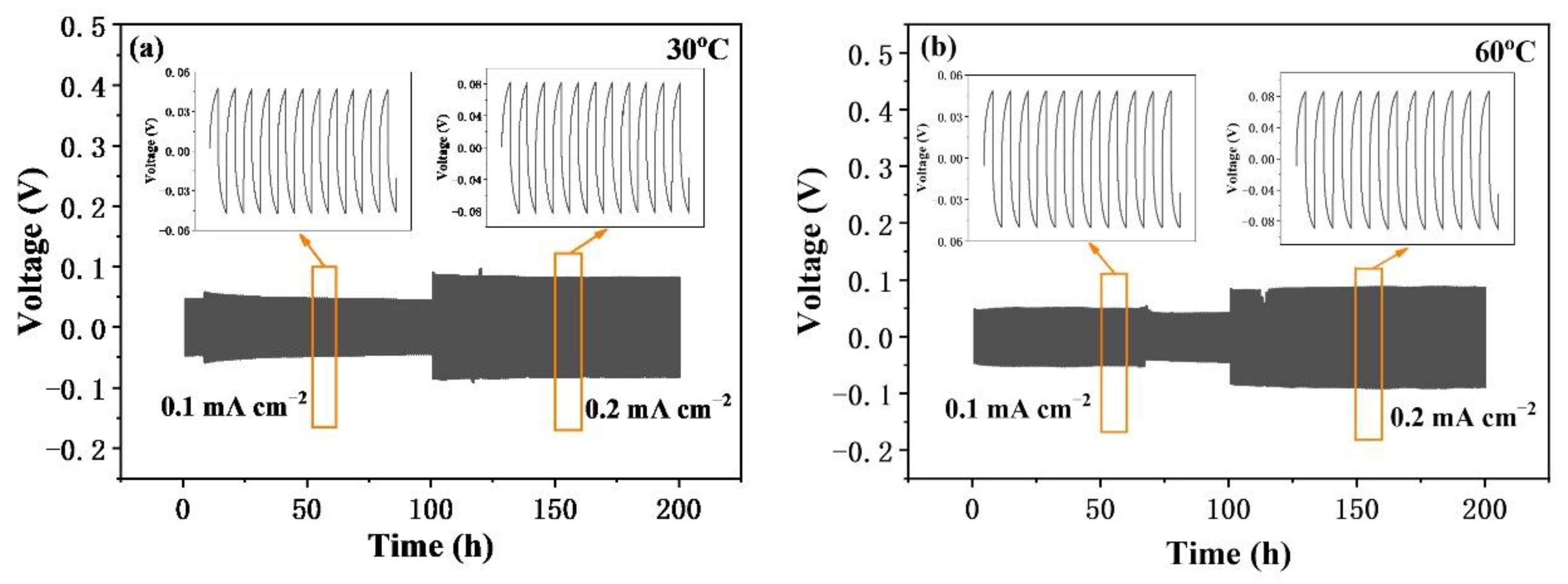
2.3. Electrochemical Tests
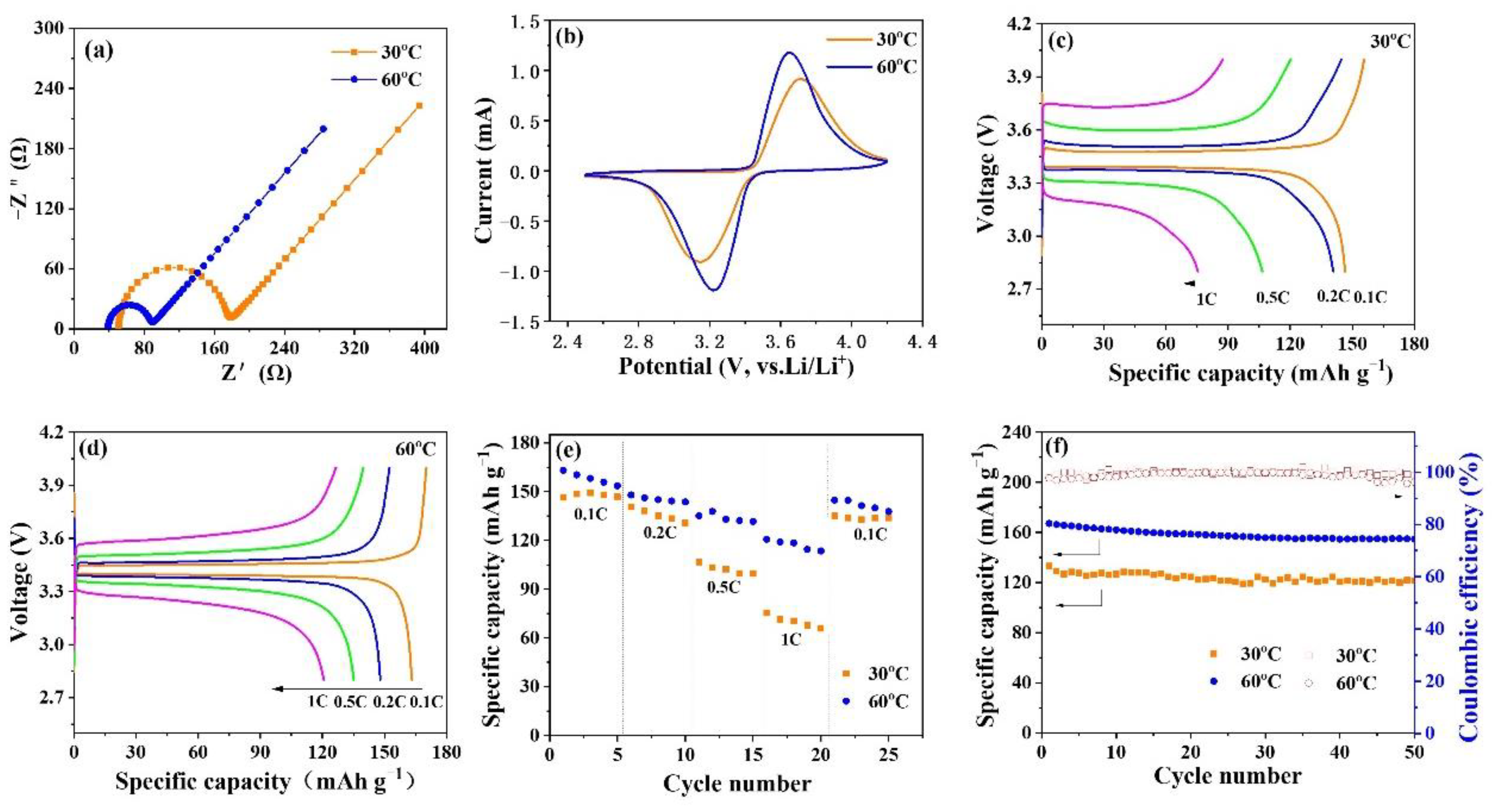
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Das, A.; Goswami, M.; Illath, K.; Ajithkumar, T.; Arya, A.; Krishnan, M. Synthesis and characterization of LAGP-glass-ceramics-based composite solid polymer electrolyte for solid-state Li-ion battery application. J. Non-Cryst. Solids 2021, 558, 120654. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Jie, Y.; Meng, X.; Omar, A.; Mikhailova, D.; Cao, R.; Jiao, S.; Lu, Y.; Xu, Y. Carbon materials for stable Li metal anodes: Challenges, solutions, and outlook. Carbon Energy 2021, 3, 957–975. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Chang, Z.; Wang, Y.; Gao, J.; Zhu, Y.; Wu, Y.; Huang, W. A Sandwich PVDF/HEC/PVDF Gel Polymer Electrolyte for Lithium Ion Battery. Electrochim. Acta 2017, 245, 752–759. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Wu, M.M.; Itoh, T.; Kubo, M.; Lin, Z.X.; Yamamoto, O. Effects of alumina whisker in (PEO)8-LiClO4-based composite polymer electrolytes. Solid State Ion. 2002, 148, 185–191. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lin, S.-J.; Wu, G.-M. Study of ionic transport properties of alkaline poly(vinyl) alcohol-based polymer electrolytes. Mater. Chem. Phys. 2005, 92, 251–255. [Google Scholar] [CrossRef]
- Huang, B.Y.; Wang, Z.X.; Li, G.B.; Huang, H.; Xue, R.J.; Chen, L.Q.; Wang, F.S. Lithium-ion conduction in polymer electrolytes based on PAN. Solid State Ion. 1996, 85, 79–84. [Google Scholar] [CrossRef]
- Kobayashi, K.; Pagot, G.; Vezzù, K.; Bertasi, F.; Di Noto, V.; Tominaga, Y. Effect of plasticizer on the ion-conductive and dielectric behavior of poly(ethylene carbonate)-based Li electrolytes. Polym. J. 2020, 53, 149–155. [Google Scholar] [CrossRef]
- Jacob, M.M.E.; Prabaharan, S.R.S.; Radhakrishna, S. Effect of PEO addition on the electrolytic and thermal properties of PVDF-LiClO4 polymer electrolytes. Solid State Ion. 1997, 104, 267–276. [Google Scholar] [CrossRef]
- Liu, T.; Chang, Z.; Yin, Y.; Chen, K.; Zhang, Y.; Zhang, X. The PVDF-HFP gel polymer electrolyte for Li-O 2 battery. Solid State Ionics 2018, 318, 88–94. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Song, Z.; Rui, K.; Wang, Q.; Xiu, T.; Badding, M.E.; Wen, Z. Manipulating Li2O atmosphere for sintering dense Li7La3Zr2O12 solid electrolyte. Energy Storage Mater. 2019, 22, 207–217. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, N.; Ma, L.; Fu, Z.; Xu, F.; Wang, C.; Guo, X. Interface engineering on cathode side for solid garnet batteries. Chem. Eng. J. 2020, 387, 124089. [Google Scholar] [CrossRef]
- MJia, M.; Zhao, N.; Huo, H.; Guo, X. Comprehensive Investigation into Garnet Electrolytes Toward Application-Oriented Solid Lithium Batteries. Electrochem. Energy Rev. 2020, 3, 656–689. [Google Scholar]
- Lu, W.; Xue, M.; Zhang, C. Modified Li7La3Zr2O12 (LLZO) and LLZO-polymer composites for solid-state lithium batteries. Energy Storage Mater. 2021, 39, 108–129. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Xu, R.; Han, F.; Ji, X.; Fan, X.; Tu, J.; Wang, C. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries. Nano Energy 2018, 53, 958–966. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.F.; Xiao, W.; Hu, J.H.; Zhang, L.L.; Yang, X.L. Design, synthesis, and application of metal sulfides for Li-S batteries: Progress and prospects. J. Mater. Chem. A 2020, 8, 17848–17882. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2018, 136, 27–46. [Google Scholar] [CrossRef]
- Keller, M.; Varzi, A.; Passerini, S. Hybrid electrolytes for lithium metal batteries. J. Power Sources 2018, 392, 206–225. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Shen, L.; Liu, Q.; Ma, J.; Lv, W.; He, Y.; Yang, Q. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Li, L.S.; Deng, Y.F.; Duan, H.H.; Qian, Y.X.; Chen, G.H. LiF and LiNO3 as synergistic additives for PEO-PVDF/LLZTO-based composite electrolyte towards high-voltage lithium batteries with dual interfaces stability. J. Energy Chem. 2022, 65, 319–328. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, H.; Duan, H.; Lu, X.; Xin, S.; Zhou, W.; Xue, L.; Fu, G.; Manthiram, A.; et al. Hybrid Polymer/Garnet Electrolyte with a Small Interfacial Resistance for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 56, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, G.; Guan, J.; Wang, L.; Chen, J.; Zheng, J. Garnet-doped composite polymer electrolyte with high ionic conductivity for dendrite-free lithium batteries. J. Energy Storage 2019, 24, 100767. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, S.F.; Huang, X.; Xu, B.Q.; Lin, Y.H.; Xu, B.; Li, L.L.; Nan, C.W.; Shen, Y. Ynergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly (vinylidenefluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef]
- Gu, Y.; She, S.; Hong, Z.; Huang, Y.; Wu, Y. Enabling lithium metal battery with flexible polymer/garnet type solid oxide composite electrolyte. Solid State Ion. 2021, 368, 115710. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Y.; Yao, P.; Ding, Z.; Tang, Q.; Wu, J.; Ye, Z.; Huang, K.; Liu, X. Hybridizing poly(vinylidene fluoride-co-hexafluoropropylene) with Li6.5La3Zr1.5Ta0.5O12 as a lithium-ion electrolyte for solid state lithium metal batteries. Chem. Eng. J. 2019, 367, 230–238. [Google Scholar] [CrossRef]
- Huggins, R.A. Simple method to determine electronic and ionic components of the conductivity in mixed conductors a review. Ionics 2002, 8, 300–313. [Google Scholar] [CrossRef]
- Tao, X.Y.; Liu, Y.Y.; Liu, W.; Zhou, G.M.; Zhao, J.; Lin, D.C.; Zu, C.X.; Sheng, O.W.; Zhang, W.K.; Lee, H.W.; et al. Solid-State Lithium−Sulfur Batteries Operated at 37 °C with Composites of Nanostructured Li7La3Zr2O12/Carbon Foam and Polymer. Nano Lett. 2017, 17, 2967–2972. [Google Scholar] [CrossRef]
- Jiang, G.S.; Qu, C.Z.; Xu, F.; Zhang, E.; Lu, Q.Q.; Cai, X.R.; Hausdorf, S.; Wang, H.Q.; Kaskel, S. Glassy metal-organic-framework-based quasi-solid-state electrolyte for high-performance Lithium-metal batteries. Adv. Funct. Mater. 2021, 31, 2104300. [Google Scholar] [CrossRef]
- Chen, F.; Yang, D.; Zha, W.; Zhu, B.; Zhang, Y.; Li, J.; Gu, Y.; Shen, Q.; Zhang, L.; Sadoway, D.R. Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries. Electrochim. Acta 2017, 258, 1106–1114. [Google Scholar] [CrossRef]
- Liang, Y.F.; Deng, S.J.; Xia, Y.; Wang, X.L. A superior composite gel polymer electrolyte of Li7La3Zr2O12-poly (vinylidenefluoride-hexafluoropropylene)(PVDF-HFP) for re-chargeable solid-state lithium ion batteries. Mater. Res. Bull. 2018, 102, 412–417. [Google Scholar] [CrossRef]
- Yan, H.; Huang, L.B.; Huang, Z.Y.; Wang, C.A. Enhanced mechanical strength and ionic conductivity of LLZO solid electrolytes by oscillatory pressure sintering. Ceram. Int. 2019, 45, 18115–18118. [Google Scholar]
- Liu, L.X.; Wang, J.W.; Oswald, S.; Hu, J.P.; Tang, H.M.; Wang, J.H.; Yin, Y.; Lu, Q.Q.; Liu, L.F.; Argibay, E.C.; et al. Decoding of oxygen network distortion in a layered high-rate anode by in situ investigation of a single microelectrode. ACS Nano 2020, 14, 11753–11764. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Ning, Y.; Lan, L.; Yang, G.; Li, M.; Tang, S.; Huang, J. Electrochemical Performance of a PVDF-HFP-LiClO4-Li6.4La3.0Zr1.4Ta0.6O12 Composite Solid Electrolyte at Different Temperatures. Nanomaterials 2022, 12, 3390. https://doi.org/10.3390/nano12193390
Liang X, Ning Y, Lan L, Yang G, Li M, Tang S, Huang J. Electrochemical Performance of a PVDF-HFP-LiClO4-Li6.4La3.0Zr1.4Ta0.6O12 Composite Solid Electrolyte at Different Temperatures. Nanomaterials. 2022; 12(19):3390. https://doi.org/10.3390/nano12193390
Chicago/Turabian StyleLiang, Xinghua, Yujuan Ning, Linxiao Lan, Guanhua Yang, Minghua Li, Shufang Tang, and Jianling Huang. 2022. "Electrochemical Performance of a PVDF-HFP-LiClO4-Li6.4La3.0Zr1.4Ta0.6O12 Composite Solid Electrolyte at Different Temperatures" Nanomaterials 12, no. 19: 3390. https://doi.org/10.3390/nano12193390
APA StyleLiang, X., Ning, Y., Lan, L., Yang, G., Li, M., Tang, S., & Huang, J. (2022). Electrochemical Performance of a PVDF-HFP-LiClO4-Li6.4La3.0Zr1.4Ta0.6O12 Composite Solid Electrolyte at Different Temperatures. Nanomaterials, 12(19), 3390. https://doi.org/10.3390/nano12193390




