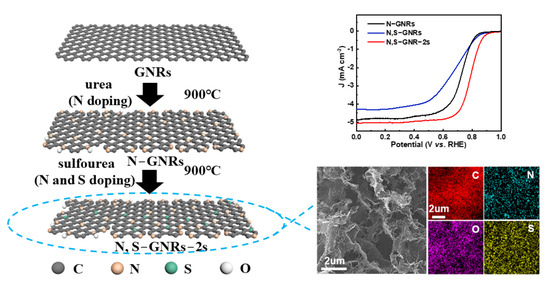
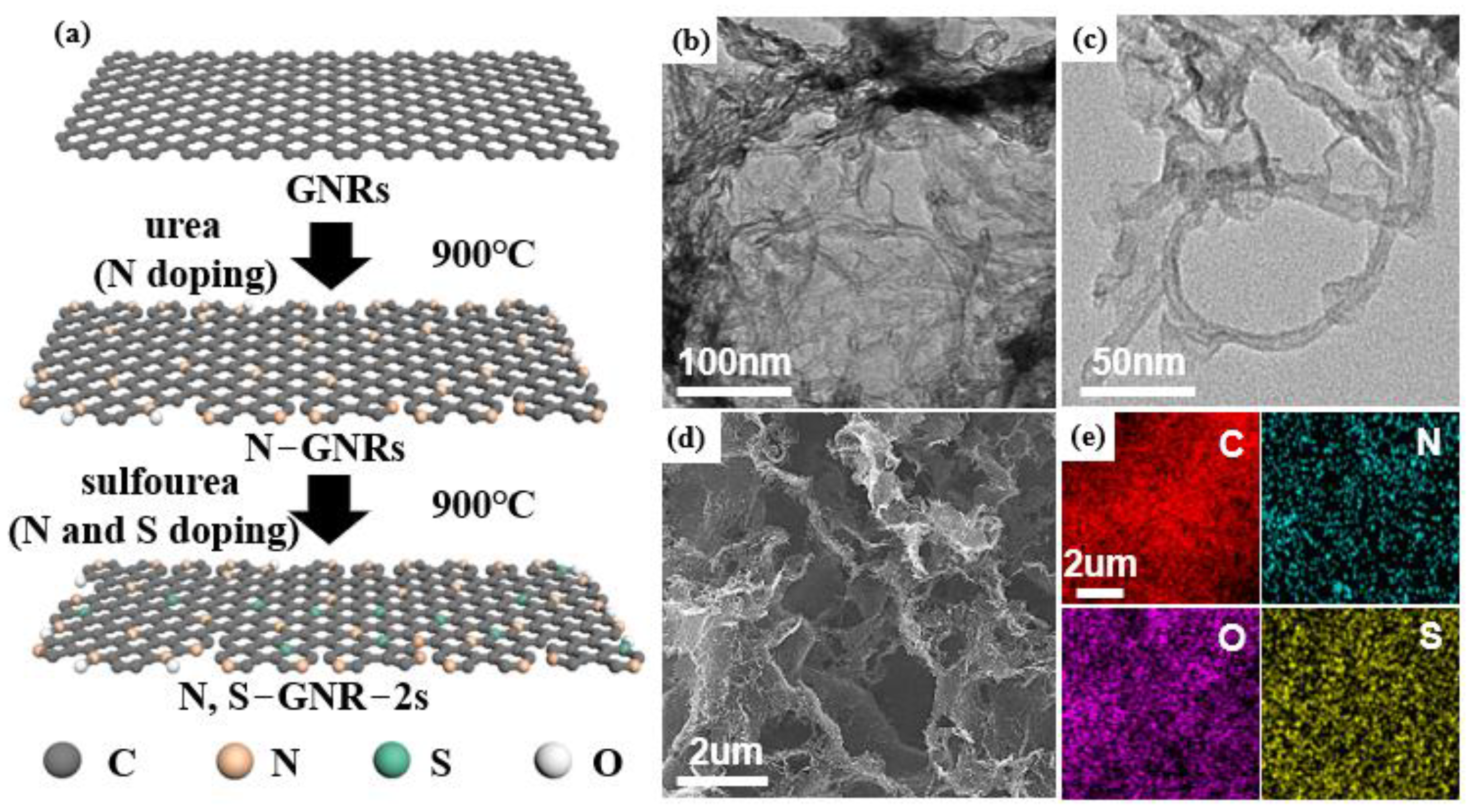
Secondary-Heteroatom-Doping-Derived Synthesis of N, S Co-Doped Graphene Nanoribbons for Enhanced Oxygen Reduction Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of N-GNRs, N, S-GNRs and N, S-GNR-2s
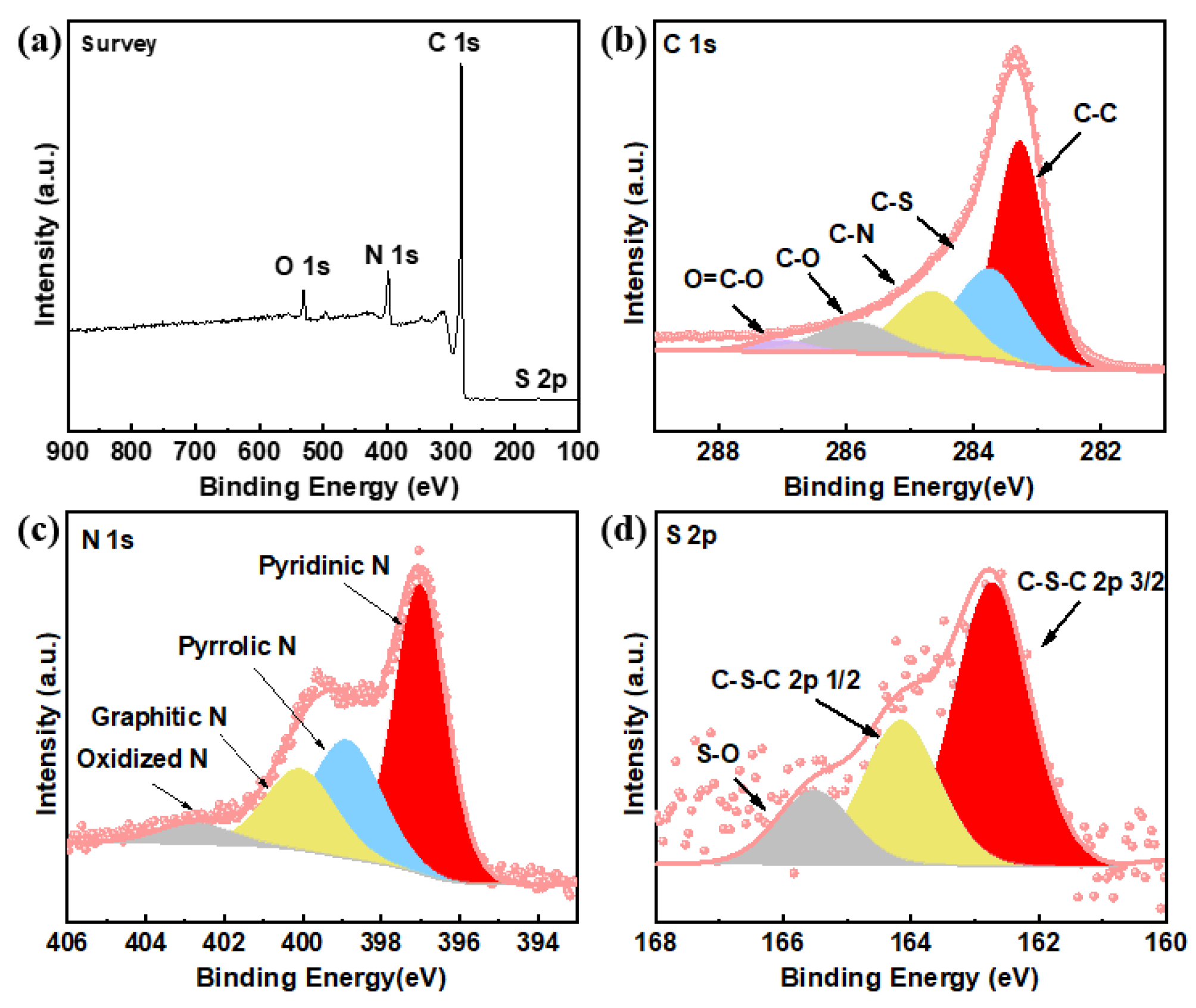
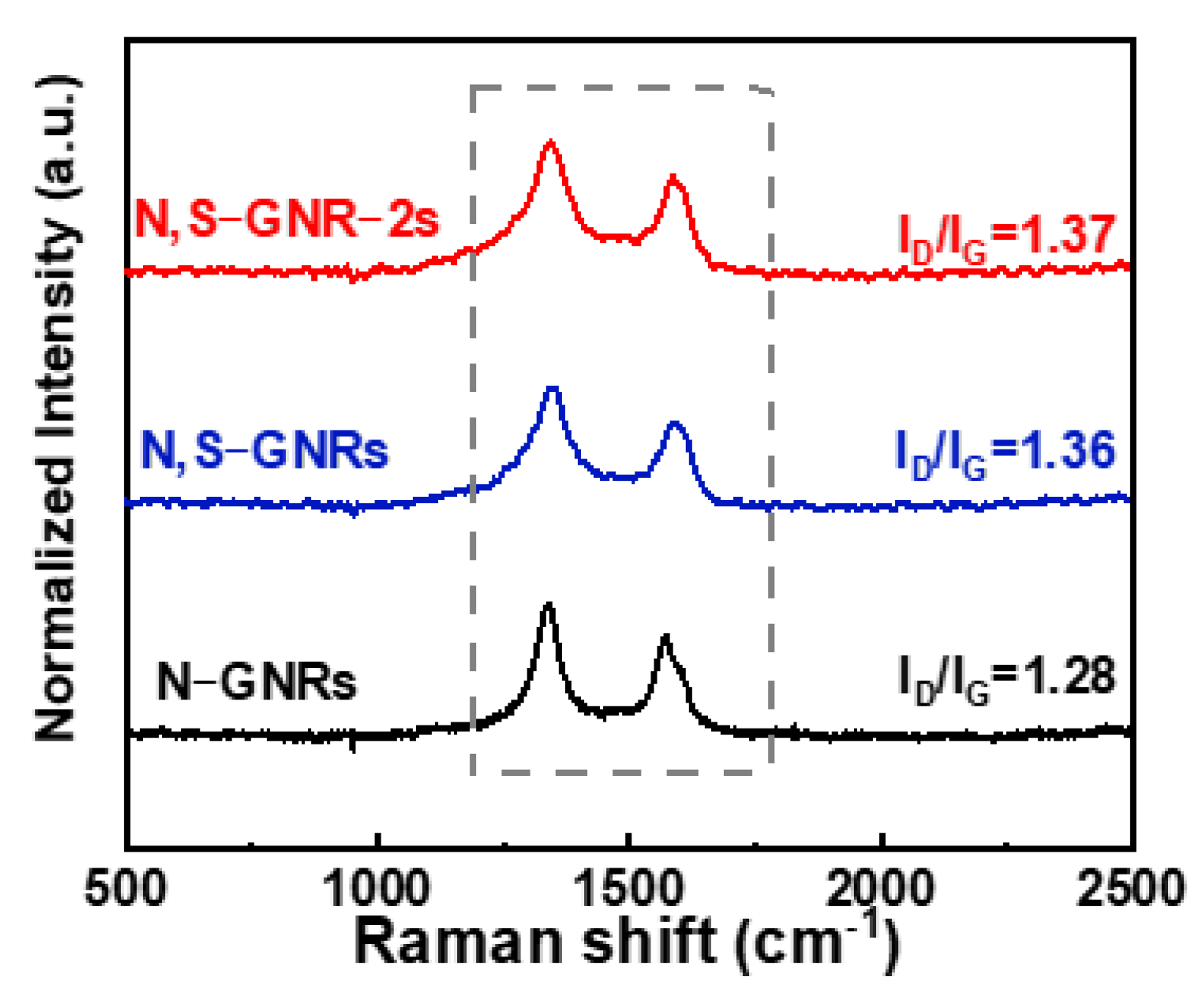
2.2. Structural Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fazendeiro, L.M.; Simoes, S.G. Historical Variation of IEA Energy and CO2 Emission Projections: Implications for Future Energy Modeling. Sustainability 2021, 13, 27. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Nayoze-Coynel, C.; Shaigan, N.; Fisher, D.; Zhao, N.N.; Zamel, N.; Gazdzicki, P.; Ulsh, M.; Friedrich, K.A.; Girard, F.; et al. A review of functions, attributes, properties and measurements for the quality control of proton exchange membrane fuel cell components. J. Power Sources 2021, 491, 39. [Google Scholar] [CrossRef]
- Fan, J.T.; Chen, M.; Zhao, Z.L.; Zhang, Z.; Ye, S.Y.; Xu, S.Y.; Wang, H.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Tao, Z.W.; Wang, C.Y.; Zhao, X.Y.; Li, J.; Guiver, M.D. Progress in High-Performance Anion Exchange Membranes Based on the Design of Stable Cations for Alkaline Fuel Cells. Adv. Mater. Technol. 2021, 6, 14. [Google Scholar] [CrossRef]
- Borchers, N.; Clark, S.; Horstmann, B.; Jayasayee, K.; Juel, M.; Stevens, P. Innovative zinc-based batteries. J. Power Sources 2021, 484, 22. [Google Scholar] [CrossRef]
- Yang, D.; Chen, D.; Jiang, Y.; Ang, E.H.X.; Feng, Y.Z.; Rui, X.H.; Yu, Y. Carbon-based materials for all-solid-state zinc-air batteries. Carbon Energy 2021, 3, 50–65. [Google Scholar] [CrossRef]
- Kundu, A.; Mallick, S.; Ghora, S.; Raj, C.R. Advanced Oxygen Electrocatalyst for Air-Breathing Electrode in Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 40172–40199. [Google Scholar] [CrossRef]
- Galos, J.; Pattarakunnan, K.; Best, A.S.; Kyratzis, I.L.; Wang, C.H.; Mouritz, A.P. Energy Storage Structural Composites with Integrated Lithium-Ion Batteries: A Review. Adv. Mater. Technol. 2021, 6, 19. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, Z.; Luo, L.A.; Fan, Y.L.; Du, Z.Y. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022, 238, 12. [Google Scholar] [CrossRef]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A perspective on the sustainability of cathode materials used in lithium-ion batteries. Adv. Energy Mater. 2021, 11, 17. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, H.L.; Yang, J.Y.; Bo, Z.; Huang, F.; Islam, M.S.; Lu, X.; Dai, L.; Amal, R.; Wang, C.H.; et al. Two-birds-one-stone: Multifunctional supercapacitors beyond traditional energy storage. Energy Environ. Sci. 2021, 14, 1854–1896. [Google Scholar] [CrossRef]
- Park, J.; Kim, W. History and Perspectives on Ultrafast Supercapacitors for AC Line Filtering. Adv. Energy Mater. 2021, 11, 28. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Su, F.Y.; Chen, C.M.; Liu, F.Y.; Wu, Z.-S.; Ma, Y. Recent advances in carbon nanostructures prepared from carbon dioxide for high-performance supercapacitors. J. Energy Chem. 2021, 54, 352–367. [Google Scholar] [CrossRef]
- Neatu, S.; Neatu, F.; Chirica, I.M.; Borbath, I.; Talas, E.; Tompos, A.; Somacescu, S.; Osiceanu, P.; Folgado, M.A.; Chaparro, A.M.; et al. Recent progress in electrocatalysts and electrodes for portable fuel cells. J. Mater. Chem. A 2021, 9, 17065–17128. [Google Scholar] [CrossRef]
- Hu, B.B.; Xia, C.R. Factors influencing the measured surface reaction kinetics parameters. Asia-Pac. J. Chem. Eng. 2016, 11, 327–337. [Google Scholar] [CrossRef]
- Li, M.R.; Zhou, W.; Zhu, Z.H. Recent development on perovskite-type cathode materials based on SrCoO3-delta parent oxide for intermediate-temperature solid oxide fuel cells. Asia-Pac. J. Chem. Eng. 2016, 11, 370–381. [Google Scholar] [CrossRef]
- Li, C.L.; Tan, H.B.; Lin, J.J.; Luo, X.L.; Wang, S.P.; You, J.; Kang, Y.-M.; Bando, Y.; Yamauchi, Y.; Kim, J. Emerging Pt-based electrocatalysts with highly open nanoarchitectures for boosting oxygen reduction reaction. Nano Today 2018, 21, 91–105. [Google Scholar] [CrossRef]
- Ly, A.; Asset, T.; Atanassov, P. Integrating nanostructured Pt-based electrocatalysts in proton exchange membrane fuel cells. J. Power Sources 2020, 478, 9. [Google Scholar] [CrossRef]
- Devi, N.; Sahoo, S.; Kumar, R.; Singh, R.K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 2021, 13, 11679–11711. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, M.Y.; Zhang, J.T.; Tu, J.G.; Ge, J.B.; Jiao, S.Q. Green and sustainable molten salt electrochemistry for the conversion of secondary carbon pollutants to advanced carbon materials. J. Mater. Chem. A 2021, 9, 14119–14146. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L.M. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 12. [Google Scholar] [CrossRef]
- Liu, M.J.; Lee, J.Y.; Yang, T.C.; Zheng, F.Y.; Zhao, J.; Yang, C.M.; Lee, L.Y.S. Synergies of Fe Single Atoms and Clusters on N-Doped Carbon Electrocatalyst for pH-Universal Oxygen Reduction. Small Methods 2021, 5, 10. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Z.N.; He, S.L.; Hao, F.; Yang, Y.Z.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for nitrobenzene hydrogenation. Appl. Catal. B Environ. 2020, 260, 118105. [Google Scholar] [CrossRef]
- Yi, S.J.; Qin, X.P.; Liang, C.H.; Li, J.S.; Rajagopalan, R.; Zhang, Z.J.; Song, J.; Tang, Y.; Cheng, F.; Wang, H.; et al. Insights into KMnO4 etched N-rich carbon nanotubes as advanced electrocatalysts for Zn-air batteries. Appl. Catal. B Environ. 2020, 264, 118537. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Tao, H.S.; Hsu, R.S.; Chen, Z.W. Highly Active Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction in Fuel Cell Applications. J. Phys. Chem. C 2009, 113, 21008–21013. [Google Scholar] [CrossRef]
- Kim, H.W.; Bukas, V.J.; Park, H.; Park, S.; Diederichsen, K.M.; Lim, J.; Cho, Y.H.; Kim, J.; Kim, W.; Han, T.H.; et al. Mechanisms of Two-Electron and Four-Electron Electrochemical Oxygen Reduction Reactions at Nitrogen-Doped Reduced Graphene Oxide. ACS Catal. 2019, 10, 852. [Google Scholar] [CrossRef]
- Gasnier, A.; Luguet, M.; Pereira, A.G.; Troiani, H.; Zampieri, G.; Gennari, F.C. Entanglement of N-doped graphene in resorcinol-formaldehyde: Effect over nanoconfined LiBH4 for hydrogen storage. Carbon 2019, 147, 284–294. [Google Scholar] [CrossRef]
- Wu, X.X.; Chen, K.Q.; Lin, Z.P.; Zhang, Y.M.; Meng, H. Nitrogen doped graphitic carbon from biomass as non noble metal catalyst for oxygen reduction reaction. Mater. Today Energy 2019, 13, 100–108. [Google Scholar] [CrossRef]
- Wang, S.H.; Yan, X.; Wu, K.H.; Chen, X.M.; Feng, J.M.; Lu, P.Y.; Feng, H.; Cheng, H.-M.; Liang, J.; Dou, S.X. A hierarchical porous Fe-N impregnated carbon-graphene hybrid for high-performance oxygen reduction reaction. Carbon 2019, 144, 798–804. [Google Scholar] [CrossRef]
- Liu, Y.M.; Xu, Q.; Fan, X.F.; Quan, X.; Su, Y.; Chen, S.; Yu, H.; Cai, Z. Electrochemical reduction of N-2 to ammonia on Co single atom embedded N-doped porous carbon under ambient conditions. J. Mater. Chem. A 2019, 7, 26358–26363. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Nitrogen-Doped Carbon Nanoparticle-Carbon Nanofiber Composite as an Efficient Metal-Free Cathode Catalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Int. 2016, 8, 6962–6971. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.J.; Gracia-Espino, E.; Ma, J.Y.; Zang, K.T.; Luo, J.; Wang, L.; Gao, S.; Mamat, X.; Hu, G.; Wagberg, T.; et al. Synergistic Effects between Atomically Dispersed Fe-N-C and C-S-C for the Oxygen Reduction Reaction in Acidic Media. Angew. Chem. Int. Edit. 2017, 56, 13800–13804. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.P.; Shan, H.B.; Lin, Y.M.; Zhang, K.; Zhou, B.J.; Zhong, Q.; Wang, P. A Fe single atom on N,S-doped carbon catalyst for performing N-alkylation of aromatic amines under solvent-free conditions. J. Mater. Chem. A 2021, 9, 25128–25135. [Google Scholar] [CrossRef]
- Chen, Z.P.; Narita, A.; Mullen, K. Graphene Nanoribbons: On-Surface Synthesis and Integration into Electronic Devices. Adv. Mater. 2020, 32, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Wang, H.S.; Ma, C.X.; Chen, L.X.; Jiang, C.X.; Chen, C.; Xie, X.; Li, A.-P.; Wang, X. Graphene nanoribbons for quantum electronics. Nat. Rev. Phys. 2021, 3, 791–802. [Google Scholar] [CrossRef]
- Xiang, T.; Wu, Z.R.; Sun, Z.T.; Cheng, C.; Wang, W.W.; Liu, Z.Z.; Yang, J.; Li, B. The Synergistic Effect of Carbon Edges and Dopants Towards Efficient Oxygen Reduction Reaction. J. Colloid Inter. Sci. 2021, 610, 486–494. [Google Scholar] [CrossRef]
- Yazdi, A.Z.; Roberts, E.P.L.; Sundararaj, U. Nitrogen/sulfur co-doped helical graphene nanoribbons for efficient oxygen reduction in alkaline and acidic electrolytes. Carbon 2016, 100, 99–108. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xu, C.X.; Hou, Z.H.; Zhou, M.J.; He, B.H.; Wang, W.; Ren, W.; Liu, Y.; Chen, L.; Xu, W. 3D N, S-co-doped carbon nanotubes/graphene/MnS ternary hybrid derived from Hummers’ method for highly efficient oxygen reduction reaction. Mater. Today Energy 2020, 16, 9100402. [Google Scholar] [CrossRef]
- Gong, Y.J.; Fei, H.L.; Zou, X.L.; Zhou, W.; Yang, S.B.; Ye, G.L.; Liu, Z.; Peng, Z.; Lou, J.; Vajtai, R.; et al. Boron- and Nitrogen-Substituted Graphene Nanoribbons as Efficient Catalysts for Oxygen Reduction Reaction. Chem. Mater. 2015, 27, 1181–1186. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef]
- Zhang, C.K.; Lin, W.Y.; Zhao, Z.J.; Zhuang, P.P.; Zhan, L.J.; Zhou, Y.H.; Cai, W. CVD synthesis of nitrogen-doped graphene using urea. Sci. China-Phys. Mech. Astron. 2015, 58, 107801. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Jiang, X.M.; Hu, J.H.; Yue, C.J.; Zhang, J.T. Controlled Synthesis of Mesoporous Nitrogen-Doped Carbon Supported Ni-Mo Sulfides for Hydrodesulfurization of Dibenzenethiophene. Catal. Lett. 2017, 147, 2515–2522. [Google Scholar] [CrossRef]
- Li, W.; Min, C.G.; Tan, F.; Li, Z.P.; Zhang, B.S.; Si, R.; Xu, M.; Kiu, W.; Zhou, L.; Yang, X.K. Bottom-Up Construction of Active Sites in a Cu-N4-C Catalyst for Highly Efficient Oxygen Reduction Reaction. ACS Nano 2019, 13, 3177–3187. [Google Scholar] [CrossRef]
- Abdelkader-Fernandez, V.K.; Domingo-Garcia, M.; Lopez-Garzon, F.J.; Fernandes, D.M.; Freire, C.; de la Torre, M.D.L.; Melguizo, M.; Godino-Salido, M.L.; Pérez-Mendoza, M. Expanding graphene properties by a simple S-doping methodology based on cold CS2 plasma. Carbon 2019, 144, 269–279. [Google Scholar] [CrossRef]
- Poh, H.L.; Simek, P.; Sofer, Z.; Pumera, M. Sulfur-Doped Graphene via Thermal Exfoliation of Graphite Oxide in H2S, SO2, or CS2 Gas. ACS Nano 2013, 7, 5262–5272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Weng, C.C.; Ren, J.T.; Ge, L.; Liu, Y.P.; Yuan, Z.Y. Phosphonate-derived nitrogen-doped cobalt phosphate/carbon nanotube hybrids as highly active oxygen reduction reaction electrocatalysts. Chin. J. Catal. 2020, 41, 259–267. [Google Scholar] [CrossRef]
- Li, R.R.; Liu, F.; Zhang, Y.H.; Guo, M.M.; Liu, D. Nitrogen, Sulfur Co-Doped Hierarchically Porous Carbon as a Metal-Free Electrocatalyst for Oxygen Reduction and Carbon Dioxide Reduction Reaction. ACS Appl. Mater. Inter. 2020, 12, 44578–44587. [Google Scholar] [CrossRef]
- Liu, J.T.; Wei, L.L.; Wang, H.Q.; Lan, G.J.; Yang, H.J.; Shen, J.Q. In-situ synthesis of heteroatom co-doped mesoporous dominated carbons as efficient electrocatalysts for oxygen reduction reaction. Electrochim. Acta 2020, 364, 11137335. [Google Scholar] [CrossRef]
- Patil, I.M.; Reddy, V.; Lokanathan, M.; Kakade, B. Nitrogen and Sulphur co-doped multiwalled carbon nanotubes as an efficient electrocatalyst for improved oxygen electroreduction. Appl. Surf. Sci. 2018, 449, 697–704. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xu, H.B.; Huang, H.Y.; Gao, L.G.; Zhao, Y.Y.; Ma, T.L. Facile synthesis of N, S co-doped porous carbons from a dual-ligand metal organic framework for high performance oxygen reduction reaction catalysts. Electrochim. Acta 2017, 254, 148–154. [Google Scholar] [CrossRef]
- Zhang, H.H.; Liu, X.Q.; He, G.L.; Zhang, X.X.; Bao, S.J.; Hu, W.H. Bioinspired synthesis of nitrogen/sulfur co-doped graphene as an efficient electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 279, 252–258. [Google Scholar] [CrossRef]
- Huang, B.B.; Hu, X.; Liu, Y.C.; Qi, W.; Xie, Z.L. Biomolecule-derived N/S co-doped CNT-graphene hybrids exhibiting excellent electrochemical activities. J. Power Sources 2019, 413, 408–417. [Google Scholar] [CrossRef]
- Wang, S.T.; Liu, Y.; Liu, X.P.; Chen, Y.; Zhao, Y.L.; Gao, S.Y. Fabricating N, S Co-Doped Hierarchical Macro-Meso-Micro Carbon Materials as pH-Universal ORR Electrocatalysts. ChemistrySelect 2022, 7, e202200044. [Google Scholar] [CrossRef]
- Zhang, X.R.; Wang, Y.Q.; Du, Y.H.; Qing, M.; Yu, F.; Tian, Z.Q.; Shen, P.K. Highly active N,S co-doped hierarchical porous carbon nanospheres from green and template-free method for super capacitors and oxygen reduction reaction. Electrochim. Acta 2019, 318, 272–280. [Google Scholar] [CrossRef]
- Nong, J.; Zhu, M.; He, K.; Zhu, A.S.; Xie, P.; Rong, M.Z.; Zhang, M.Q. N/S co-doped 3D carbon framework prepared by a facile morphology-controlled solid-state pyrolysis method for oxygen reduction reaction in both acidic and alkaline media. J. Energy Chem. 2019, 34, 220–226. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Xiang, T.; Shao, Y.; Lv, F.; Cheng, C.; Zhang, J.; Zhu, Q.; Zhang, Y.; Yang, J. Secondary-Heteroatom-Doping-Derived Synthesis of N, S Co-Doped Graphene Nanoribbons for Enhanced Oxygen Reduction Activity. Nanomaterials 2022, 12, 3306. https://doi.org/10.3390/nano12193306
Li B, Xiang T, Shao Y, Lv F, Cheng C, Zhang J, Zhu Q, Zhang Y, Yang J. Secondary-Heteroatom-Doping-Derived Synthesis of N, S Co-Doped Graphene Nanoribbons for Enhanced Oxygen Reduction Activity. Nanomaterials. 2022; 12(19):3306. https://doi.org/10.3390/nano12193306
Chicago/Turabian StyleLi, Bing, Tingting Xiang, Yuqi Shao, Fei Lv, Chao Cheng, Jiali Zhang, Qingchao Zhu, Yifan Zhang, and Juan Yang. 2022. "Secondary-Heteroatom-Doping-Derived Synthesis of N, S Co-Doped Graphene Nanoribbons for Enhanced Oxygen Reduction Activity" Nanomaterials 12, no. 19: 3306. https://doi.org/10.3390/nano12193306
APA StyleLi, B., Xiang, T., Shao, Y., Lv, F., Cheng, C., Zhang, J., Zhu, Q., Zhang, Y., & Yang, J. (2022). Secondary-Heteroatom-Doping-Derived Synthesis of N, S Co-Doped Graphene Nanoribbons for Enhanced Oxygen Reduction Activity. Nanomaterials, 12(19), 3306. https://doi.org/10.3390/nano12193306