Electrocatalysis of Methanol Oxidation in Alkaline Electrolytes over Novel Amorphous Fe/Ni Biphosphate Material Prepared by Different Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Chemical Fabrication of FeNiP-R and FeNiP-S Materials
2.2.1. Fabrication of FeNiP Based on Reflux Methodology (FeNiP-R)
2.2.2. Fabrication of FeNiP Based on Sol–Gel Strategy (FeNiP-S)
2.3. Physicochemical Characterization
2.4. Electrochemical Investigation
3. Results and Discussion
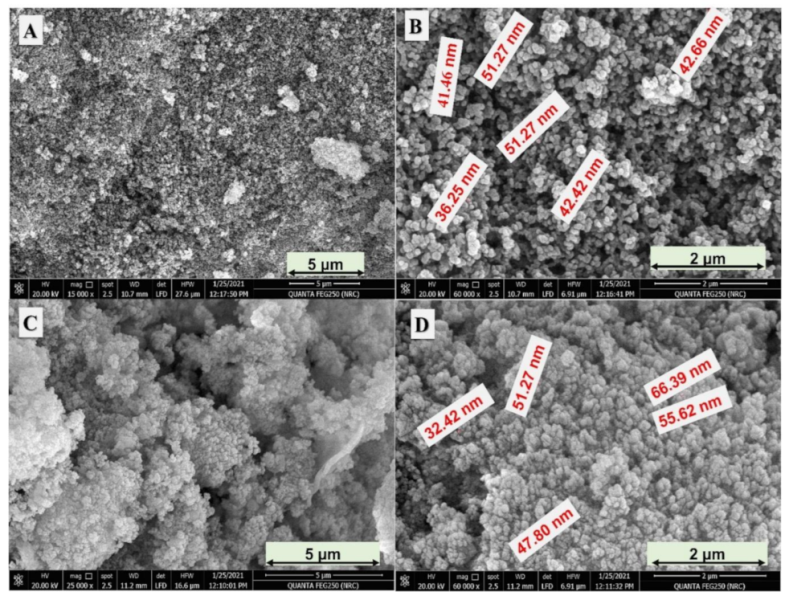
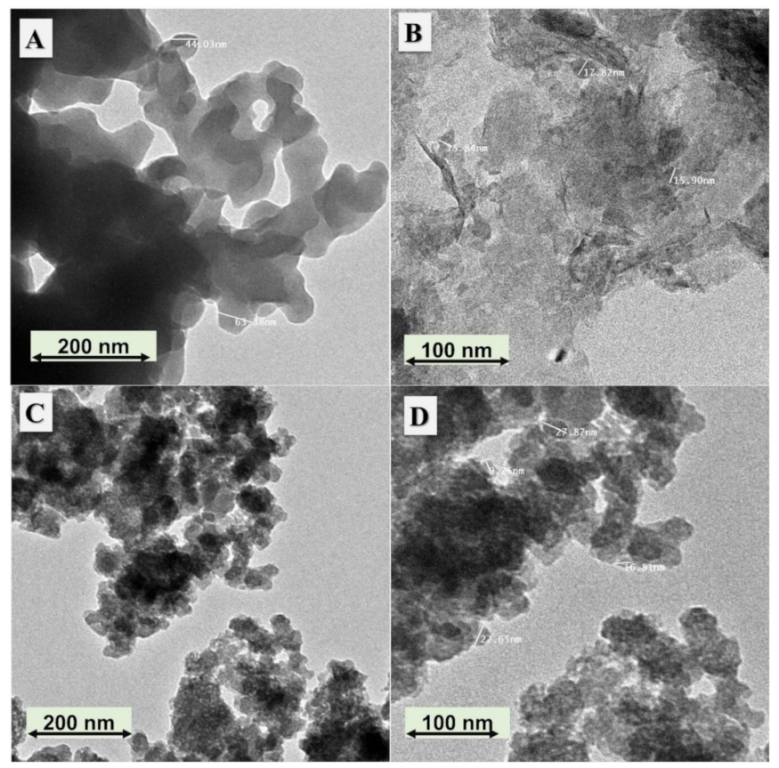
3.1. Morphological and Chemical Analyses
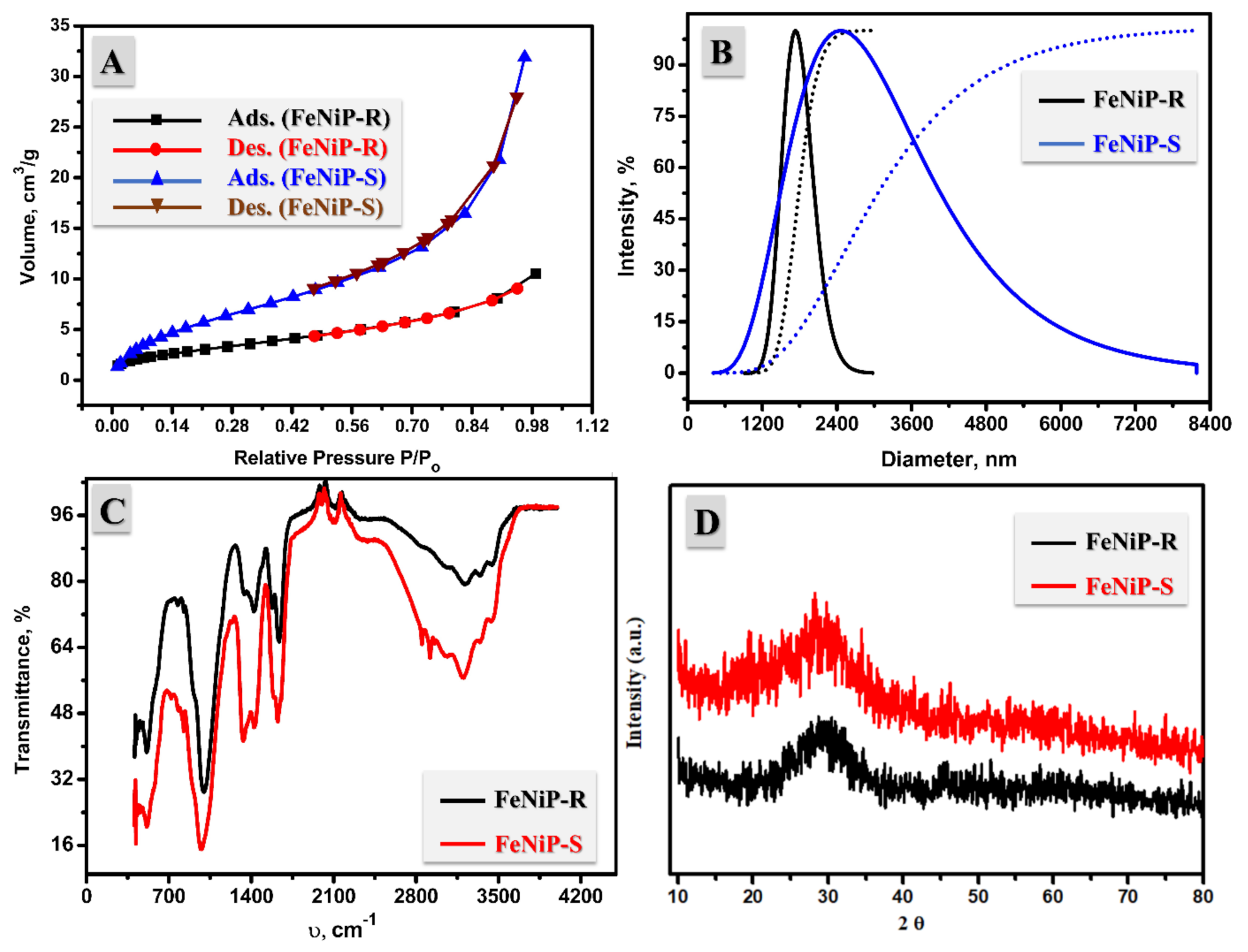
3.2. Surface Area, DLS, FT-IR, XRD Analyses
3.3. Electrochemical Analyses
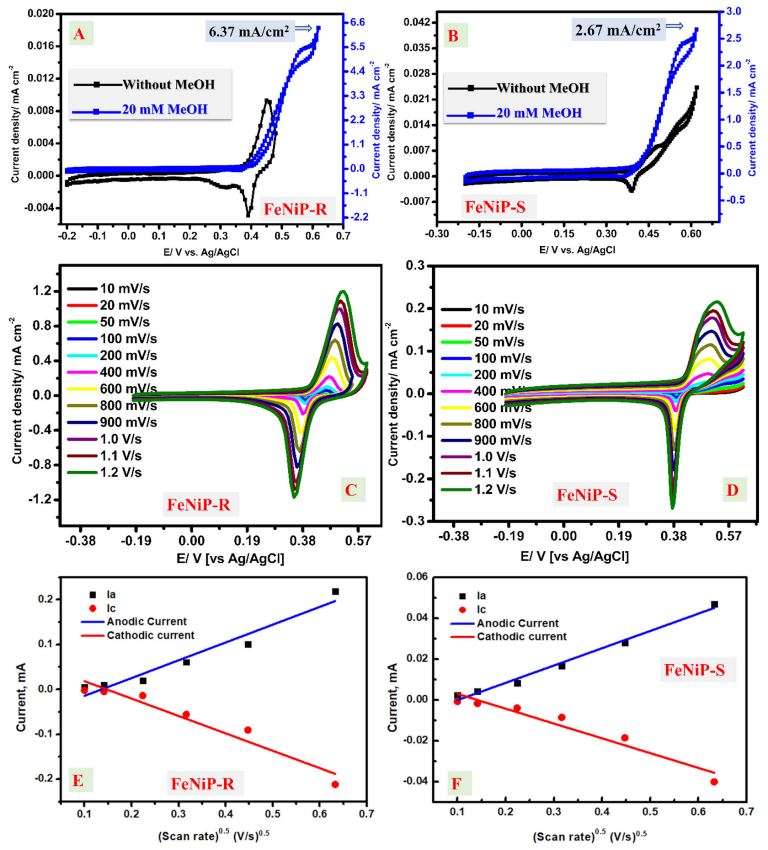
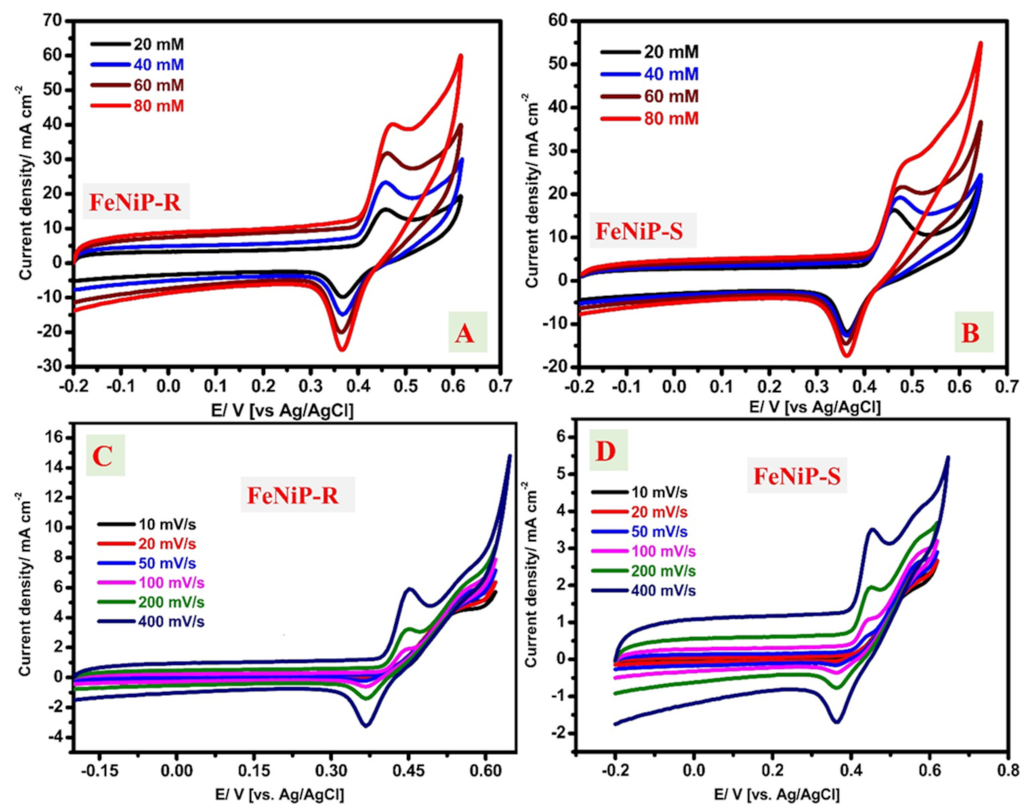
3.3.1. Cyclic Voltammetry (CV) Studies
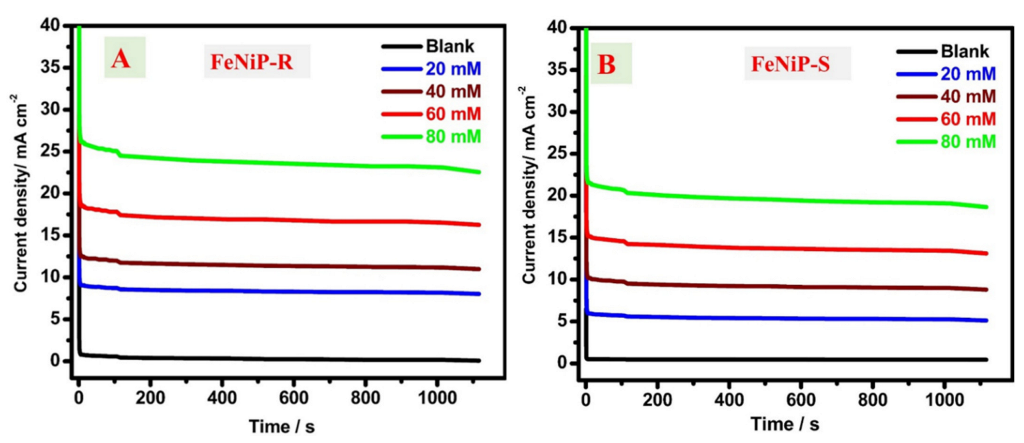
3.3.2. Chronoamperometric (CA) Studies
3.3.3. Electrochemical Impedance Spectroscopic (EIS) Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huynh, T.T.; Dang, N.N.; Pham, H.Q. Bimetallic PtIr nanoalloy on TiO2-based solid solution oxide with enhanced oxygen reduction and ethanol electro-oxidation performance in direct ethanol fuel cells. Catal. Sci. Technol. 2021, 11, 1571–1579. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, C.; Yang, Y.; Zhang, L.; Zhang, B.W.; Wu, K.H.; Zhao, S. Pt3Co@ Pt core@ shell nanoparticles as efficient oxygen reduction electrocatalysts in direct methanol fuel cell. ChemCatChem 2021, 13, 1587–1594. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, L.; Yuan, Q.; Yang, X.; Zhang, Q.; Xu, H.; Guo, Y.; Yang, S.; Zhou, Z.; Gu, L. Ultrathin PdAuBiTe Nanosheets as High-Performance Oxygen Reduction Catalysts for a Direct Methanol Fuel Cell Device. Adv. Mater. 2021, 33, 2103383. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Jin, C.; Yan, B.; Cai, J.; Li, M.; Peng, X.; Wang, Y. Tailoring Reaction Pathways by Tuning the Surface Composition of AuPt Nanocatalysts for Enhanced Formic Acid Oxidation. ACS Sustain. Chem. Eng. 2021, 9, 11062–11069. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn metal atom doping on the surface plane of one-dimesional NiMoO4 nanorods with improved redox chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Al Otaibi, N.; Prakasam, T. Electrocatalytic Properties of 3D Hierarchical Graphitic Carbon–Cobalt Nanoparticles for Urea Oxidation. ACS Omega 2020, 5, 26038–26048. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Pant, D.; Ghosh, P.C.; Adeloju, S.B. Polyaniline–Copper Composite: A Non-precious Metal Cathode Catalyst for Low-Temperature Fuel Cells. Energy Fuels 2021, 35, 3385–3395. [Google Scholar] [CrossRef]
- Mohamed, I.M.; Motlak, M.; Fouad, H.; Barakat, N.A. Cobalt/chromium nanoparticles-incorporated carbon nanofibers as effective nonprecious catalyst for methanol electrooxidation in alkaline medium. Nano 2016, 11, 1650049. [Google Scholar] [CrossRef]
- Mohammed, F.A.; Khalaf, M.M.; Mohamed, I.M.A.; Saleh, M.M.; El-Lateef, H.M.A. Synthesis of mesoporous nickel ferrite nanoparticles by use of citrate framework methodology and application for electrooxidation of glucose in alkaline media. Microchem. J. 2020, 153, 104507. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Touny, A.H.; Saleh, M.M.; Mohamed, I.M.A. Electrocatalytic performance of inorganic nanoflakes nickel phosphates under adjusted synthetic parameters towards urea and methanol oxidation in alkaline media. Microchem. J. 2021, 163, 105901. [Google Scholar] [CrossRef]
- Kottayintavida, R.; Gopalan, N.K. Nickel phosphate modified carbon supported Pd catalyst for enhanced alcohol electro oxidation. Int. J. Hydrogen Energy 2020, 45, 11116–11126. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Wu, M.-S. Zeolitic nickel phosphate nanorods with open-framework structure (VSB-5) for catalytic application in electro-oxidation of urea. Appl. Surf. Sci. 2020, 529, 147175. [Google Scholar] [CrossRef]
- Zhou, Z.; Zeng, L.; Xiong, G.; Yang, L.; Yuan, H.; Yu, J.; Xu, S.; Wang, D.; Zhang, X.; Liu, H. Multifunctional Electrocatalyst of NiCo-NiCoP Nanoparticles Embedded into P-doped Carbon Nanotubes for Energy-Saving Hydrogen Production and Upgraded Conversion of Formaldehyde. Chem. Eng. J. 2021, 426, 129214. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Wang, X. Electrostatic Interaction-Directed Growth of Nickel Phosphate Single-Walled Nanotubes for High Performance Oxygen Evolution Reaction Catalysts. Small 2016, 12, 2969–2974. [Google Scholar] [CrossRef]
- Kucernak, A.R.; Sundaram, V.N.N. Nickel phosphide: The effect of phosphorus content on hydrogen evolution activity and corrosion resistance in acidic medium. J. Mater. Chem. A 2014, 2, 17435–17445. [Google Scholar] [CrossRef]
- Li, C.; Mei, X.; Lam, F.L.-Y.; Hu, X. Hybridizing amorphous nickel cobalt phosphate and nickel phosphide as an efficient bifunctional nanocatalyst towards overall water splitting. Catal. Today 2020, 358, 215–220. [Google Scholar] [CrossRef]
- He, L.; Gong, L.; Gao, M.; Yang, C.-W.; Sheng, G.-P. In situ formation of NiCoP@ phosphate nanocages as an efficient bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2020, 337, 135799. [Google Scholar] [CrossRef]
- Pujari, S.S.; Kadam, S.A.; Ma, Y.-R.; Khalate, S.A.; Katkar, P.K.; Marje, S.J.; Patil, U.M. Highly sensitive hydrothermally prepared nickel phosphate electrocatalyst as non-enzymatic glucose sensing electrode. J. Porous Mater. 2021, 28, 369–381. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Gu, J.; Li, Y.; Tian, Z.; Pang, H. Porous rod-like Ni2P/Ni assemblies for enhanced urea electrooxidation. Nano Res. 2021, 14, 1405–1412. [Google Scholar] [CrossRef]
- Chinnadurai, D.; Rajendiran, R.; Li, O.L.; Prabakar, K. Mn-Co bimetallic phosphate on electrodeposited PANI nanowires with composition modulated structural morphology for efficient electrocatalytic water splitting. Appl. Catal. B Environ. 2021, 292, 120202. [Google Scholar] [CrossRef]
- Sun, C.; Sun, L.; Zhang, Y.; Si, H.; Fan, K.; Shi, Y.; Gu, J.; Zhang, Y. Reduced graphene oxide-modified NiCo-phosphates on Ni foam enabling high areal capacitances for asymmetric supercapacitors. J. Mater. Sci. Technol. 2020, 90, 255–263. [Google Scholar] [CrossRef]
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Li, J.; Luo, Z.; Zuo, Y.; Liu, J.; Zhang, T.; Tang, P.; Arbiol, J.; Llorca, J.; Cabot, A. NiSn bimetallic nanoparticles as stable electrocatalysts for methanol oxidation reaction. Appl. Catal. B Environ. 2018, 234, 10–18. [Google Scholar] [CrossRef]
- Sgroi, M.F.; Zedde, F.; Barbera, O.; Stassi, A.; Sebastián, D.; Lufrano, F.; Baglio, V.; Aricò, A.S.; Bonde, J.L.; Schuster, M. Cost analysis of direct methanol fuel cell stacks for mass production. Energies 2016, 9, 1008. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Yasin, A.S.; Barakat, N.A.M.; Song, S.A.; Lee, H.E.; Kim, S.S. Electrocatalytic behavior of a nanocomposite of Ni/Pd supported by carbonized PVA nanofibers towards formic acid, ethanol and urea oxidation: A physicochemical and electro-analysis study. Appl. Surf. Sci. 2018, 435, 122–129. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Liu, C. Chemical design of novel electrospun CoNi/Cr nanoparticles encapsulated in C-nanofibers as highly efficient material for urea oxidation in alkaline media. Appl. Surf. Sci. 2019, 475, 532–541. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, P.; Wang, M.; Li, F.; Wu, X.; Fan, K.; Sun, L. Selective Electro-oxidation of Alcohols to the Corresponding Aldehydes in Aqueous Solution via Cu (III) Intermediates from CuO Nanorods. ACS Sustain. Chem. Eng. 2021, 9, 11855–11861. [Google Scholar] [CrossRef]
- Sreekanth, T.; Dillip, G.; Nagajyothi, P.; Yoo, K.; Kim, J. Integration of Marigold 3D flower-like Ni-MOF self-assembled on MWCNTs via microwave irradiation for high-performance electrocatalytic alcohol oxidation and oxygen evolution reactions. Appl. Catal. B Environ. 2021, 285, 119793. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Liu, Y.; Wang, Y.; Yan, K. Electrodeposited 3D hierarchical NiFe microflowers assembled from nanosheets robust for the selective electrooxidation of furfuryl alcohol. Green Energy Environ. 2021. [Google Scholar] [CrossRef]
- Shi, Y.; Li, H.; Ao, D.; Chang, Y.; Xu, A.; Jia, M.; Jia, J. 3D nickel diselenide architecture on nitrogen-doped carbon as a highly efficient electrode for the electrooxidation of methanol and urea. J. Alloys Compd. 2021, 885, 160919. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, D.; Gu, Q.; Zhu, S.; Wang, L.; Peng, H.; Zhao, Y. Implanting cation vacancies in Ni-Fe LDHs for efficient oxygen evolution reactions of lithium-oxygen batteries. Appl. Catal. B Environ. 2021, 285, 119792. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Jiang, X.; Jiang, M.; Zhan, X.; Fu, G.; Lee, J.-M.; Tang, Y. Gd-induced electronic structure engineering of a NiFe-layered double hydroxide for efficient oxygen evolution. J. Mater. Chem. A 2021, 9, 2999–3006. [Google Scholar] [CrossRef]
- Li, W.; Jen Shih, Y.; Sanchez Carretero, D.; Huang, C.-P. The electrochemical oxidation of chloride on Pt-Ni-Co-G electrodes and its application in in-situ disinfection of water. Chem. Eng. J. 2022, 428, 132069. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Shaheer Akhtar, M.; Mohamed, I.M.A.; Dakka, Y.A.; Hamdan, R.; El-Deen, A.G.; Elsaid, K.; Obaid, M.; Al-Meer, S. Effective and stable FeNi@ N-doped graphene counter electrode for enhanced performance dye sensitized solar cells. Mater. Lett. 2017, 191, 80–84. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Zhou, D.; Luo, J.; Zhang, D.; Xu, P.; Wei, L.; Yuan, D. Synthesis of 3D flower-like cobalt nickel phosphate grown on Ni foam as an excellent electrocatalyst for the oxygen evolution reaction. J. Mater. Sci. 2018, 53, 2077–2086. [Google Scholar] [CrossRef]
- Ren, H.; Sun, X.; Du, C.; Zhao, J.; Liu, D.; Fang, W.; Kumar, S.; Chua, R.; Meng, S.; Kidkhunthod, P. Amorphous Fe–Ni–P–B–O nanocages as efficient electrocatalysts for oxygen evolution reaction. ACS Nano 2019, 13, 12969–12979. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, S.; Zhang, S.; Yan, Y.; Zhan, K.; Yang, J.; Zhao, B. Fe-Doped Ni–Co Phosphide Nanoplates with Planar Defects as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 7436–7444. [Google Scholar] [CrossRef]
- Song, S.; Guo, M.; Zhang, S.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B.; Xu, M. Plasma-assisted synthesis of hierarchical NiCoxPy nanosheets as robust and stable electrocatalyst for hydrogen evolution reaction in both acidic and alkaline media. Electrochim. Acta 2020, 331, 135431. [Google Scholar] [CrossRef]
- Chen, S.; Yang, X.; Tong, X.; Zhang, F.; Zou, H.; Qiao, Y.; Dong, M.; Wang, J.; Fan, W. Design of 3D hollow porous heterogeneous nickel–cobalt phosphides for synergistically enhancing catalytic performance for electrooxidation of methanol. ACS Appl. Mater. Interfaces 2020, 12, 34971–34979. [Google Scholar] [CrossRef]
- Al-Omair, M.A.; Touny, A.H.; Al-Odail, F.A.; Saleh, M.M. Electrocatalytic Oxidation of Glucose at Nickel Phosphate Nano/Micro Particles Modified Electrode. Electrocatalysis 2017, 8, 340–350. [Google Scholar] [CrossRef]
- Yang, J.; Tan, J.; Yang, F.; Li, X.; Liu, X.; Ma, D. Electro-oxidation of methanol on mesoporous nickel phosphate modified GCE. Electrochem. Commun. 2012, 23, 13–16. [Google Scholar] [CrossRef]
- Touny, A.H.; Tammam, R.H.; Saleh, M.M. Electrocatalytic oxidation of formaldehyde on nanoporous nickel phosphate modified electrode. Appl. Catal. B Environ. 2018, 224, 1017–1026. [Google Scholar] [CrossRef]
- Song, D.; Hong, D.; Kwon, Y.; Kim, H.; Shin, J.; Lee, H.M.; Cho, E. Highly porous Ni–P electrode synthesized by an ultrafast electrodeposition process for efficient overall water electrolysis. J. Mater. Chem. A 2020, 8, 12069–12079. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C. Iron-Doped Nickel Phosphate as Synergistic Electrocatalyst for Water Oxidation. Chem. Mater. 2016, 28, 5659–5666. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Cardoso, E.S.F.; Fortunato, G.V.; Casagrande, G.A.; Senthilkumar, B.; Madhavan, J.; Maia, G. Highly Electroactive Ni Pyrophosphate/Pt Catalyst toward Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 4969–4982. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, C.; Zheng, J.; Wang, L.; Yang, Z.; Yang, W. 3D hierarchical Ni(PO3)2 nanosheet arrays with superior electrochemical capacitance behavior. J. Mater. Chem. A 2017, 5, 1421–1427. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M.; Mohamed, I.M.A. An efficient and non-precious anode electrocatalyst of NiO-modified carbon nanofibers towards electrochemical urea oxidation in alkaline media. Ceram. Int. 2020, 46, 20376–20384. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Almulhim, N.F.; Mohamed, I.M.A. Physicochemical and electrochemical investigations of an electrodeposited CeNi2@NiO nanomaterial as a novel anode electrocatalyst material for urea oxidation in alkaline media. J. Mol. Liq. 2019, 297, 111737. [Google Scholar] [CrossRef]
- Chinnadurai, D.; Nallal, M.; Kim, H.J.; Li, O.L.; Park, K.H.; Prabakar, K. Mn3+ Active Surface Site Enriched Manganese Phosphate Nano-polyhedrons for Enhanced Bifunctional Oxygen Electrocatalyst. ChemCatChem 2020, 12, 2348–2355. [Google Scholar] [CrossRef]
- Ojha, R.P.; Pal, S.; Prakash, R. Cu-Fe Prussian blue analog nanocube with intrinsic oxidase mimetic behaviour for the non-invasive colorimetric detection of Isoniazid in human urine. Microchem. J. 2021, 171, 106854. [Google Scholar] [CrossRef]
- Xing, X.; Song, Y.; Jiang, W.; Zhang, X. CuFe-P from a Prussian blue analogue as an electrocatalyst for efficient full water splitting. Sustain. Energy Fuels 2020, 4, 3985–3991. [Google Scholar] [CrossRef]
- Xuan, C.; Wang, J.; Xia, W.; Peng, Z.; Wu, Z.; Lei, W.; Xia, K.; Xin, H.L.; Wang, D. Porous Structured Ni-Fe-P Nanocubes Derived from a Prussian Blue Analogue as an Electrocatalyst for Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 26134–26142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, Y.; Li, X.; Wang, Y. Investigation of phosphate removal mechanisms by a lanthanum hydroxide adsorbent using p-XRD, FTIR and XPS. Appl. Surf. Sci. 2021, 557, 149838. [Google Scholar] [CrossRef]
- Iqbal, W.; Yang, B.; Zhao, X.; Rauf, M.; Mohamed, I.M.A.; Zhang, J.; Mao, Y. Facile one-pot synthesis of mesoporous g-C3N4 nanosheets with simultaneous iodine doping and N-vacancies for efficient visible-light-driven H2 evolution performance. Catal. Sci. Technol. 2020, 10, 549–559. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Yang, P.; Cheng, X. Morphological evolution of Co phosphate and its electrochemical and photocatalytic performance. CrystEngComm 2018, 20, 6982–6988. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, J.-M.; Lee, S.J.; Choi, B.G.; Lee, K.G. Protein-directed assembly of cobalt phosphate hybrid nanoflowers. J. Colloid Interface Sci. 2016, 484, 44–50. [Google Scholar] [CrossRef]
- Arunachalam, P.; Shaddad, M.N.; Alamoudi, A.S.; Ghanem, M.A.; Al-Mayouf, A.M. Microwave-Assisted Synthesis of Co3(PO4)2 Nanospheres for Electrocatalytic Oxidation of Methanol in Alkaline Media. Catalysts 2017, 7, 119. [Google Scholar] [CrossRef]
- Alsafrani, A.E.; Adeosun, W.A.; Alruwais, R.S.; Marwani, H.M.; Asiri, A.M.; Khan, I.; Khan, A. Preparation, characterization and super electrocatalytic sensing study of polyaniline@yttrium phosphate (PANI@Y(III)PO4) nanocomposite. J. Mater. Res. Technol. 2022, 16, 1686–1701. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Yassin, M.A.; Al-Mubaddel, F.S.; Amen, M.T. New electrooxidation characteristic for Ni-based electrodes for wide application in methanol fuel cells. Appl. Catal. A Gen. 2018, 555, 148–154. [Google Scholar] [CrossRef]
- Mohamed, I.M.; Kanagaraj, P.; Yasin, A.S.; Iqbal, W.; Liu, C. Electrochemical impedance investigation of urea oxidation in alkaline media based on electrospun nanofibers towards the technology of direct-urea fuel cells. J. Alloys Compd. 2020, 816, 152513. [Google Scholar] [CrossRef]
- Van der Ven, A.; Morgan, D.; Meng, Y.; Ceder, G. Phase stability of nickel hydroxides and oxyhydroxides. J. Electrochem. Soc. 2006, 153, A210–A215. [Google Scholar] [CrossRef]
- Barakat, N.A.; El-Newehy, M.H.; Yasin, A.S.; Ghouri, Z.K.; Al-Deyab, S.S. Ni&Mn nanoparticles-decorated carbon nanofibers as effective electrocatalyst for urea oxidation. Appl. Catal. A Gen. 2016, 510, 180–188. [Google Scholar]
- Barakat, N.A.; Motlak, M.; Elzatahry, A.A.; Khalil, K.A.; Abdelghani, E.A. NixCo1−x alloy nanoparticle-doped carbon nanofibers as effective non-precious catalyst for ethanol oxidation. Int. J. Hydrogen Energy 2014, 39, 305–316. [Google Scholar] [CrossRef]
- Abdi, Z.; Vandichel, M.; Sologubenko, A.S.; Willinger, M.-G.; Shen, J.-R.; Allakhverdiev, S.I.; Najafpour, M.M. The importance of identifying the true catalyst when using Randles-Sevcik equation to calculate turnover frequency. Int. J. Hydrogen Energy 2021, 46, 37774–37781. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.; Jones, R.; Alenazey, F.; Watcharatharapong, T.; Chakraborty, S.; Ahuja, R. Synthesis, structural and electrochemical properties of sodium nickel phosphate for energy storage devices. Nanoscale 2016, 8, 11291–11305. [Google Scholar] [CrossRef]
- Liu, J.G.; Zhao, T.S.; Chen, R.; Wong, C.W. The effect of methanol concentration on the performance of a passive DMFC. Electrochem. Commun. 2005, 7, 288–294. [Google Scholar] [CrossRef]
- Barakat, N.A.M. CoNi/CNTs composite as effective and stable electrode for oxygen evaluation reaction in alkaline media. Int. J. Hydrogen Energy 2018, 43, 8623–8631. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Almulhim, N.F.; Alaulamie, A.A.; Saleh, M.M.; Mohamed, I.M.A. Design of ultrafine nickel oxide nanostructured material for enhanced electrocatalytic oxidation of urea: Physicochemical and electrochemical analyses. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124092. [Google Scholar] [CrossRef]
- Barakat, N.A.; El-Newehy, M.; Al-Deyab, S.S.; Kim, H.Y. Cobalt/copper-decorated carbon nanofibers as novel non-precious electrocatalyst for methanol electrooxidation. Nanoscale Res. Lett. 2014, 9, 2. [Google Scholar] [CrossRef]
- Ghouri, Z.K.; Barakat, N.A.M.; Obaid, M.; Lee, J.H.; Kim, H.Y. Co/CeO2-decorated carbon nanofibers as effective non-precious electro-catalyst for fuel cells application in alkaline medium. Ceram. Int. 2015, 41 Pt A, 2271–2278. [Google Scholar] [CrossRef]
- Ghouri, Z.K.; Barakat, N.A.M.; Kim, H.Y.; Park, M.; Khalil, K.A.; El-Newehy, M.H.; Al-Deyab, S.S. Nano-engineered ZnO/CeO2 dots@CNFs for fuel cell application. Arab. J. Chem. 2016, 9, 219–228. [Google Scholar] [CrossRef]
- Ghouri, Z.K.; Barakat, N.A.M.; Park, M.; Kim, B.-S.; Kim, H.Y. Synthesis and characterization of Co/SrCO3 nanorods-decorated carbon nanofibers as novel electrocatalyst for methanol oxidation in alkaline medium. Ceram. Int. 2015, 41 Pt A, 6575–6582. [Google Scholar] [CrossRef]
- Sarkar, C.; Nath, J.; Bhuyan, S.; Dolui, S.K. Multifunctional ternary nanocomposites of Ni/Polypyrrole/Reduced graphene oxide as supercapacitor and electrocatalyst in methanol oxidation. ChemistrySelect 2019, 4, 2529–2537. [Google Scholar] [CrossRef]
- Mohamed, I.; Khalil, K.A.; Mousa, H.M.; Barakat, N.A. Ni/Pd-Decorated carbon NFs as an efficient electrocatalyst for methanol oxidation in alkaline medium. J. Electron. Mater. 2017, 46, 265–273. [Google Scholar] [CrossRef]
- Perales-Rondón, J.V.; Solla-Gullón, J.; Herrero, E.; Sánchez-Sánchez, C.M. Enhanced catalytic activity and stability for the electrooxidation of formic acid on lead modified shape controlled platinum nanoparticles. Appl. Catal. B Environ. 2017, 201, 48–57. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, I.M.A.; Park, C.H.; Kim, C.S. Design of novel electrode for capacitive deionization using electrospun composite titania/zirconia nanofibers doped-activated carbon. Mater. Lett. 2018, 213, 62–66. [Google Scholar] [CrossRef]
- Zhan, W.; Ma, L.; Gan, M.; Xie, F. Ultra-fine bimetallic FeCoP supported by N-doped MWCNTs Pt-based catalyst for efficient electrooxidation of methanol. Appl. Surf. Sci. 2022, 585, 152621. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Du, M.; Huang, X.; Cheng, K.; Wang, G.; Cao, D. Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium. Electrochim. Acta 2016, 210, 474–482. [Google Scholar] [CrossRef]
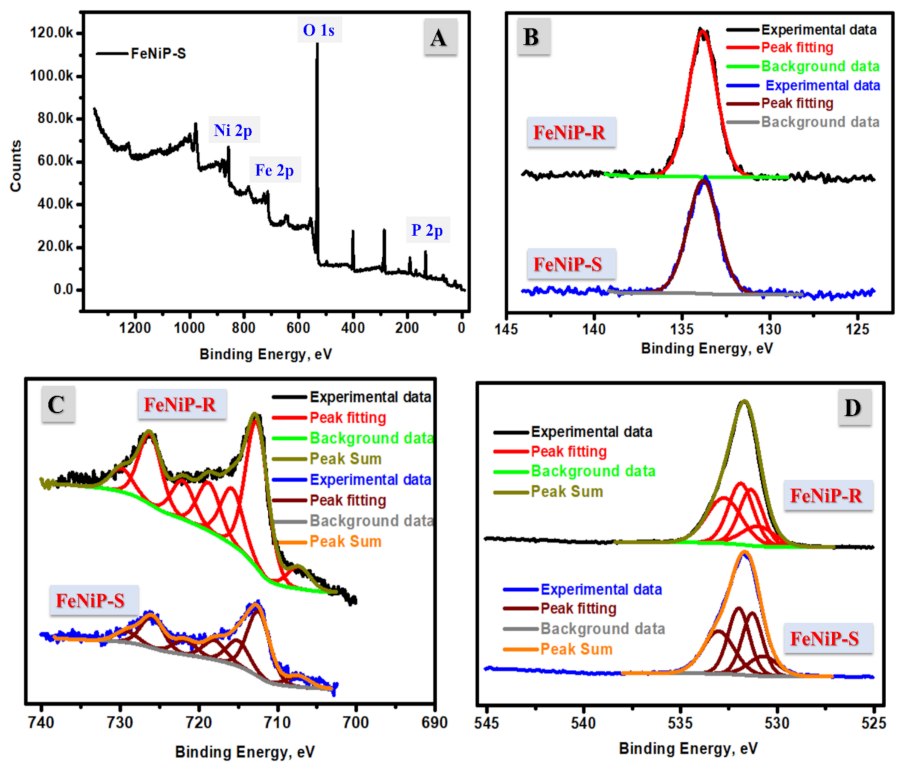


| Peak | Start BE | Peak BE | End BE | Height CPS | FWHM eV | Atomic % |
|---|---|---|---|---|---|---|
| O1s | 539.08 | 532.96 | 523.08 | 95,796.76 | 3.41 | 44.35 |
| Ni2p | 871.08 | 858.1 | 843.08 | 16,208.02 | 4.62 | 22.18 |
| P2p | 140.58 | 134.82 | 129.08 | 11,595.97 | 3.22 | 1.192 |
| Fe2p | 736.08 | 714.08 | 702.08 | 11,659.48 | 6.66 | 16.42 |
| No. | Material | MeOH in KOH | Current Density, mA/cm2 | Peak Potential, V |
|---|---|---|---|---|
| 1 | FeNiP-R | 20 mM | 15.60 | 0.458 |
| 2 | 40 mM | 23.40 | 0.458 | |
| 3 | 60 mM | 31.80 | 0.458 | |
| 4 | 80 mM | 40.25 | 0.472 | |
| 5 | FeNiP-S | 20 mM | 16.40 | 0.465 |
| 6 | 40 mM | 19.25 | 0.472 | |
| 7 | 60 mM | 21.65 | 0.479 | |
| 8 | 80 mM | 27.39 | 0.486 |
| No. | Electrocatalyst Material | MeOH Concentration | Current Density, mA/cm2 | Reference |
|---|---|---|---|---|
| 1 | Co/Cu @CNFs | 2 M | 17.5 | [70] |
| 2 | Co/CeO2-decorated C fibers | 3 M | 9.5 | [71] |
| 3 | ZnO/CeO2 dots@CNFs | 3 M | 16.3 | [72] |
| 4 | Co/SrCO3 nanorods | 3 M | 10.0 | [73] |
| 5 | Ni/Polypyrrole/RGO | 1 M | 32.94 | [74] |
| 6 | Ni/PdCNFs | 1 M | 7.15 | [75] |
| 7 | FeNiP-R | 0.08 M | 40.25 | This work |
| 8 | FeNiP-S | 0.08 M | 27.39 |
| No. | Material | Rh/Ω | R1/Ω | R2/kΩ | CPE1-T(+), µF | CPE1-P(+)/F | CPE2-T(+)/µF | CPE2-P(+)/F |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.0 mM | 16.20 | 10.63 | 13144.00 | 59.16 | 0.92 | 49.59 | 0.90 |
| Error, % | 0.17 | 27.74 | 1.84 | 31.67 | 3.22 | 37.91 | 3.33 | |
| 2 | 20.0 mM | 16.71 | 9.79 | 10.08 | 50.08 | 0.96 | 53.81 | 0.94 |
| Error, % | 0.21 | 25.82 | 0.35 | 37.90 | 3.86 | 35.41 | 3.14 | |
| 3 | 40 mM | 16.32 | 9.56 | 9.10 | 50.35 | 0.96 | 53.96 | 0.94 |
| Error, % | 0.21 | 25.21 | 0.48 | 36.93 | 3.75 | 34.58 | 3.07 | |
| 4 | 60 mM | 17.16 | 9.17 | 8.69 | 49.09 | 0.96 | 53.87 | 0.94 |
| Error, % | 0.22 | 26.81 | 0.48 | 40.21 | 4.06 | 36.77 | 3.26 | |
| 5 | 80 mM | 18.09 | 8.95 | 7.19 | 45.13 | 0.96 | 55.25 | 0.94 |
| Error, % | 0.24 | 31.21 | 0.51 | 52.03 | 5.28 | 43.01 | 3.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Khalaf, M.; M. Abd El-Lateef, H.; Dao, V.-D.; Mohamed, I.M.A. Electrocatalysis of Methanol Oxidation in Alkaline Electrolytes over Novel Amorphous Fe/Ni Biphosphate Material Prepared by Different Techniques. Nanomaterials 2022, 12, 3429. https://doi.org/10.3390/nano12193429
M. Khalaf M, M. Abd El-Lateef H, Dao V-D, Mohamed IMA. Electrocatalysis of Methanol Oxidation in Alkaline Electrolytes over Novel Amorphous Fe/Ni Biphosphate Material Prepared by Different Techniques. Nanomaterials. 2022; 12(19):3429. https://doi.org/10.3390/nano12193429
Chicago/Turabian StyleM. Khalaf, Mai, Hany M. Abd El-Lateef, Van-Duong Dao, and Ibrahim M. A. Mohamed. 2022. "Electrocatalysis of Methanol Oxidation in Alkaline Electrolytes over Novel Amorphous Fe/Ni Biphosphate Material Prepared by Different Techniques" Nanomaterials 12, no. 19: 3429. https://doi.org/10.3390/nano12193429
APA StyleM. Khalaf, M., M. Abd El-Lateef, H., Dao, V.-D., & Mohamed, I. M. A. (2022). Electrocatalysis of Methanol Oxidation in Alkaline Electrolytes over Novel Amorphous Fe/Ni Biphosphate Material Prepared by Different Techniques. Nanomaterials, 12(19), 3429. https://doi.org/10.3390/nano12193429









