Co-Existence of Atomic Pt and CoPt Nanoclusters on Co/SnOx Mix-Oxide Demonstrates an Ultra-High-Performance Oxygen Reduction Reaction Activity
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Preparation of CSPP Nanocatalysts
2.2. Physical Inspections of CSPP Nanocatalysts
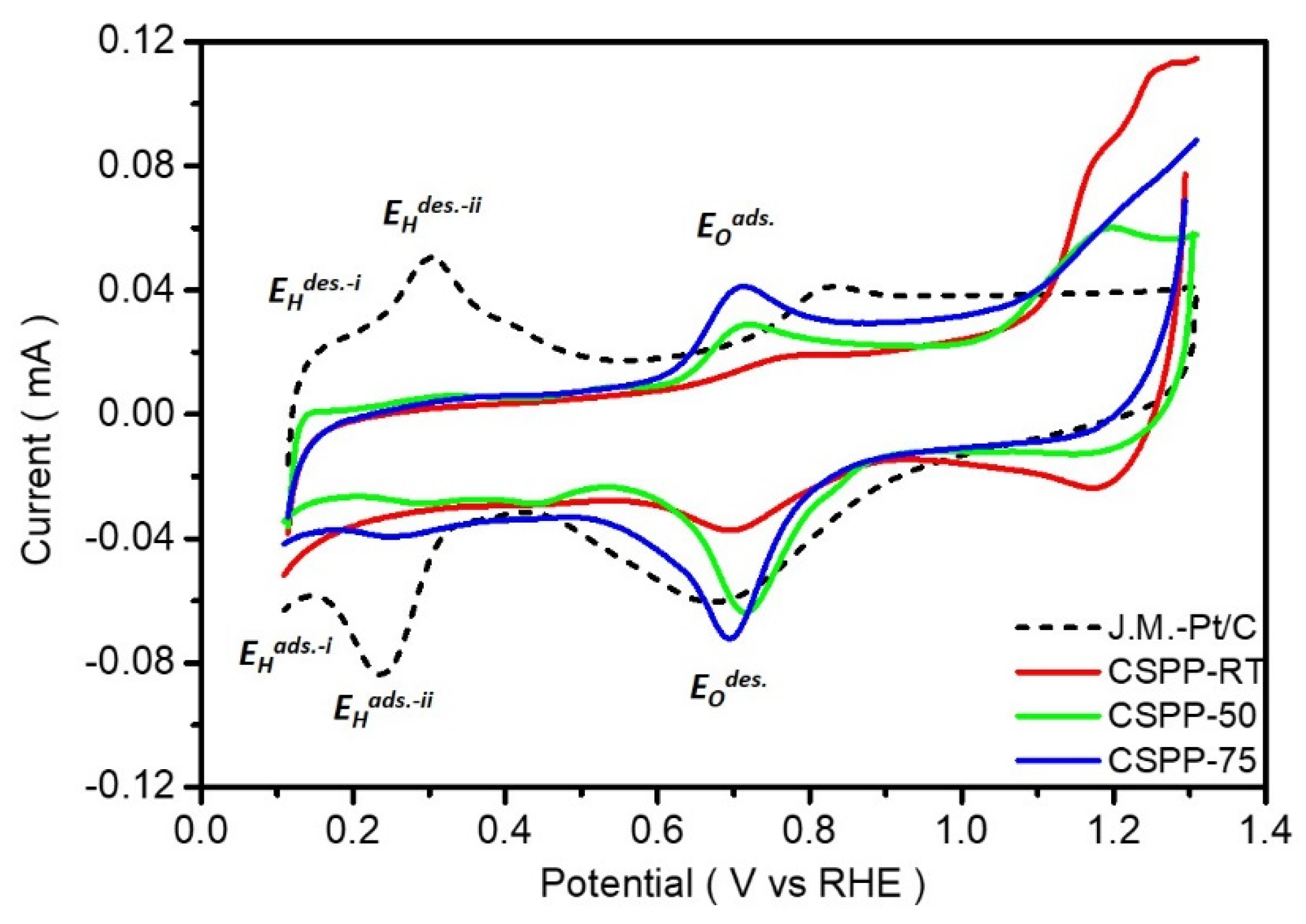
2.3. Electrochemical Analysis of CSPP Nanocatalysts
2.4. Preparation of the Electrode and the Calculation for ORR Mass Activity
3. Results and Discussion
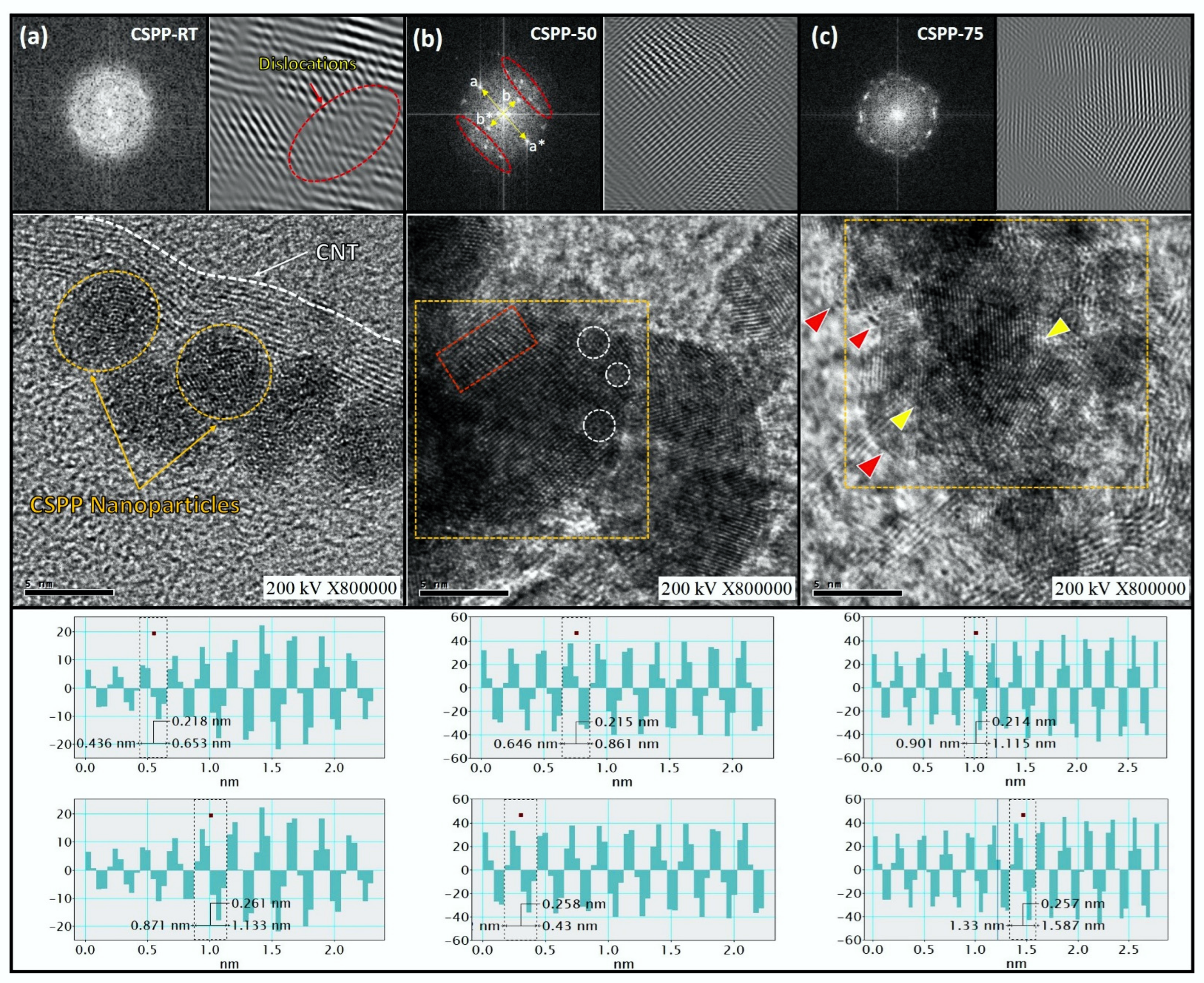
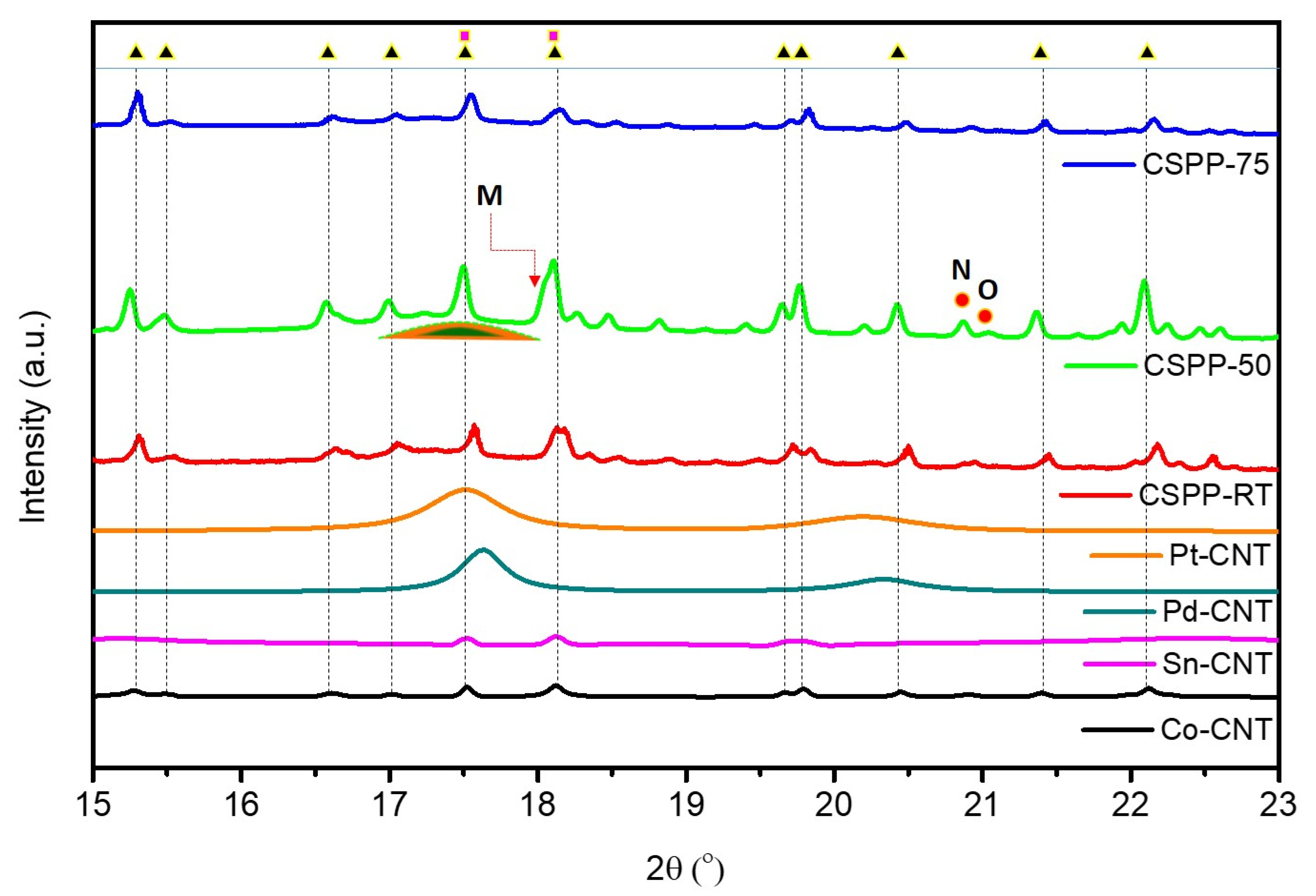
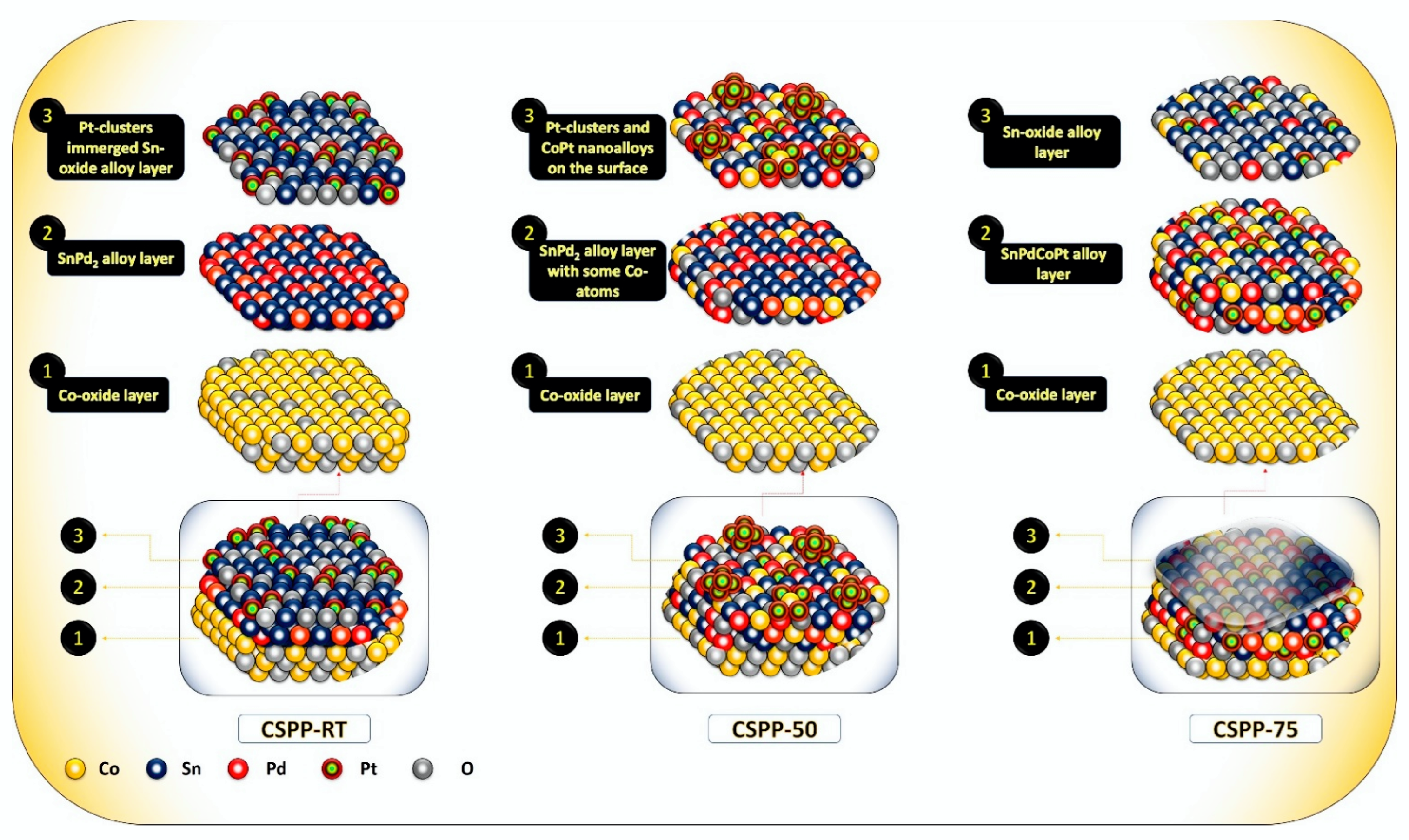
Surface Morphology and Crystal Structure of CSPP Nanocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells: Principles, Types, Fuels, and Applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef]
- Bhalothia, D.; Dai, S.; Wang, S.-P.; Yan, C.; Huang, T.-H.; Chen, P.-C.; Hiraoka, N.; Wang, K.-W.; Chen, T.-Y. Sub-nanometer Pt cluster decoration enhances the oxygen reduction reaction performances of NiOx supported Pd nano-islands. Sustain. Energy Fuels 2020, 4, 809–823. [Google Scholar] [CrossRef]
- Yi, S.; Jiang, H.; Bao, X.; Zou, S.; Liao, J.; Zhang, Z. Recent progress of Pt-based catalysts for oxygen reduction reaction in preparation strategies and catalytic mechanism. J. Electroanal. Chem. 2019, 848, 113279. [Google Scholar] [CrossRef]
- Bhalothia, D.; Krishnia, L.; Yang, S.-S.; Yan, C.; Hsiung, W.-H.; Wang, K.-W.; Chen, T.-Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Appl. Sci. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Bhalothia, D.; Huang, T.-H.; Chang, C.-W.; Lin, T.-H.; Wu, S.-C.; Wang, K.-W.; Chen, T.-Y. High-Performance and Stable Hydrogen Evolution Reaction Achieved by Pt Trimer Decoration on Ultralow-Metal Loading Bimetallic PtPd Nanocatalysts. ACS Appl. Energy Mater. 2020, 3, 11142–11152. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chen, P.-C.; Yan, C.; Yeh, W.; Tsai, D.-L.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y. Heterogeneous assembly of Pt-clusters on hierarchically structured CoOx@SnPd2@SnO2 quaternary nanocatalysts manifesting oxygen reduction reaction performance. New J. Chem. 2020, 44, 9712–9724. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, 2006494. [Google Scholar] [CrossRef]
- Gómez–Marín, A.M.; Ticianelli, E.A. A reviewed vision of the oxygen reduction reaction mechanism on Pt-based catalysts. Curr. Opin. Electrochem. 2018, 9, 129–136. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Leng, D.; Yin, F. The enhanced activity of Pt–Ce nanoalloy for oxygen electroreduction. Sci. Rep. 2020, 10, 14837. [Google Scholar] [CrossRef] [PubMed]
- Bhalothia, D.; Huang, T.-H.; Chou, P.-H.; Wang, K.-W.; Chen, T.-Y. Promoting formic acid oxidation performance of Pd nanoparticles via Pt and Ru atom mediated surface engineering. RSC Adv. 2020, 10, 17302–17310. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Nørskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Bhalothia, D.; Tsai, D.-L.; Wang, S.-P.; Yan, C.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y.; Chen, P.-C. Ir-oxide mediated surface restructure and corresponding impacts on durability of bimetallic NiOx@Pd nanocatalysts in oxygen reduction reaction. J. Alloy. Compd. 2020, 844, 156160. [Google Scholar] [CrossRef]
- Song, L.; Liang, Z.; Nagamori, K.; Igarashi, H.; Vukmirovic, M.B.; Adzic, R.R.; Sasaki, K. Enhancing Oxygen Reduction Performance of Pt Monolayer Catalysts by Pd(111) Nanosheets on WNi Substrates. ACS Catal. 2020, 10, 4290–4298. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, P.; Huang, X. Opportunities and Challenges of Interface Engineering in Bimetallic Nanostructure for Enhanced Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1806419. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Yu, H.; Mao, Q.; Xu, Y.; Li, X.; Wang, Z.; Wang, L. Interface engineering of polyaniline-functionalized porous Pd metallene for alkaline oxygen reduction reaction. Appl. Catal. B Environ. 2022, 307, 121172. [Google Scholar] [CrossRef]
- Li, H.; Dai, S.; Bhalothia, D.; Chou, J.-P.; Hu, A.; Chen, T.-Y. Collaboration between a Pt-dimer and neighboring Co–Pd atoms triggers efficient pathways for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2021, 23, 1822–1834. [Google Scholar] [CrossRef]
- Rodriguez, J. Electronic and chemical properties of Pt, Pd and Ni in bimetallic surfaces. Surf. Sci. 1996, 345, 347–362. [Google Scholar] [CrossRef]
- Bhalothia, D.; Fan, Y.-J.; Lai, Y.-C.; Yang, Y.-T.; Yang, Y.-W.; Lee, C.-H.; Chen, T.-Y. Conformational Effects of Pt-Shells on Nanostructures and Corresponding Oxygen Reduction Reaction Activity of Au-Cluster-Decorated NiOx@Pt Nanocatalysts. Nanomaterials 2019, 9, 1003. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chou, J.-P.; Yan, C.; Hu, A.; Yang, Y.-T.; Chen, T.-Y. Programming ORR Activity of Ni/NiOx@Pd Electrocatalysts via Controlling Depth of Surface-Decorated Atomic Pt Clusters. ACS Omega 2018, 3, 8733–8744. [Google Scholar] [CrossRef] [PubMed]
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. H2 Reduction Annealing Induced Phase Transition and Improvements on Redox Durability of Pt Cluster-Decorated Cu@Pd Electrocatalysts in Oxygen Reduction Reaction. ACS Omega 2019, 4, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Chou, J.-P.; Wang, K.-W.; Hsu, Y.-Y.; Hu, A.; Pan, X.; Chen, T.-Y. Platinum-trimer decorated cobalt-palladium core-shell nanocatalyst with promising performance for oxygen reduction reaction. Nat. Commun. 2019, 10, 440. [Google Scholar] [CrossRef]
- Najam, T.; Shoaib Ahmad Shah, S.; Sufyan Javed, M.; Chen, P.-T.; Chuang, C.; Saad, A.; Song, Z.; Liu, W.; Cai, X. Modulating the electronic structure of zinc single atom catalyst by P/N coordination and Co2P supports for efficient oxygen reduction in Zn-Air battery. Chem. Eng. J. 2022, 440, 135928. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Bashir, M.S.; Peng, L.; Nazir, M.A.; Javed, M.S. Single-atom catalysts for next-generation rechargeable batteries and fuel cells. Energy Storage Mater. 2022, 45, 301–322. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Javed, M.S.; Rahman, M.M.; Tsiakaras, P. Novel Mn-/Co-Nx Moieties Captured in N-Doped Carbon Nanotubes for Enhanced Oxygen Reduction Activity and Stability in Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2021, 13, 23191–23200. [Google Scholar] [CrossRef]
- Bhalothia, D.; Shuan, L.; Wu, Y.-J.; Yan, C.; Wang, K.-W.; Chen, T.-Y. A highly mismatched NiO2-to-Pd hetero-structure as an efficient nanocatalyst for the hydrogen evolution reaction. Sustain. Energy Fuels 2020, 4, 2541–2550. [Google Scholar] [CrossRef]
- Huang, T.-H.; Bhalothia, D.; Dai, S.; Yan, C.; Wang, K.-W.; Chen, T.-Y. Bifunctional Pt–SnOx nanorods for enhanced oxygen reduction and hydrogen evolution reactions. Sustain. Energy Fuels 2021, 5, 2960–2971. [Google Scholar] [CrossRef]
- Bhalothia, D.; Yu, Y.-M.; Lin, Y.-R.; Huang, T.-H.; Yan, C.; Lee, J.-F.; Wang, K.-W.; Chen, T.-Y. NiOx-supported PtRh nanoalloy enables high-performance hydrogen evolution reaction under universal pH conditions. Sustain. Energy Fuels 2021, 5, 5490–5504. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, S.-M.; Xu, Y.; Tan, L.; Wang, X.; Zhao, Y.; Duan, H.; Song, Y.-F. Single Ru atoms with precise coordination on a monolayer layered double hydroxide for efficient electrooxidation catalysis. Chem. Sci. 2019, 10, 378–384. [Google Scholar] [CrossRef]
- Sarkar, C.; Koley, P.; Shown, I.; Lee, J.; Liao, Y.-F.; An, K.; Tardio, J.; Nakka, L.; Chen, K.-H.; Mondal, J. Integration of Interfacial and Alloy Effects to Modulate Catalytic Performance of Metal–Organic-Framework-Derived Cu–Pd Nanocrystals toward Hydrogenolysis of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 10349–10362. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Tong, H.; Liu, X.; Du, L.; Chen, N.; Wang, J.; Sun, T.; Regier, T.; Sun, S. Cobalt (II) oxide nanosheets with rich oxygen vacancies as highly efficient bifunctional catalysts for ultra-stable rechargeable Zn-air flow battery. Nano Energy 2021, 79, 105409. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Liu, P.; Bi, X.; Yan, X.; Wang, W.; Ge, X.; Chen, M.; Ding, Y. Dispersing Pt atoms onto nanoporous gold for high performance direct formic acid fuel cells. Chem. Sci. 2014, 5, 403–409. [Google Scholar] [CrossRef]
- Yang, T.; Cao, G.; Huang, Q.; Ma, Y.; Wan, S.; Zhao, H.; Li, N.; Sun, X.; Yin, F. Surface-Limited Synthesis of Pt Nanocluster Decorated Pd Hierarchical Structures with Enhanced Electrocatalytic Activity toward Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 17162–17170. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Ultrafine Pt-Based Nanowires for Advanced Catalysis. Adv. Funct. Mater. 2020, 30, 2000793. [Google Scholar] [CrossRef]
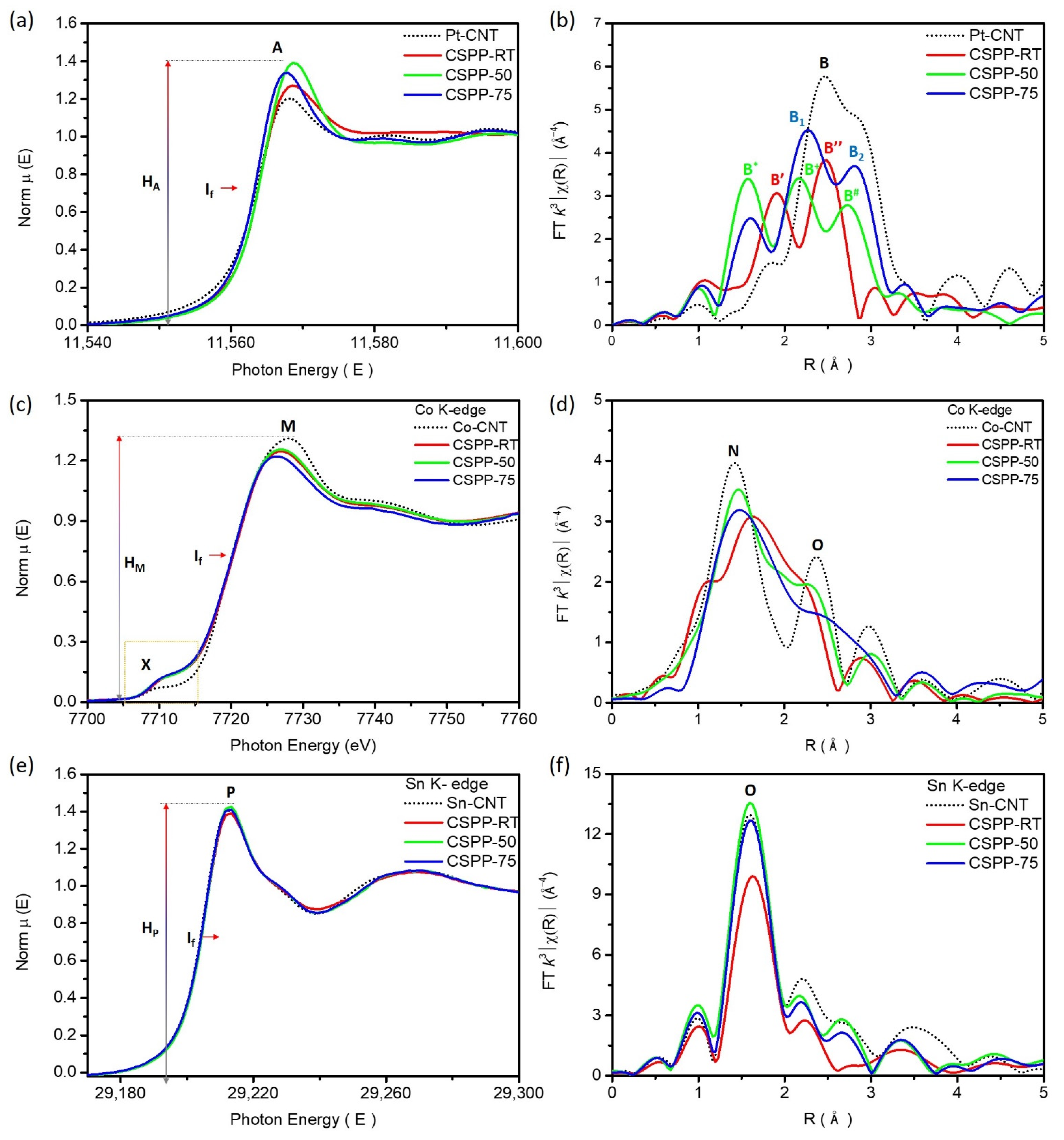

| Sample | Bond Pair | CN | R (Å) | R-Factor | ΔE0 |
|---|---|---|---|---|---|
| CSPP-RT | Pt–Pt | 1.316 | 2.686 | 0.011 | 2.045 |
| Pt–Pd | 0.795 | 2.730 | |||
| Pt–Sn | 2.274 | 2.567 | |||
| CSPP-50 | Pt–Pt | 5.759 | 2.676 | 0.015 | −0.972 |
| Pt–Pd | 1.574 | 2.643 | |||
| Pt–Sn | 0.801 | 2.494 | |||
| Pt–Co | 0.821 | 2.489 | |||
| CSPP-75 | Pt–Pt | 3.906 | 2.670 | 0.006 | −1.137 |
| Pt–Pd | 0.784 | 2.578 | |||
| Pt–Sn | 0.267 | 2.687 | |||
| Pt–Co | 1.436 | 2.565 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beniwal, A.; Bhalothia, D.; Yeh, W.; Cheng, M.; Yan, C.; Chen, P.-C.; Wang, K.-W.; Chen, T.-Y. Co-Existence of Atomic Pt and CoPt Nanoclusters on Co/SnOx Mix-Oxide Demonstrates an Ultra-High-Performance Oxygen Reduction Reaction Activity. Nanomaterials 2022, 12, 2824. https://doi.org/10.3390/nano12162824
Beniwal A, Bhalothia D, Yeh W, Cheng M, Yan C, Chen P-C, Wang K-W, Chen T-Y. Co-Existence of Atomic Pt and CoPt Nanoclusters on Co/SnOx Mix-Oxide Demonstrates an Ultra-High-Performance Oxygen Reduction Reaction Activity. Nanomaterials. 2022; 12(16):2824. https://doi.org/10.3390/nano12162824
Chicago/Turabian StyleBeniwal, Amisha, Dinesh Bhalothia, Wei Yeh, Mingxing Cheng, Che Yan, Po-Chun Chen, Kuan-Wen Wang, and Tsan-Yao Chen. 2022. "Co-Existence of Atomic Pt and CoPt Nanoclusters on Co/SnOx Mix-Oxide Demonstrates an Ultra-High-Performance Oxygen Reduction Reaction Activity" Nanomaterials 12, no. 16: 2824. https://doi.org/10.3390/nano12162824
APA StyleBeniwal, A., Bhalothia, D., Yeh, W., Cheng, M., Yan, C., Chen, P.-C., Wang, K.-W., & Chen, T.-Y. (2022). Co-Existence of Atomic Pt and CoPt Nanoclusters on Co/SnOx Mix-Oxide Demonstrates an Ultra-High-Performance Oxygen Reduction Reaction Activity. Nanomaterials, 12(16), 2824. https://doi.org/10.3390/nano12162824






