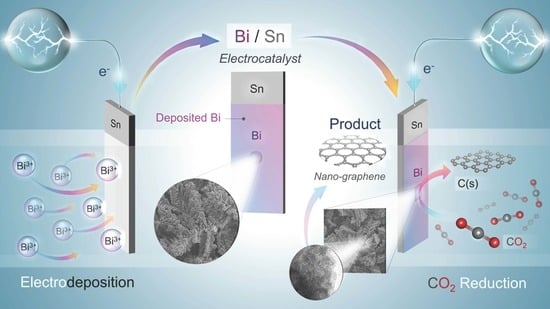
Room Temperature Nanographene Production via CO2 Electrochemical Reduction on the Electrodeposited Bi on Sn Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bi/Sn Electrodes
2.2. Electrochemical Measurements and Electrocatalytic Tests
2.3. Structure and Products Characterization
3. Results and Discussion
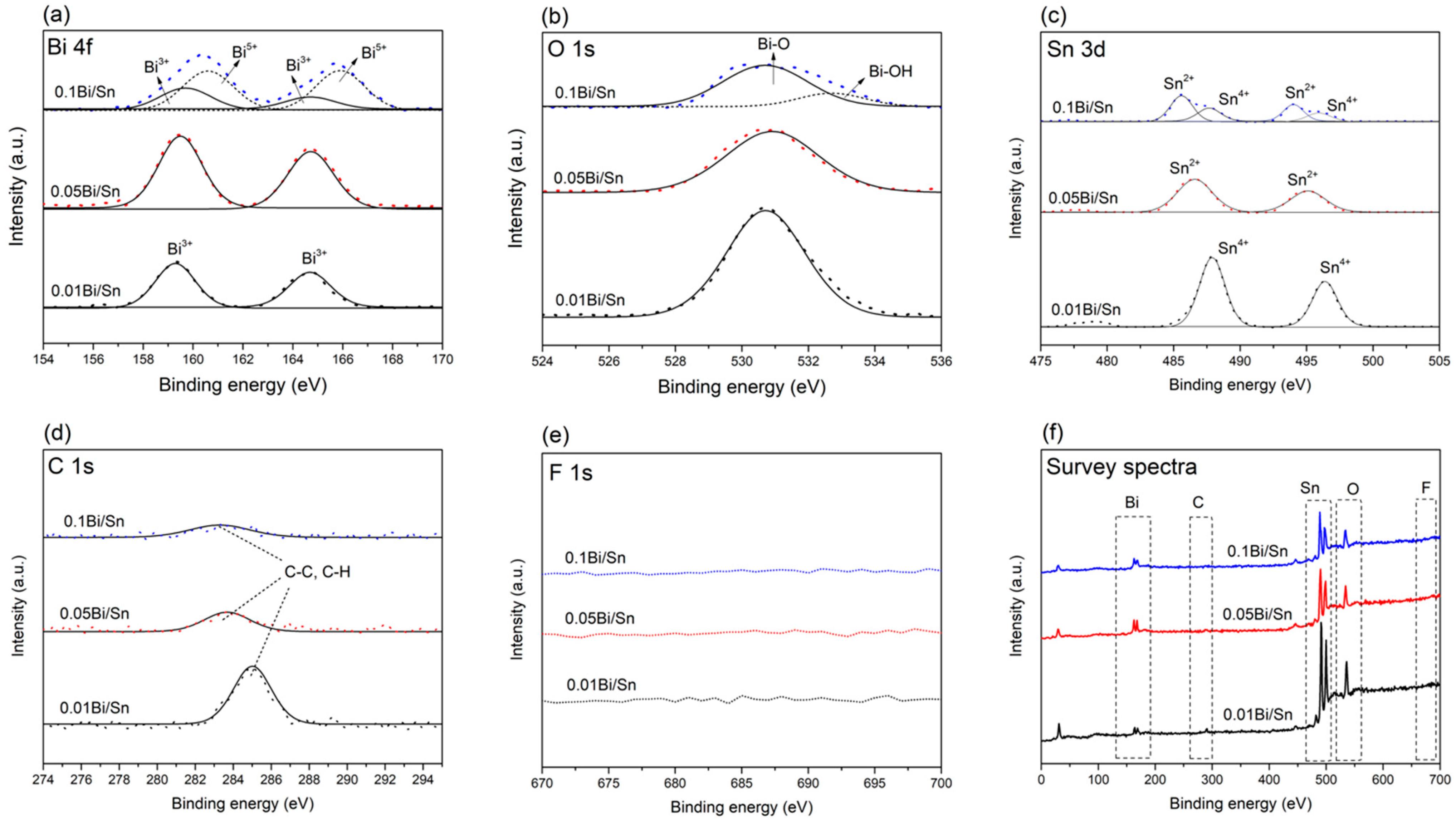
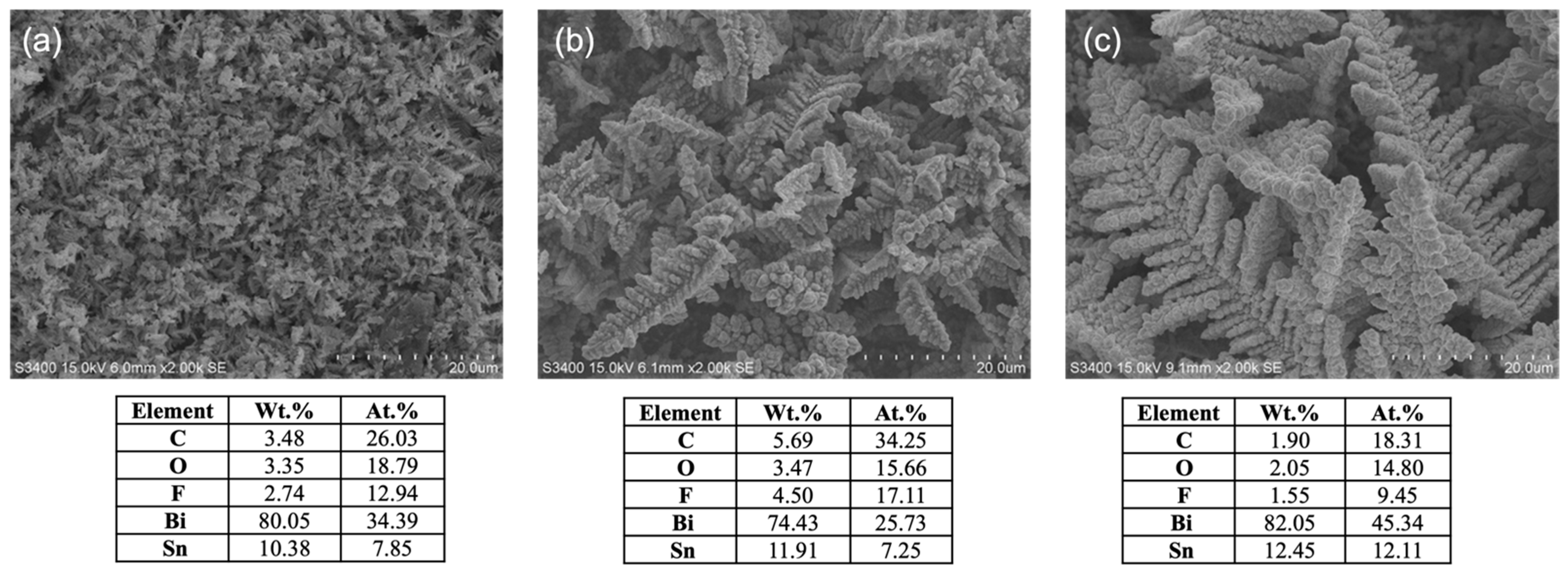
3.1. Effect of Bi Concentration on Bi/Sn Electrode Morphology
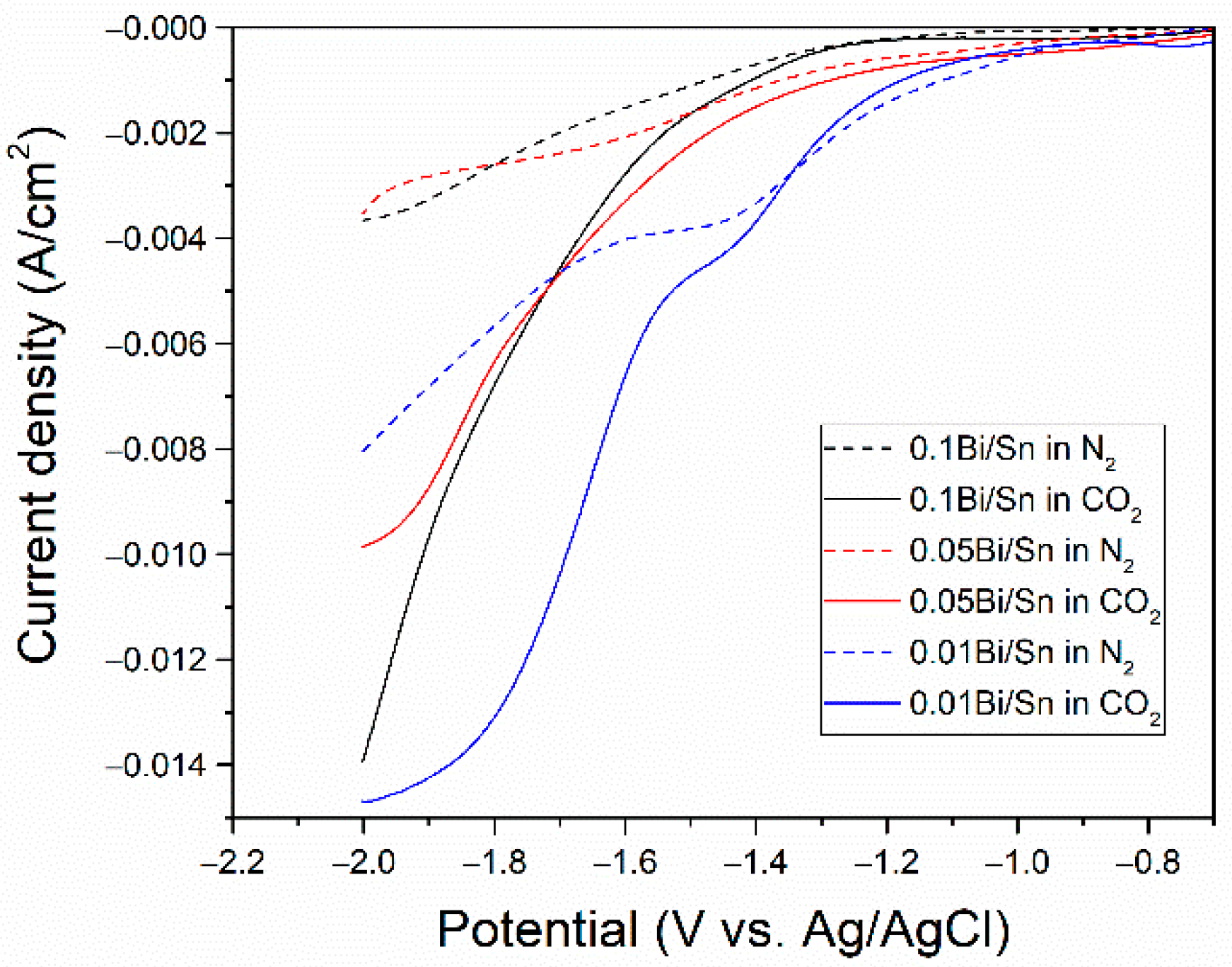
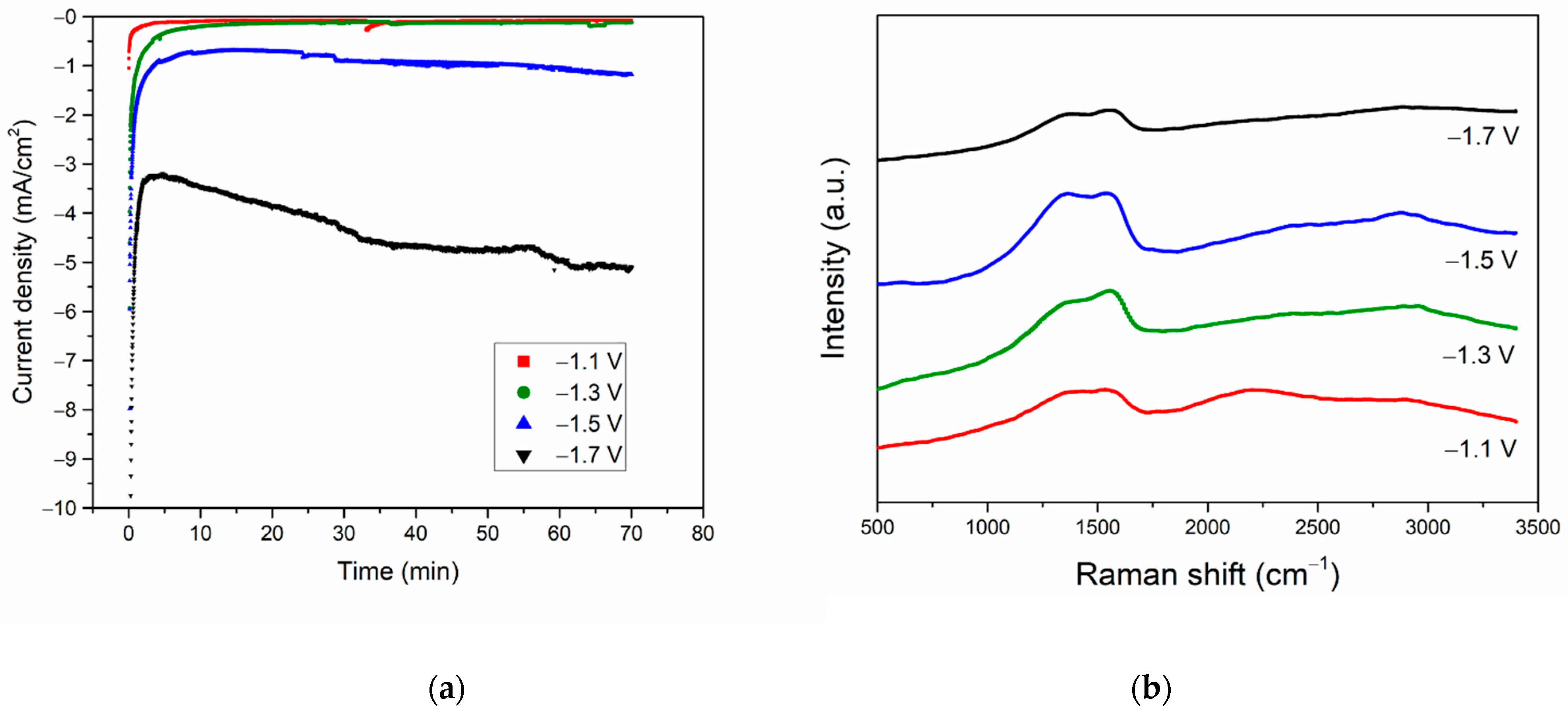
3.2. Electrochemical Measurement
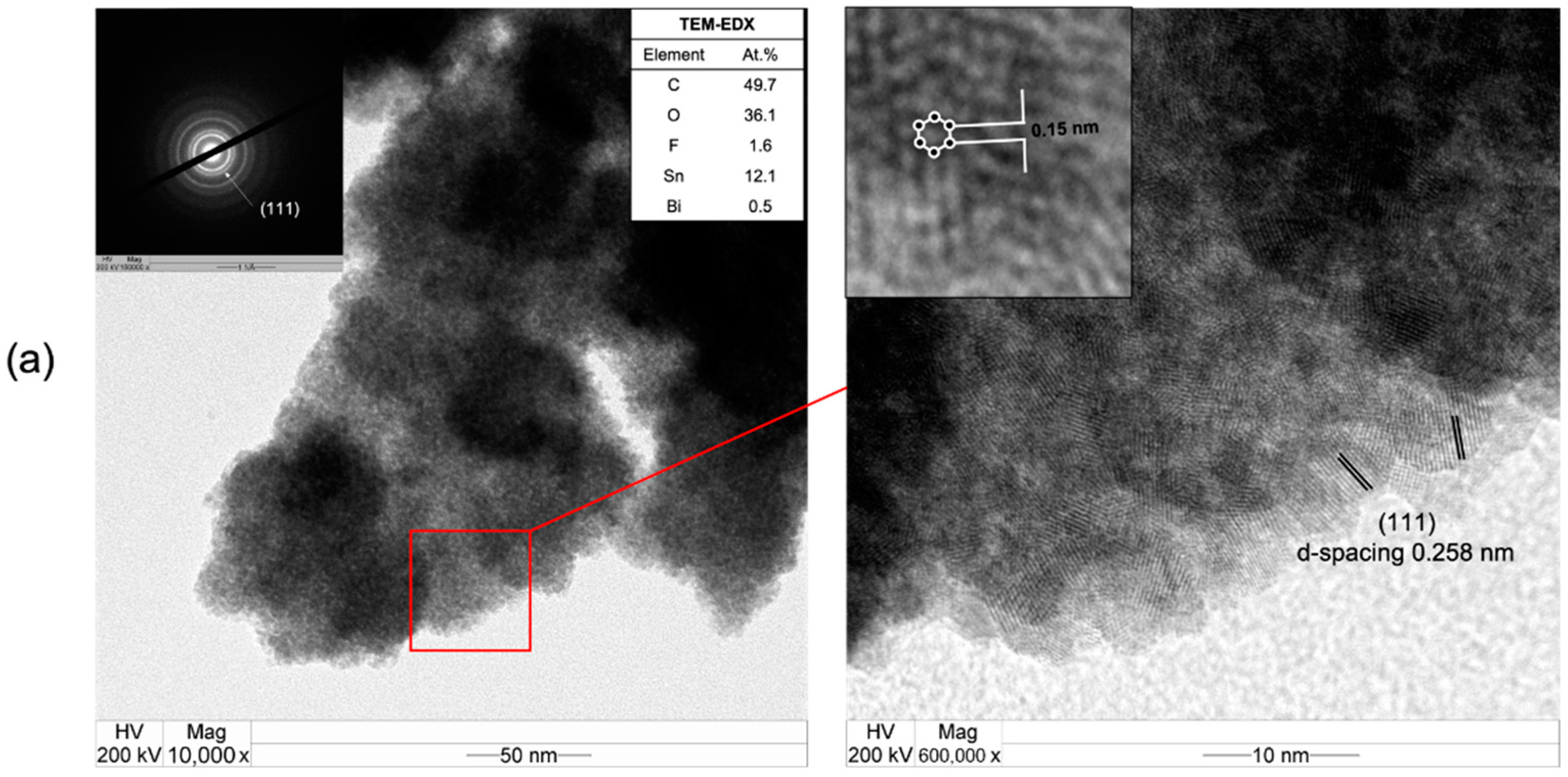
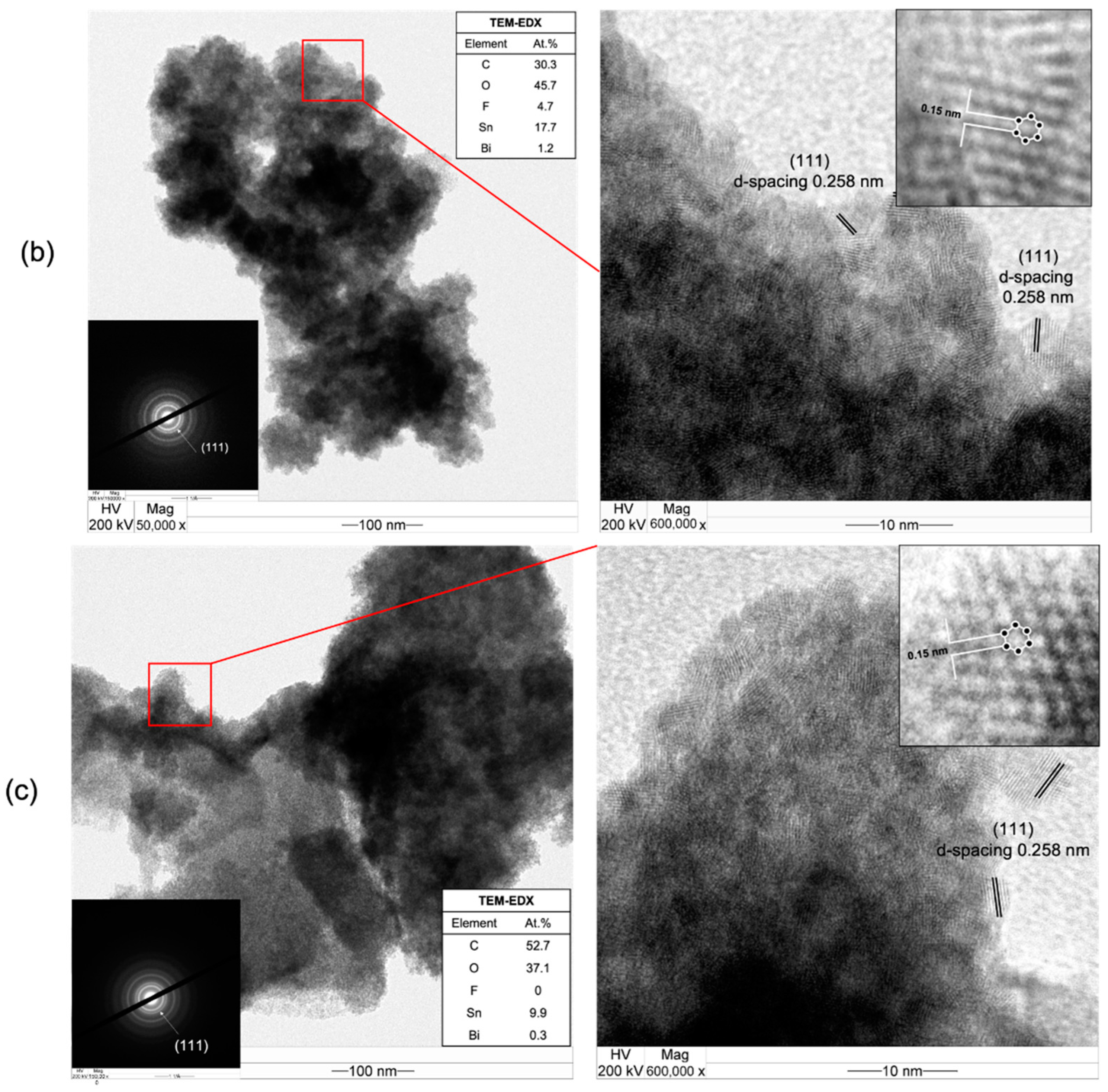
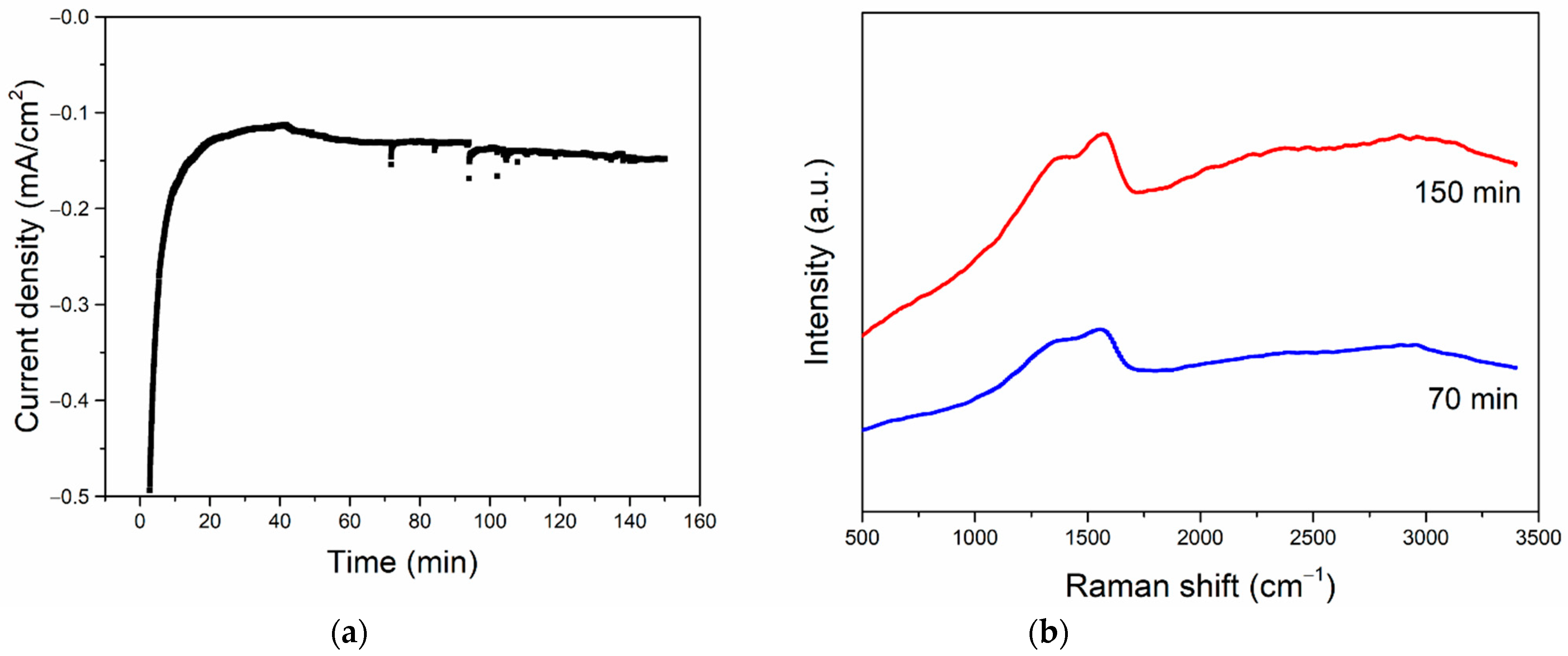
3.3. CO2RR toward Nanographene on the Bi/Sn Electrodes
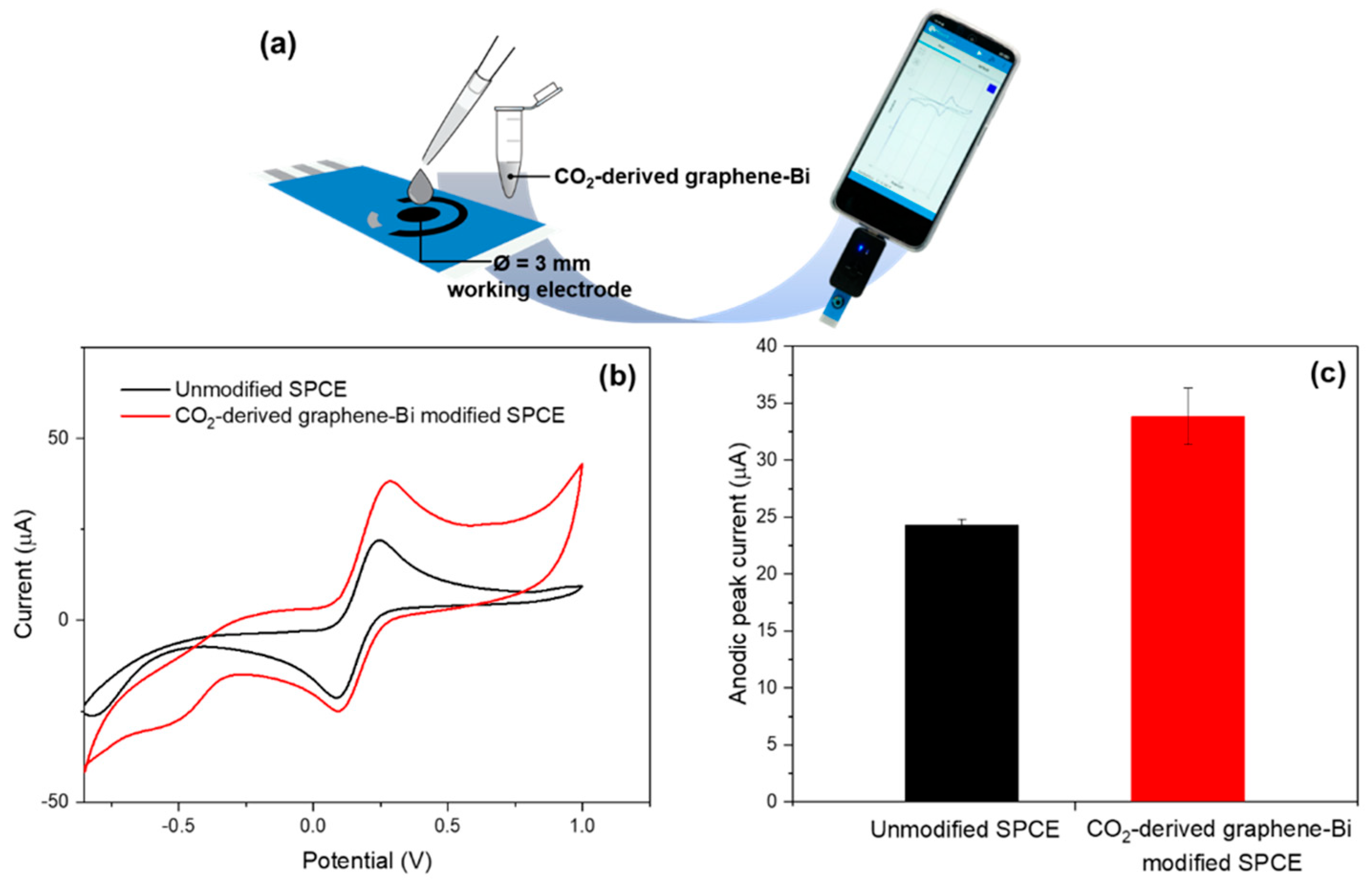
3.4. Application of CO2-Derived Nanographene–Bi Composite in Electrochemical Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wasmus, S.; Cattaneo, E.; Vielstich, W. Reduction of carbon dioxide to methane and ethene—An on-line MS study with rotating electrodes. Electrochim. Acta 1990, 35, 771–775. [Google Scholar] [CrossRef]
- Esrafilzadeh, D.; Zavabeti, A.; Jalili, R.; Atkin, P.; Choi, J.; Carey, B.J.; Brkljača, R.; O’Mullane, A.P.; Dickey, M.D.; Officer, D.L. Room temperature CO2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces. Nat. Commun. 2019, 10, 865. [Google Scholar] [CrossRef]
- Nganglumpoon, R.; Watmanee, S.; Teerawatananond, T.; Pinthong, P.; Poolboon, K.; Hongrutai, N.; Tungasmita, D.N.; Tungasmita, S.; Boonyongmaneerat, Y.; Jantaping, N. Growing 3D-nanostructured carbon allotropes from CO2 at room temperature under the dynamic CO2 electrochemical reduction environment. Carbon 2022, 187, 241–255. [Google Scholar] [CrossRef]
- Watmanee, S.; Nganglumpoon, R.; Hongrutai, N.; Pinthong, P.; Praserthdam, P.; Wannapaiboon, S.; Szilágyi, P.Á.; Morikawa, Y.; Panpranot, J. Formation and growth characteristics of nanostructured carbon films on nascent Ag clusters during room-temperature electrochemical CO2 reduction. Nanoscale Adv. 2022, 4, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tang, R.; Chen, Y.; Wang, S.; Wang, W.; Chen, X.; Mitsuzaki, N.; Chen, Z. Electrochemical reduction of CO2 to carbon films on stainless steel around room temperature. Electrochem. Commun. 2020, 110, 106606. [Google Scholar] [CrossRef]
- DiMeglio, J.L.; Rosenthal, J. Selective conversion of CO2 to CO with high efficiency using an inexpensive bismuth-based electrocatalyst. J. Am. Chem. Soc. 2013, 135, 8798–8801. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; DiMeglio, J.L.; Rosenthal, J. Efficient reduction of CO2 to CO with high current density using in situ or ex situ prepared Bi-based materials. J. Am. Chem. Soc. 2014, 136, 8361–8367. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Li, S.; Hao, X.; Xie, Z.; Du, X.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Common strategies for improving the performances of tin and bismuth-based catalysts in the electrocatalytic reduction of CO2 to formic acid/formate. Renew. Sust. Energ. Rev. 2021, 143, 110952. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.-K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 electro-reduction to formate via oxygen bidentate intermediate stabilized by high-index planes of Bi dendrite catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Qiu, Y.; Du, J.; Dong, W.; Dai, C.; Tao, C. Selective conversion of CO2 to formate on a size tunable nano-Bi electrocatalyst. J. CO2 Util. 2017, 20, 328–335. [Google Scholar] [CrossRef]
- Su, P.; Xu, W.; Qiu, Y.; Zhang, T.; Li, X.; Zhang, H. Ultrathin bismuth nanosheets as a highly efficient CO2 reduction electrocatalyst. ChemSusChem 2018, 11, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Hong, J.S.; Yang, K.D.; Jin, K.; Lee, J.H.; Ahn, H.-Y.; Seo, H.; Sung, N.-E.; Nam, K.T. Selective electrochemical production of formate from carbon dioxide with bismuth-based catalysts in an aqueous electrolyte. ACS Catal. 2018, 8, 931–937. [Google Scholar] [CrossRef]
- Bera, D.; Patil, S.; Scammon, K.; Seal, S. Diffusion-limited growth of FTO-nanofilm-coated tin fractals. Electrochem. Solid-State Lett. 2005, 8, D31. [Google Scholar] [CrossRef]
- Won, D.H.; Choi, C.H.; Chung, J.; Chung, M.W.; Kim, E.H.; Woo, S.I. Rational design of a hierarchical tin dendrite electrode for efficient electrochemical reduction of CO2. ChemSusChem 2015, 8, 3092–3098. [Google Scholar] [CrossRef]
- Teller, H.; Ohanona, S.; Kashyap, D.; Schechter, A. Morphological study of branched Sn structure formed under selected electrochemical conditions. J. Mater. Sci. 2016, 51, 8471–8483. [Google Scholar] [CrossRef]
- Djanashvili, M.; Ma, K.; Smith, W.A. Selective electrochemical reduction of CO2 to CO on CuO-derived Cu nanowires. Phys. Chem. Chem. Phys. 2015, 17, 20861–20867. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Y.; Zhang, T.; Li, X.; Zhang, H.; Chen, X. Bismuth nanodendrites as a high performance electrocatalyst for selective conversion of CO2 to formate. J. Mater. Chem. A 2016, 4, 13746–13753. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Electrochim. Acta 2002, 47, 2901–2912. [Google Scholar] [CrossRef]
- Deng, P.; Wang, H.; Qi, R.; Zhu, J.; Chen, S.; Yang, F.; Zhou, L.; Qi, K.; Liu, H.; Xia, B.Y. Bismuth oxides with enhanced bismuth–oxygen structure for efficient electrochemical reduction of carbon dioxide to formate. ACS Catal. 2019, 10, 743–750. [Google Scholar] [CrossRef]
- Firet, N.J.; Venugopal, A.; Blommaert, M.A.; Cavallari, C.; Sahle, C.J.; Longo, A.; Smith, W.A. Chemisorption of anionic species from the electrolyte alters the surface electronic structure and composition of photocharged BiVO4. Chem. Mater. 2019, 31, 7453–7462. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhang, X.; Hu, S.; Li, M.; Smart, S.; Zhu, Z.; Yuan, Z. Shape-tuned electrodeposition of bismuth-based nanosheets on flow-through hollow fiber gas diffusion electrode for high-efficiency CO2 reduction to formate. Appl. Catal. B 2021, 286, 119945. [Google Scholar] [CrossRef]
- Hong, S.; Lee, S.; Kim, S.; Lee, J.K.; Lee, J. Anion dependent CO/H2 production ratio from CO2 reduction on Au electro-catalyst. Catal. Today 2017, 295, 82–88. [Google Scholar] [CrossRef]
- Banerjee, S.; Han, X.; Thoi, V.S. Modulating the electrode–electrolyte interface with cationic surfactants in carbon dioxide reduction. ACS Catal. 2019, 9, 5631–5637. [Google Scholar] [CrossRef]
- Bienen, F.; Kopljar, D.; Geiger, S.; Wagner, N.; Friedrich, K.A. Investigation of CO2 electrolysis on tin foil by electrochemical impedance spectroscopy. ACS Sustain. Chem. Eng. 2020, 8, 5192–5199. [Google Scholar] [CrossRef]
- Piao, G.; Yoon, S.H.; Han, D.S.; Park, H. Ion-enhanced conversion of CO2 into formate on porous dendritic bismuth electrodes with high efficiency and durability. ChemSusChem 2020, 13, 698–706. [Google Scholar] [CrossRef]
- Li, Z.; Feng, Y.; Li, Y.; Chen, X.; Li, N.; He, W.; Liu, J. Fabrication of Bi/Sn bimetallic electrode for high-performance electrochemical reduction of carbon dioxide to formate. Chem. Eng. J. 2022, 428, 130901. [Google Scholar] [CrossRef]
- Yang, Y.; Ping, Y.; Gong, Y.; Wang, Z.; Fu, Q.; Pan, C. Ag/graphene composite based on high-quality graphene with high electrical and mechanical properties. Prog. Nat. Sci. 2019, 29, 384–389. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Kononenko, O.; Matveev, V.; Zotov, A.; Khodos, I.I.; Levashov, V.; Volkov, V.; Bozhko, S.I.; Chekmazov, S.V.; Roshchupkin, D. Engineering of Numerous Moiré Superlattices in Twisted Multilayer Graphene for Twistronics and Straintronics Applications. ACS Nano 2021, 15, 12358–12366. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Kapitanova, O.; Kononenko, O.; Koveshnikov, S.; Korepanov, V.; Roshchupkin, D. Large-scalable graphene oxide films with resistive switching for non-volatile memory applications. J. Alloys Compd. 2020, 849, 156699. [Google Scholar] [CrossRef]
- Oh, W.; Rhee, C.K.; Han, J.W.; Shong, B. Atomic and molecular adsorption on the Bi(111) surface: Insights into catalytic CO2 reduction. J. Phys. Chem. C 2018, 122, 23084–23090. [Google Scholar] [CrossRef]
- Popović, S.; Smiljanić, M.; Jovanovič, P.; Vavra, J.; Buonsanti, R.; Hodnik, N. Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 reduction. Angew. Chem. 2020, 132, 14844–14854. [Google Scholar] [CrossRef]
- Ren, D.; Gao, J.; Zakeeruddin, S.M.; Gratzel, M. New insights into the interface of electrochemical flow cells for carbon dioxide reduction to ethylene. J. Phys. Chem. Lett. 2021, 12, 7583–7589. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, D.; Wu, J.; Liu, J.; Li, C.; Liu, G.; Chen, D.; Feng, Y. Electroreduction of CO2 to formate with excellent selectivity and stability on nano-dendrite Bi film electrode. J. CO2 Util. 2021, 43, 101360. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, L.; Jiang, H.; Li, F.; Zhao, T.; Zhuo, M.; Chen, Q.; Mao, M.; Xu, Y. Design of counter oxidation vs. CO2 electroreduction for efficient formate production on a tin cathode. J. Electroanal. Chem. 2019, 847, 113264. [Google Scholar] [CrossRef]
- Kildahl, H.; Wang, L.; Tong, L.; Cao, H.; Ding, Y. Industrial Carbon Monoxide Production by Thermochemical CO2 Splitting—A Techno-Economic Assessment. Available online: https://ssrn.com/abstract=4075927 (accessed on 19 September 2022).
- Wyss, K.M.; De Kleine, R.D.; Couvreur, R.L.; Kiziltas, A.; Mielewski, D.F.; Tour, J.M. Upcycling end-of-life vehicle waste plastic into flash graphene. Commun. Eng. 2022, 1, 3. [Google Scholar] [CrossRef]

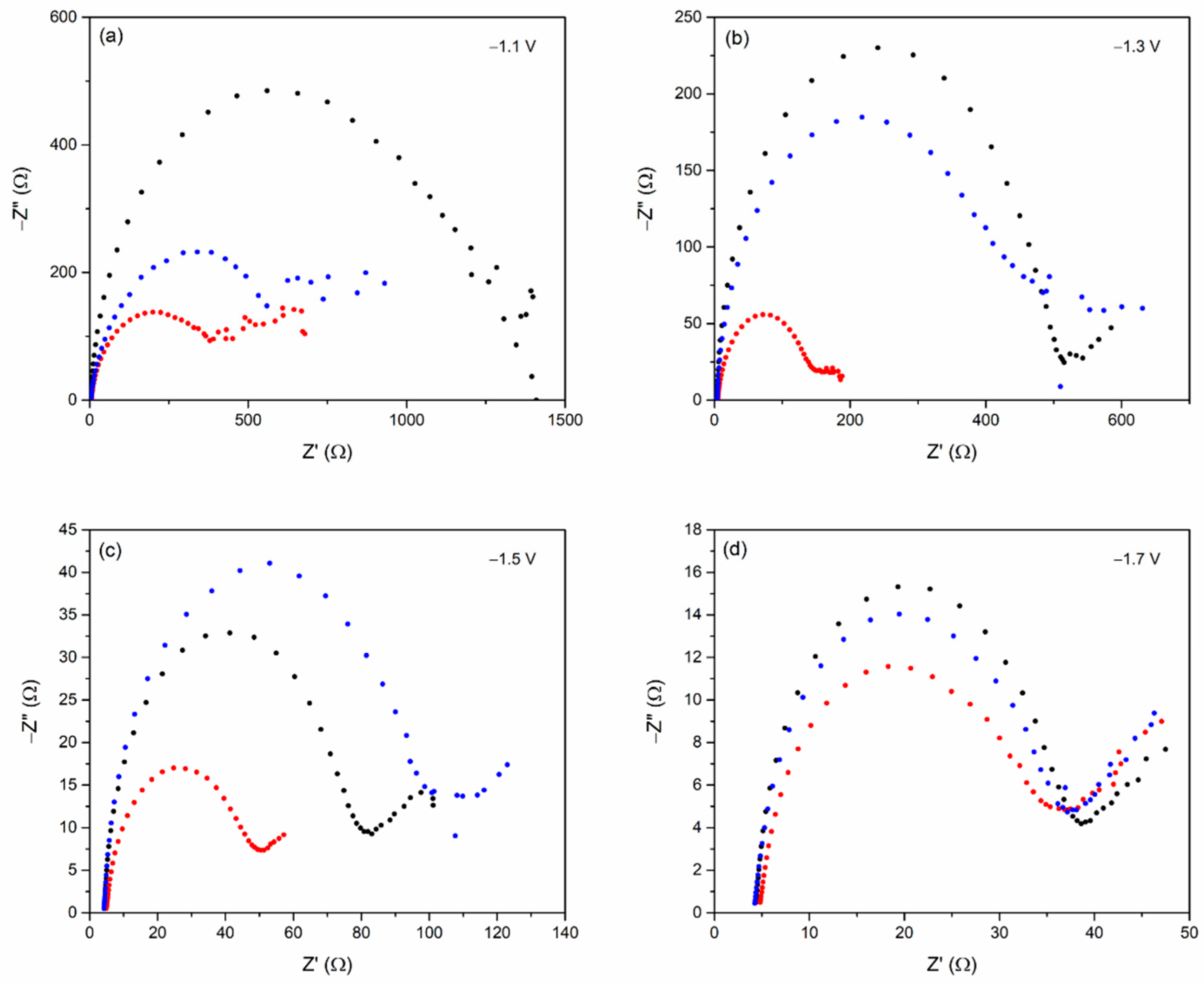

| Electrode | Rct (Charge-Transfer Resistances) (Ω) at Each Potential (vs. Ag/AgCl) | |||
|---|---|---|---|---|
| −1.1 V | −1.3 V | −1.5 V | −1.7 V | |
| 0.01Bi/Sn | 597.48 | 477.61 | 51.96 | 33.23 |
| 0.05Bi/Sn | 392.77 | 144.40 | 46.04 | 31.89 |
| 0.1Bi/Sn | 1015.44 | 516.42 | 78.68 | 34.41 |
| Applied Potential (V) vs. Ag/AgCl | Production Rate (µmole/min) | |
|---|---|---|
| H2 | CO | |
| −1.1 | 0.00 | 0.00 |
| −1.3 | 0.00 | 0.01 |
| −1.5 | 0.00 | 0.12 |
| −1.7 | 2.87 | 0.47 |
| Products | FE (%) | Input Energy (kWh/kg Product) | Energy Price * (Electricity) (USD/kg Product) | Product Price (USD/kg Product) |
|---|---|---|---|---|
| CO | 21.4 | 8.22 | 0.82 | 0.6 [35] |
| C (graphene) | 78.6 | 5.22 | 0.52 | 60–200 [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinthong, P.; Phupaichitkun, S.; Watmanee, S.; Nganglumpoon, R.; Tungasmita, D.N.; Tungasmita, S.; Boonyongmaneerat, Y.; Promphet, N.; Rodthongkum, N.; Panpranot, J. Room Temperature Nanographene Production via CO2 Electrochemical Reduction on the Electrodeposited Bi on Sn Substrate. Nanomaterials 2022, 12, 3389. https://doi.org/10.3390/nano12193389
Pinthong P, Phupaichitkun S, Watmanee S, Nganglumpoon R, Tungasmita DN, Tungasmita S, Boonyongmaneerat Y, Promphet N, Rodthongkum N, Panpranot J. Room Temperature Nanographene Production via CO2 Electrochemical Reduction on the Electrodeposited Bi on Sn Substrate. Nanomaterials. 2022; 12(19):3389. https://doi.org/10.3390/nano12193389
Chicago/Turabian StylePinthong, Piriya, Sarita Phupaichitkun, Suthasinee Watmanee, Rungkiat Nganglumpoon, Duangamol N. Tungasmita, Sukkaneste Tungasmita, Yuttanant Boonyongmaneerat, Nadtinan Promphet, Nadnudda Rodthongkum, and Joongjai Panpranot. 2022. "Room Temperature Nanographene Production via CO2 Electrochemical Reduction on the Electrodeposited Bi on Sn Substrate" Nanomaterials 12, no. 19: 3389. https://doi.org/10.3390/nano12193389
APA StylePinthong, P., Phupaichitkun, S., Watmanee, S., Nganglumpoon, R., Tungasmita, D. N., Tungasmita, S., Boonyongmaneerat, Y., Promphet, N., Rodthongkum, N., & Panpranot, J. (2022). Room Temperature Nanographene Production via CO2 Electrochemical Reduction on the Electrodeposited Bi on Sn Substrate. Nanomaterials, 12(19), 3389. https://doi.org/10.3390/nano12193389