Solution-Processed SnO2 Quantum Dots for the Electron Transport Layer of Flexible and Printed Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SnO2 QDs Synthesis
2.3. Ink Preparations
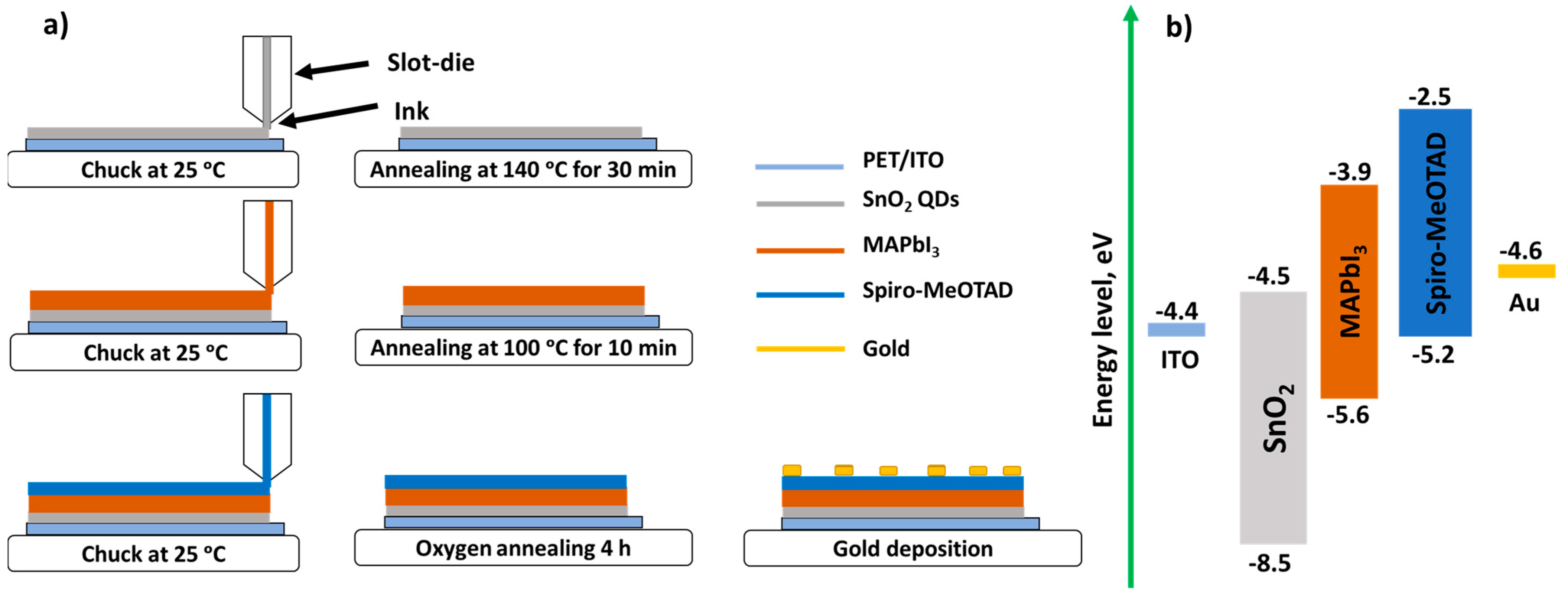
2.4. Device Fabrication
2.5. Characterization
3. Results and Discussion
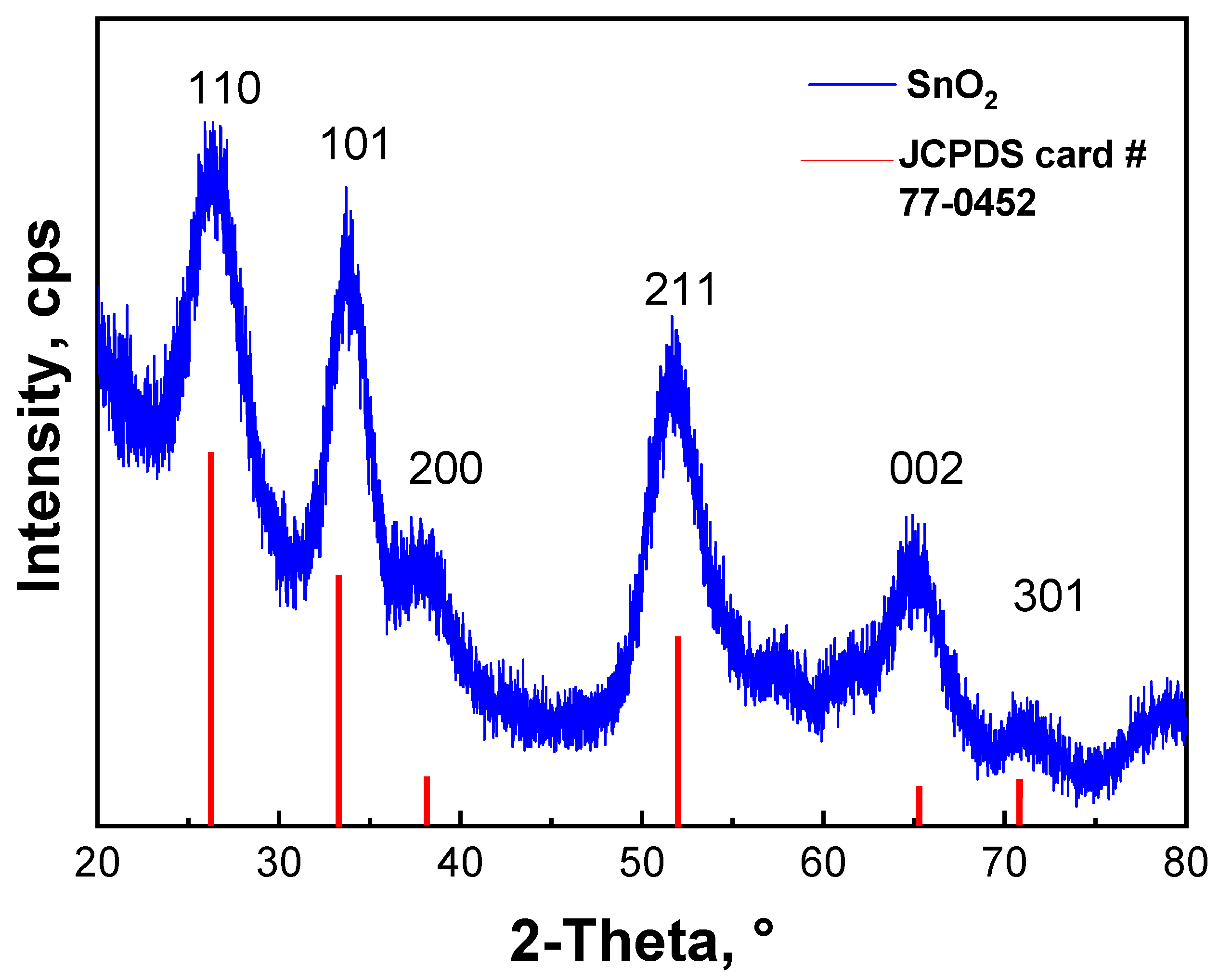
3.1. Crystallinity of SnO2 QDs
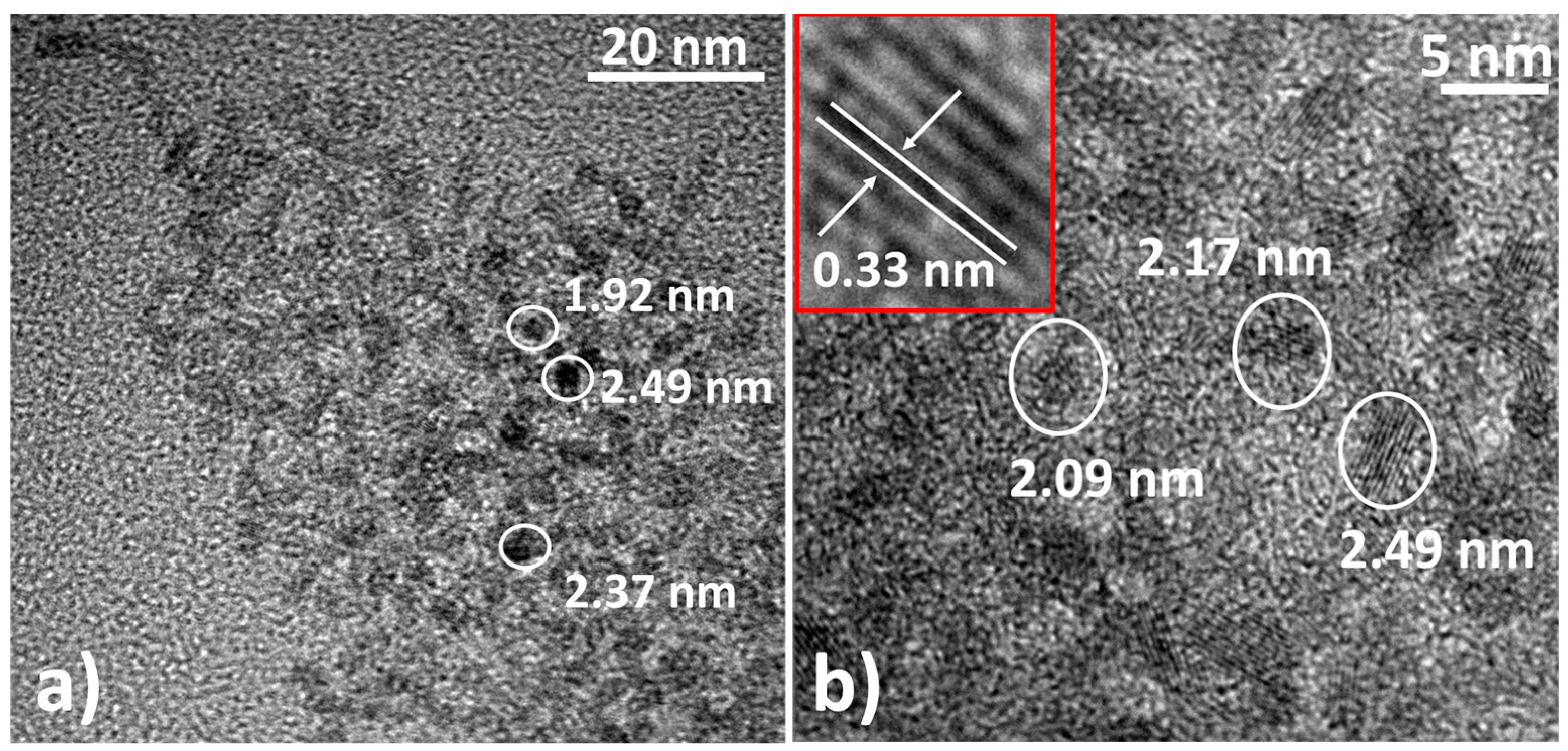
3.2. Transmission Electron Microscopy
3.3. Optical Analysis
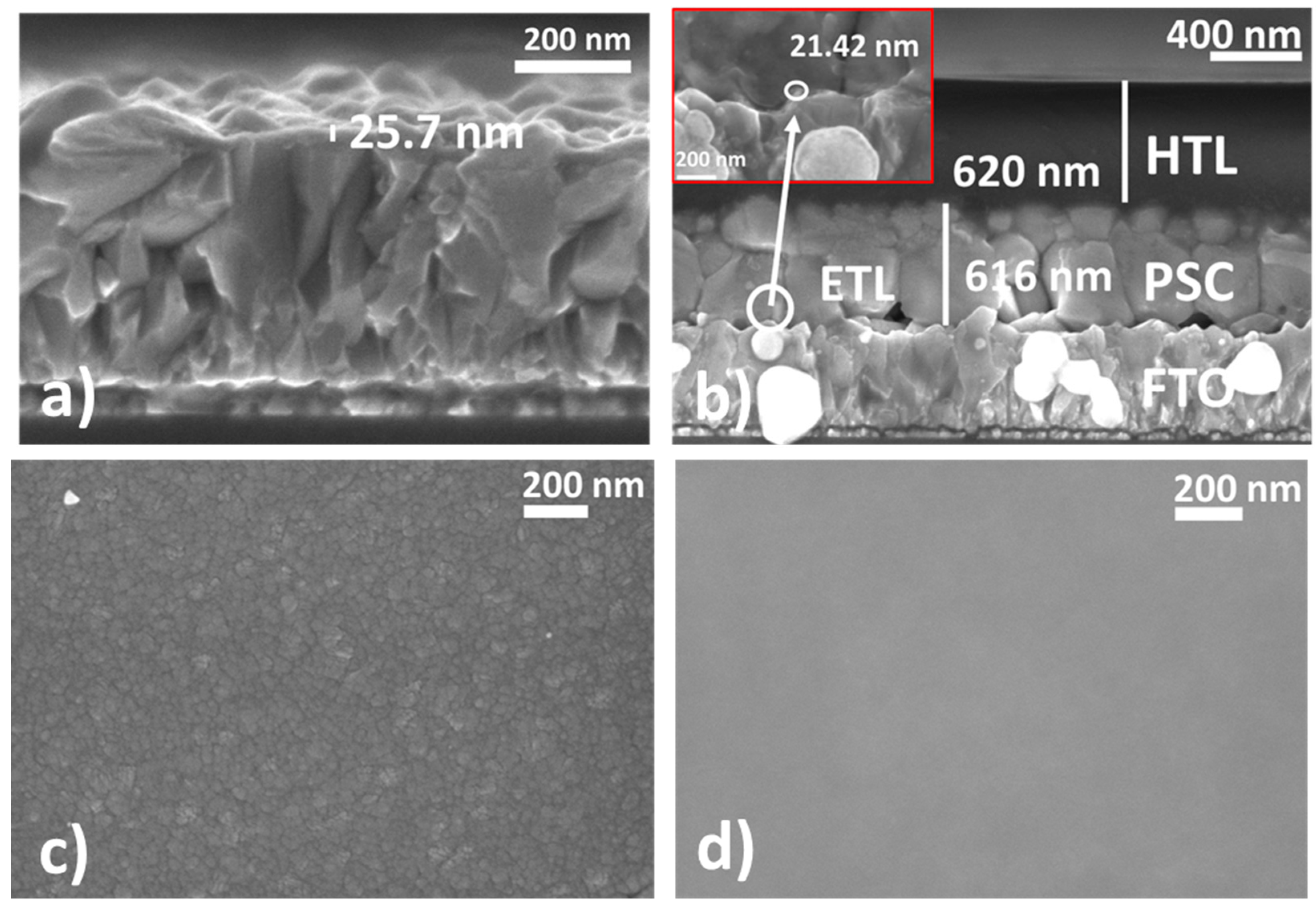
3.4. Scanning Electron Microscopy
3.5. Device Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashina, A.; Battula, R.K.; Ramasamy, E.; Chundi, N.; Sakthivel, S.; Veerappan, G. Dip Coated SnO2 Film as Electron Transport Layer for Low Temperature Processed Planar Perovskite Solar Cells. Appl. Surf. Sci. Adv. 2021, 4, 100066. [Google Scholar] [CrossRef]
- Cao, J.-J.; Wang, K.-L.; Dong, C.; Li, X.-M.; Yang, W.-F.; Wang, Z.-K. Bottom-Contact Passivation for High-Performance Perovskite Solar Cells Using TaCl5-Doped SnO2 as Electron-Transporting Layer. Org. Electron. 2021, 88, 105972. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, Stable and Scalable Perovskite Solar Cells Using Poly(3-Hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Razza, S.; Castro-Hermosa, S.; Di Carlo, A.; Brown, T.M. Research Update: Large-Area Deposition, Coating, Printing, and Processing Techniques for the Upscaling of Perovskite Solar Cell Technology. APL Mater. 2016, 4, 091508. [Google Scholar] [CrossRef]
- Cai, B.; Zhang, W.-H.; Qiu, J. Solvent Engineering of Spin-Coating Solutions for Planar-Structured High-Efficiency Perovskite Solar Cells. Chin. J. Catal. 2015, 36, 1183–1190. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, D.; Xie, B.; Yue, X.; Li, M.; Song, D.; Li, Y. High Reproducibility of Perovskite Solar Cells via a Complete Spin-Coating Sequential Solution Deposition Process. Sol. Energy 2015, 122, 97–103. [Google Scholar] [CrossRef]
- Gong, C.; Tong, S.; Huang, K.; Li, H.; Huang, H.; Zhang, J.; Yang, J. Flexible Planar Heterojunction Perovskite Solar Cells Fabricated via Sequential Roll-to-Roll Microgravure Printing and Slot-Die Coating Deposition. Sol. RRL 2020, 4, 1900204. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, M.; Lau, C.F.J.; Deng, X.; Kim, J.; Ma, Q.; Chen, C.; Green, M.A.; Huang, S.; Ho-Baillie, A.W.Y. Spin-Coating Free Fabrication for Highly Efficient Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2017, 168, 165–171. [Google Scholar] [CrossRef]
- Whitaker, J.B.; Hoe Kim, D.; Larson, B.W.; Zhang, F.; Berry, J.J.; van Hest, M.F.A.M.; Zhu, K. Scalable Slot-Die Coating of High Performance Perovskite Solar Cells. Sustain. Energy Fuels 2018, 2, 2442–2449. [Google Scholar] [CrossRef]
- Sandström, A.; Dam, H.F.; Krebs, F.C.; Edman, L. Ambient Fabrication of Flexible and Large-Area Organic Light-Emitting Devices Using Slot-Die Coating. Nat. Commun. 2012, 3, 1002. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, J.; Zhang, J.; Gao, C.; Liu, X. Improving the Interfacial Contact of Screen-Printed Carbon Electrodes for Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 5554–5559. [Google Scholar] [CrossRef]
- Cao, K.; Zuo, Z.; Cui, J.; Shen, Y.; Moehl, T.; Zakeeruddin, S.M.; Grätzel, M.; Wang, M. Efficient Screen Printed Perovskite Solar Cells Based on Mesoscopic TiO2/Al2O3/NiO/Carbon Architecture. Nano Energy 2015, 17, 171–179. [Google Scholar] [CrossRef]
- Yang, J.; Lim, E.L.; Tan, L.; Wei, Z. Ink Engineering in Blade-Coating Large-Area Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2200975. [Google Scholar] [CrossRef]
- Gao, B.; Meng, J. Flexible CH3NH3PbI3 Perovskite Solar Cells with High Stability Based on All Inkjet Printing. Sol. Energy 2021, 230, 598–604. [Google Scholar] [CrossRef]
- Gao, L.; Huang, K.; Long, C.; Zeng, F.; Liu, B.; Yang, J. Fully Slot-Die-Coated Perovskite Solar Cells in Ambient Condition. Appl. Phys. A 2020, 126, 452. [Google Scholar] [CrossRef]
- Mathiazhagan, G.; Seeber, A.; Gengenbach, T.; Mastroianni, S.; Vak, D.; Chesman, A.S.R.; Gao, M.; Angmo, D.; Hinsch, A. Improving the Stability of Ambient Processed, SnO2-Based, Perovskite Solar Cells by the UV-Treatment of Sub-Cells. Sol. RRL 2020, 4, 2000262. [Google Scholar] [CrossRef]
- Bahadur, J.; Ghahremani, A.H.; Martin, B.; Druffel, T.; Sunkara, M.K.; Pal, K. Solution Processed Mo Doped SnO2 as an Effective ETL in the Fabrication of Low Temperature Planer Perovskite Solar Cell under Ambient Conditions. Org. Electron. 2019, 67, 159–167. [Google Scholar] [CrossRef]
- Tzounis, L.; Stergiopoulos, T.; Zachariadis, A.; Gravalidis, C.; Laskarakis, A.; Logothetidis, S. Perovskite Solar Cells from Small Scale Spin Coating Process towards Roll-to-Roll Printing: Optical and Morphological Studies. Mater. Today Proc. 2017, 4, 5082–5089. [Google Scholar] [CrossRef]
- Brunetti, F.; Operamolla, A.; Castro-Hermosa, S.; Lucarelli, G.; Manca, V.; Farinola, G.M.; Brown, T.M. Printed Solar Cells and Energy Storage Devices on Paper Substrates. Adv. Funct. Mater. 2019, 29, 1806798. [Google Scholar] [CrossRef]
- Maafa, I.M. All-Inorganic Perovskite Solar Cells: Recent Advancements and Challenges. Nanomaterials 2022, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Afre, R.A.; Sharma, N.; Sharon, M.; Sharon, M. Transparent Conducting Oxide Films for Various Applications: A Review. Rev. Adv. Mater. Sci. 2018, 53, 79–89. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, H.Y.; Kim, S.; Song, P.K. Controlled Lattice Thermal Conductivity of Transparent Conductive Oxide Thin Film via Localized Vibration of Doping Atoms. Nanomaterials 2021, 11, 2363. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, J.; Singh, C.R.; Gujar, T.P.; Heinrich, C.D.; Thelakkat, M. Nanostructured Hybrid Metal Mesh as Transparent Conducting Electrodes: Selection Criteria Verification in Perovskite Solar Cells. Nanomaterials 2021, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Qi, X.; Zhang, Y.; Liu, G.; Wu, C.; Wang, D.; Guo, X.; Luo, W.; Li, X.; Hu, H.; et al. High Efficiency (18.53%) of Flexible Perovskite Solar Cells via the Insertion of Potassium Chloride between SnO2 and CH3NH 3PbI 3 Layers. ACS Appl. Energy Mater. 2019, 2, 3676–3682. [Google Scholar] [CrossRef]
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.-P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully Inkjet Printed SnO2 Gas Sensor on Plastic Substrate. Sens. Actuators B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef]
- Dong, J.; Jia, J.; Feng, X.; Shi, B.; Wu, Y.; Zhang, Y.; Wu, J.; Cao, B. Ligand Exchange of SnO2 Effectively Improving the Efficiency of Flexible Perovskite Solar Cells. J. Alloys Compd. 2021, 883, 160827. [Google Scholar] [CrossRef]
- Wang, H.; Cao, S.; Yang, B.; Li, H.; Wang, M.; Hu, X.; Sun, K.; Zang, Z. NH4Cl-Modified ZnO for High-Performance CsPbIBr2 Perovskite Solar Cells via Low-Temperature Process. Sol. RRL 2020, 4, 1900363. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Li, X.; Li, Y. Solvent-Assisted Preparation of Low-Temperature SnO2 Electron Transport Layers for Efficient and Stable Perovskite Solar Cells Made in Ambient Conditions. New J. Chem. 2022, 46, 9841–9850. [Google Scholar] [CrossRef]
- Cao, Z.; Li, C.; Deng, X.; Wang, S.; Yuan, Y.; Chen, Y.; Wang, Z.; Liu, Y.; Ding, L.; Hao, F. Metal Oxide Alternatives for Efficient Electron Transport in Perovskite Solar Cells: Beyond TiO2 and SnO2. J. Mater. Chem. A 2020, 8, 19768–19787. [Google Scholar] [CrossRef]
- Lin, H.-K.; Li, J.-X.; Wang, H.-C.; Su, Y.-W.; Wu, K.-H.; Wei, K.-H. Dual Nanocomposite Carrier Transport Layers Enhance the Efficiency of Planar Perovskite Photovoltaics. RSC Adv. 2018, 8, 12526–12534. [Google Scholar] [CrossRef]
- Wang, P.; Shao, Z.; Ulfa, M.; Pauporté, T. Insights into the Hole Blocking Layer Effect on the Perovskite Solar Cell Performance and Impedance Response. J. Phys. Chem. C 2017, 121, 9131–9141. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Q.; Zhang, L.; Han, C.; Gao, H.; Zhang, Y.; Yan, H. SnO2-Based Electron Transporting Layer Materials for Perovskite Solar Cells: A Review of Recent Progress. J. Energy Chem. 2019, 35, 144–167. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Bu, T.; Yang, S.; Xiao, J.; Peng, Y.; Li, W.; Zhong, J.; Ku, Z.; Cheng, Y.-B.; et al. Room-Temperature Synthesized SnO2 Electron Transport Layers for Efficient Perovskite Solar Cells. RSC Adv. 2019, 9, 9946–9950. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Zhang, Y.; Song, J.; Kong, T.; Li, Y.; Xie, H.; Bi, D. In Situ Perovskitoid Engineering at SnO2 Interface toward Highly Efficient and Stable Formamidinium Lead Triiodide Perovskite Solar Cells. J. Phys. Chem. Lett. 2021, 12, 10567–10573. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, W.; Kloo, L.; Belova, L. Inkjet-Printed Electron Transport Layers for Perovskite Solar Cells. Materials 2021, 14, 7525. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, L.; Jiang, F.; Xie, C.; Hu, L.; Dong, X.; Qin, F.; Liu, T.; Hu, L.; Jiang, X.; et al. Photocatalytic Effect of ZnO on the Stability of Nonfullerene Acceptors and Its Mitigation by SnO2 for Nonfullerene Organic Solar Cells. Mater. Horiz. 2019, 6, 1438–1443. [Google Scholar] [CrossRef]
- Dkhissi, Y.; Huang, F.; Rubanov, S.; Xiao, M.; Bach, U.; Spiccia, L.; Caruso, R.A.; Cheng, Y.-B. Low Temperature Processing of Flexible Planar Perovskite Solar Cells with Efficiency over 10%. J. Power Sources 2015, 278, 325–331. [Google Scholar] [CrossRef]
- Halvani Anaraki, E.; Kermanpur, A.; Steier, L.; Domanski, K.; Matsui, T.; Tress, W.; Saliba, M.; Abate, A.; Grätzel, M.; Hagfeldt, A.; et al. Highly Efficient and Stable Planar Perovskite Solar Cells by Solution-Processed Tin Oxide. Energy Environ. Sci. 2016, 9, 3128–3134. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef]
- Bu, T.; Li, J.; Zheng, F.; Chen, W.; Wen, X.; Ku, Z.; Peng, Y.; Zhong, J.; Cheng, Y.-B.; Huang, F. Universal Passivation Strategy to Slot-Die Printed SnO2 for Hysteresis-Free Efficient Flexible Perovskite Solar Module. Nat. Commun. 2018, 9, 4609. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.N.; Wall, J.; Li, L.; Xing, G.; Zhang, Q.; Yan, F. Low-Temperature Processed Highly Efficient Hole Transport Layer Free Carbon-Based Planar Perovskite Solar Cells with SnO2 Quantum Dot Electron Transport Layer. Mater. Today Phys. 2020, 13, 100204. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, K.; Hu, H.; Guo, X.; Gao, Y.; Fong, P.W.K.; Liang, Q.; Tang, H.; Huang, J.; Zhang, H.; et al. Room-Temperature Multiple Ligands-Tailored SnO2 Quantum Dots Endow in Situ Dual-Interface Binding for Upscaling Efficient Perovskite Photovoltaics with High VOC. Light Sci. Appl. 2021, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.P.; Reddy, V.R.; Sen, P. Size-Dependent Nonlinear Optical Absorption in SnO2 Quantum Dots. J. Opt. Soc. Am. B 2020, 37, 2788–2795. [Google Scholar] [CrossRef]
- Tong, G.; Ono, L.K.; Liu, Y.; Zhang, H.; Bu, T.; Qi, Y. Up-Scalable Fabrication of SnO2 with Multifunctional Interface for High Performance Perovskite Solar Modules. Nano-Micro Lett. 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Cheng, H.-M.; Silva, S.R.P. Critical Review of Recent Progress of Flexible Perovskite Solar Cells. Mater. Today 2020, 39, 66–88. [Google Scholar] [CrossRef]
- Ahsan Saeed, M.; Hyeon Kim, S.; Baek, K.; Hyun, J.K.; Youn Lee, S.; Won Shim, J. PEDOT:PSS: CuNW-Based Transparent Composite Electrodes for High-Performance and Flexible Organic Photovoltaics under Indoor Lighting. Appl. Surf. Sci. 2021, 567, 150852. [Google Scholar] [CrossRef]
- Liu, J.; Xue, W.; Jin, G.; Zhai, Z.; Lv, J.; Hong, W.; Chen, Y. Preparation of Tin Oxide Quantum Dots in Aqueous Solution and Applications in Semiconductor Gas Sensors. Nanomaterials 2019, 9, 240. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Yu, G.; Zhang, H.; Yao, K. A Simple Hydrothermal Route for Synthesizing SnO2quantum Dots. Nanotechnology 2006, 17, 2386–2389. [Google Scholar] [CrossRef]
- Hussain, I.; Tran, H.P.; Jaksik, J.; Moore, J.; Islam, N.; Uddin, M.J. Functional Materials, Device Architecture, and Flexibility of Perovskite Solar Cell. Emergent Mater. 2018, 1, 133–154. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, W.; Jiang, Z.; Zhang, Y.; Ni, C. CuI/Spiro-OMeTAD Double-Layer Hole Transport Layer to Improve Photovoltaic Performance of Perovskite Solar Cells. Coatings 2021, 11, 978. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Q.; Wan, F.; Gao, T. Gas Sensing Properties and Mechanism of Nano-SnO2-Based Sensor for Hydrogen and Carbon Monoxide. J. Nanomater. 2012, 2012, 612420. [Google Scholar] [CrossRef]
- Lamdhade, G.T.; Raghuwanshi, F.C.; Agrawal, R.M.; Balkhande, V.M.; Shripath, T. SnO2 Nanoparticles Synthesis Via Liquid-Phase Co-Precipitation Technique. Adv. Mater. Lett. 2015, 6, 738–742. [Google Scholar] [CrossRef]
- Rani, R.; Sharma, S. Preparation and Characterization of SnO2 Nanofibers via Electrospinning. Adv. Nanoparticles 2016, 5, 53–59. [Google Scholar] [CrossRef][Green Version]
- Bhattacharjee, A.; Ahmaruzzaman, M. A Novel and Green Process for the Production of Tin Oxide Quantum Dots and Its Application as a Photocatalyst for the Degradation of Dyes from Aqueous Phase. J. Colloid Interface Sci. 2015, 448, 130–139. [Google Scholar] [CrossRef]
- Xie, J.; Huang, K.; Yu, X.; Yang, Z.; Xiao, K.; Qiang, Y.; Zhu, X.; Xu, L.; Wang, P.; Cui, C.; et al. Enhanced Electronic Properties of SnO2 via Electron Transfer from Graphene Quantum Dots for Efficient Perovskite Solar Cells. ACS Nano 2017, 11, 9176–9182. [Google Scholar] [CrossRef]
- Ou, X.-L.; Feng, J.; Xu, M.; Sun, H.-B. Semitransparent and Flexible Perovskite Solar Cell with High Visible Transmittance Based on Ultrathin Metallic Electrodes. Opt. Lett. 2017, 42, 1958–1961. [Google Scholar] [CrossRef]
- Remarkably High Performance of Organic Photovoltaic Devices with 3,9-Bis(2-Methylene-(3-(1,1-Dicyanomethylene)-Indanone))-5,5,11,11-Tetrakis(4-Hexyl Meta-Phenyl)-Dithieno[2,3-d:2′,3′-d′]-s-Indaceno[1,2-b:5,6-b′]Dithiophene)- Ethylhexyloxy] Photoactive Acceptor under Halogen Light Illumination-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0378775321012738 (accessed on 30 June 2022).
- Elastomeric Indoor Organic Photovoltaics with Superb Photothermal Endurance-Kim-Advanced Functional Materials-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.202201921 (accessed on 30 June 2022).
- Jeyakumar, R.; Bag, A.; Nekovei, R.; Radhakrishnan, R. Influence of Electron Transport Layer (TiO2) Thickness and Its Doping Density on the Performance of CH3NH3PbI3-Based Planar Perovskite Solar Cells. J. Electron. Mater. 2020, 49, 3533–3539. [Google Scholar] [CrossRef]
- Impact of Interfacial Layers in Perovskite Solar Cells-Cho-2017-ChemSusChem-Wiley Online Library. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/cssc.201701095 (accessed on 31 May 2022).
- Habisreutinger, S.N.; Noel, N.K.; Snaith, H.J. Hysteresis Index: A Figure without Merit for Quantifying Hysteresis in Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2472–2476. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y.; Zhang, H.; Yang, J.; Han, J.; Xu, T.; Wang, S.; Wang, Z.; Gao, B.; Zhao, J.; et al. Efficient and Stable Inverted Perovskite Solar Cells with Very High Fill Factors via Incorporation of Star-Shaped Polymer. Sci. Adv. 2021, 7, eabg0633. [Google Scholar] [CrossRef]
- Rai, M.; Wong, L.H.; Etgar, L. Effect of Perovskite Thickness on Electroluminescence and Solar Cell Conversion Efficiency. J. Phys. Chem. Lett. 2020, 11, 8189–8194. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; García de Arquer, F.P.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and Stable Solution-Processed Planar Perovskite Solar Cells via Contact Passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Rath, T.; Mangalam, J.; Kunert, B.; Coclite, A.M.; Bauch, M.; Dimopoulos, T.; Trimmel, G. Investigation of NiOx-Hole Transport Layers in Triple Cation Perovskite Solar Cells. J. Mater. Sci. Mater. Electron. 2018, 29, 1847–1855. [Google Scholar] [CrossRef]
- Bush, K.A.; Bailie, C.D.; Chen, Y.; Bowring, A.R.; Wang, W.; Ma, W.; Leijtens, T.; Moghadam, F.; McGehee, M.D. Thermal and Environmental Stability of Semi-Transparent Perovskite Solar Cells for Tandems Enabled by a Solution-Processed Nanoparticle Buffer Layer and Sputtered ITO Electrode. Adv. Mater. 2016, 28, 3937–3943. [Google Scholar] [CrossRef]
- Hoang, V.Q.; Lee, S.K.; Bark, C.W. Dispersed SnO2 Colloids Using Sodium Dodecyl Benzene Sulfonate for High-Performance Planar Perovskite Solar Cells. Sol. Energy 2021, 230, 747–753. [Google Scholar] [CrossRef]
- Chander, S.; Purohit, A.; Nehra, A.; Nehra, S.P.; Dhaka, M.S. A Study on Spectral Response and External Quantum Efficiency of Mono-Crystalline Silicon Solar Cell. Int. J. Renew. Energy Res. 2015, 4, 41–44. [Google Scholar]
- Slami, A.; Benramdane, N. Manual Method for Measuring The External Quantum Efficiency for Solar Cells. E3S Web Conf. 2021, 229, 01005. [Google Scholar] [CrossRef]
- Jo Kim, B.; Hoe Kim, D.; Lee, Y.-Y.; Shin, H.-W.; Sang Han, G.; Sug Hong, J.; Mahmood, K.; Kyu Ahn, T.; Joo, Y.-C.; Sun Hong, K.; et al. Highly Efficient and Bending Durable Perovskite Solar Cells: Toward a Wearable Power Source. Energy Environ. Sci. 2015, 8, 916–921. [Google Scholar] [CrossRef]
- Wang, C.; Guan, L.; Zhao, D.; Yu, Y.; Grice, C.R.; Song, Z.; Awni, R.A.; Chen, J.; Wang, J.; Zhao, X.; et al. Water Vapor Treatment of Low-Temperature Deposited SnO2 Electron Selective Layers for Efficient Flexible Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2118–2124. [Google Scholar] [CrossRef]
- Yang, F.; Liu, J.; Lim, H.E.; Ishikura, Y.; Shinokita, K.; Miyauchi, Y.; Wakamiya, A.; Murata, Y.; Matsuda, K. High Bending Durability of Efficient Flexible Perovskite Solar Cells Using Metal Oxide Electron Transport Layer. J. Phys. Chem. C 2018, 122, 17088–17095. Available online: https://pubs.acs.org/doi/10.1021/acs.jpcc.8b05008 (accessed on 12 July 2022). [CrossRef]
- Heo, J.H.; Shin, D.H.; Jang, M.H.; Lee, M.L.; Kang, M.G.; Im, S.H. Highly Flexible, High-Performance Perovskite Solar Cells with Adhesion Promoted AuCl3-Doped Graphene Electrodes. J. Mater. Chem. A 2017, 5, 21146–21152. [Google Scholar] [CrossRef]
- Popoola, I.K.; Gondal, M.A.; Qahtan, T.F. Recent Progress in Flexible Perovskite Solar Cells: Materials, Mechanical Tolerance and Stability. Renew. Sustain. Energy Rev. 2018, 82, 3127–3151. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Wei, W.; Hao, Y.; Liu, S.; Ouyang, J.; Chang, J. Recent Progress of Electrode Materials for Flexible Perovskite Solar Cells. Nano-Micro Lett. 2022, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Flexible Perovskite Solar Cells with Simultaneously Improved Efficiency, Operational Stability, and Mechanical Reliability-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2542435121002002?via%3Dihub (accessed on 12 July 2022).
- Record Efficiency Stable Flexible Perovskite Solar Cell Using Effective Additive Assistant Strategy-Feng-2018-Advanced Materials-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201801418 (accessed on 12 July 2022).
- Lee, G.; Kim, M.; Choi, Y.W.; Ahn, N.; Jang, J.; Yoon, J.; Kim, S.M.; Lee, J.-G.; Kang, D.; Jung, H.S.; et al. Ultra-Flexible Perovskite Solar Cells with Crumpling Durability: Toward a Wearable Power Source. Energy Environ. Sci. 2019, 12, 3182–3191. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Rolston, N.; Harwood, D.; Bush, K.A.; Dauskardt, R.H.; McGehee, M.D. Design and Understanding of Encapsulated Perovskite Solar Cells to Withstand Temperature Cycling. Energy Environ. Sci. 2018, 11, 144–150. [Google Scholar] [CrossRef]
- Jiang, N.R.; Wang, Y.F.; Dong, Q.F.; Ge, C.D.; Yang, Z.Q.; Yin, D. Enhanced Efficiency and Mechanical Robustness of Flexible Perovskite Solar Cells by Using HPbI3 Additive. Sol. RRL 2021, 5, 2000821. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/solr.202000821 (accessed on 12 July 2022). [CrossRef]
- Impact of Interlayer Application on Band Bending for Improved Electron Extraction for Efficient Flexible Perovskite Mini-Modules-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S2211285518302866?via%3Dihub (accessed on 12 July 2022).
- Sears, K.K.; Fievez, M.; Gao, M.; Weerasinghe, H.C.; Easton, C.D.; Vak, D. ITO-Free Flexible Perovskite Solar Cells Based on Roll-to-Roll, Slot-Die Coated Silver Nanowire Electrodes. Sol. RRL 2017, 9, 1700059. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Bokuniaeva, A.O.; Vorokh, A.S. Estimation of Particle Size Using the Debye Equation and the Scherrer Formula for Polyphasic TiO2 Powder. J. Phys. 2019, 1410, 012057. [Google Scholar] [CrossRef]


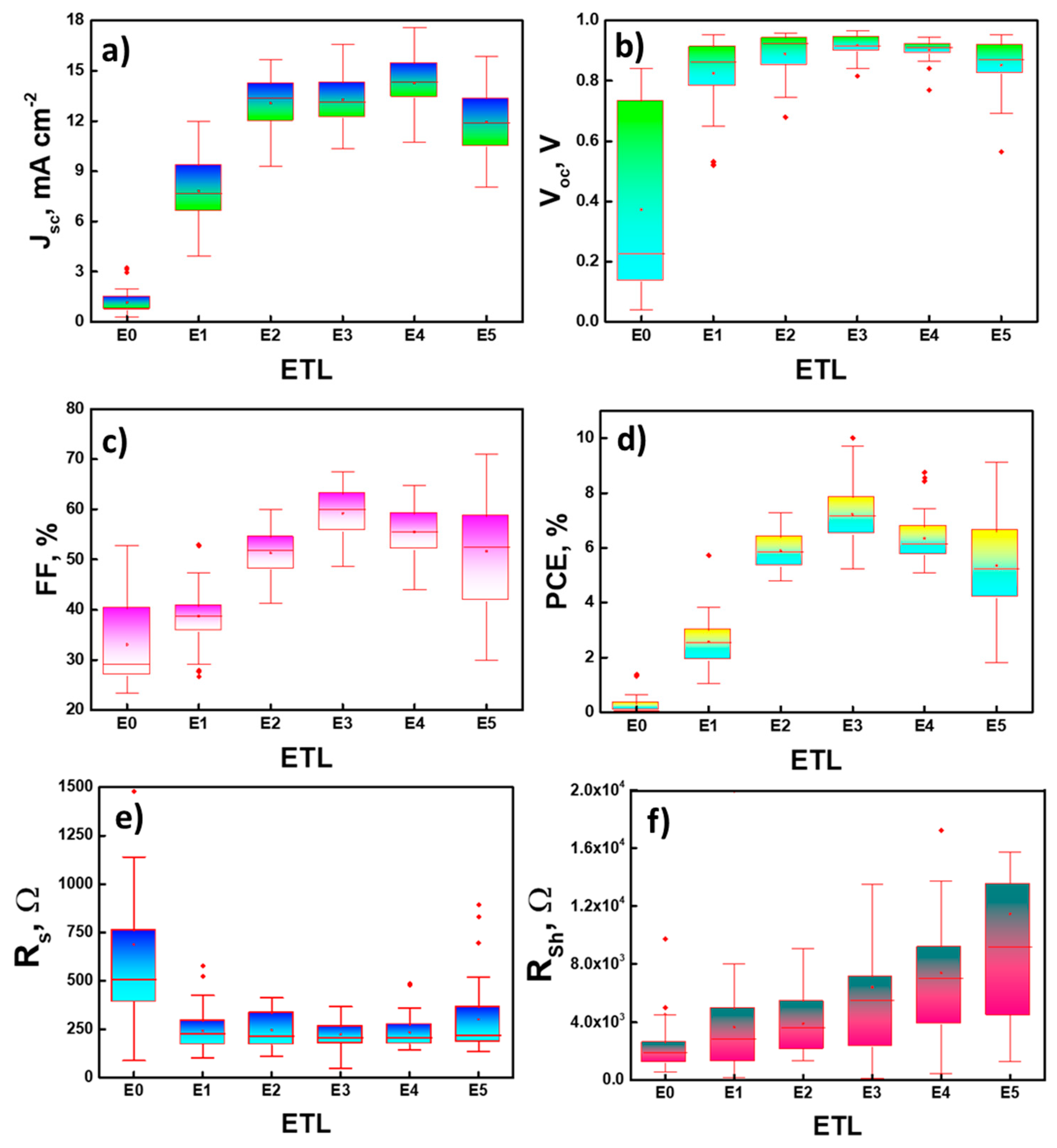

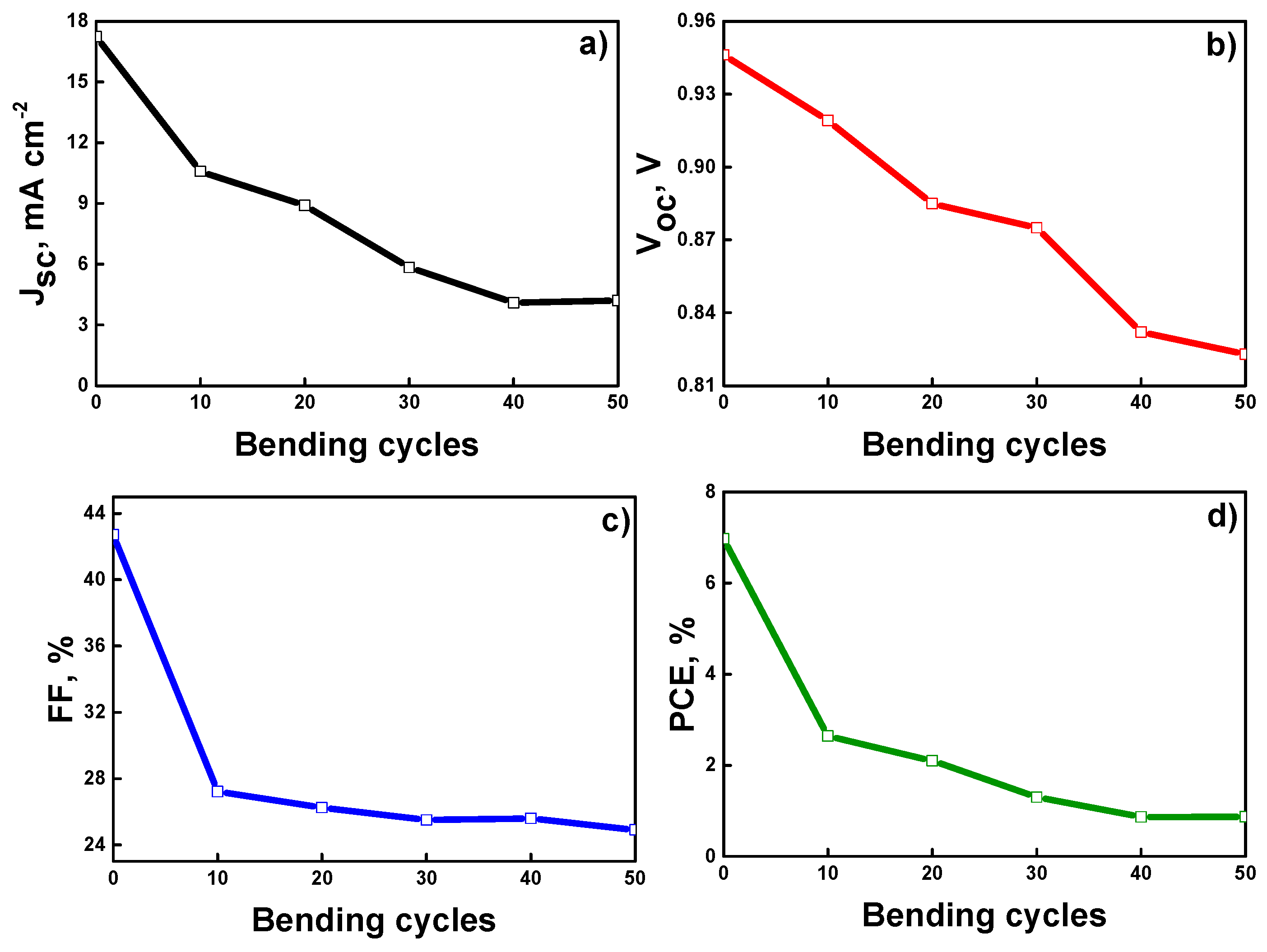
| ETLs. | Scan Direction | Jsc, mA cm−2 | Voc, V | FF, % | PCE, % | RS, Ω | RSh, kΩ |
|---|---|---|---|---|---|---|---|
| E0 (ref.) | Reverse Forward | 1.15 ± 0.113 0.94 ± 0.102 | 0.37 ± 0.046 0.33 ± 0.048 | 33.06 ± 1.326 27.95 ± 0.484 | 0.21 ± 0.050 0.12 ± 0.027 | 689.6 ± 119 994.8 ± 252.2 | 4.6 ± 2.4 16.6 ± 10.4 |
| E1 (1 wt%) | Reverse Forward | 7.72 ± 0.293 5.56 ± 0.264 | 0.82 ± 0.017 0.72 ± 0.021 | 38.69 ± 0.849 28.85 ± 0.723 | 2.52 ± 0.164 1.19 ± 0.096 | 238.4 ± 16.6 447.8 ± 121.1 | 3.7 ± 0.5 3.9 ± 0.4 |
| E2 (1.5 wt%) | Reverse Forward | 12.95 ± 0.491 10.08 ± 0.572 | 0.89 ± 0.013 0.82 ± 0.014 | 51.15 ± 1.576 36.29 ± 1.453 | 5.84 ± 0.338 2.99 ± 0.23 | 246.6 ± 14.1 230.7 ± 11.4 | 3.9 ± 0.3 3.7 ± 0.3 |
| E3 (2 wt%) | Reverse Forward | 13.28 ± 0.22 8.52 ± 0.35 | 0.93 ± 0.01 0.88 ± 0.01 | 59.22 ± 0.75 28.27 ± 0.97 | 7.23 ± 0.18 2.15 ± 0.12 | 222.7 ± 11.2 221.3 ± 10.3 | 6.3 ± 1.4 3.3 ± 0.3 |
| E4 (2.5 wt%) | Reverse Forward | 14.31 ± 0.63 10.34 ± 0.64 | 0.92 ± 0.01 0.85 ± 0.01 | 53.10 ± 1.82 32.50 ± 1.44 | 6.98 ± 0.46 3.08 ± 0.32 | 232.6 ± 12.9 223.5 ± 15.4 | 7.4 ± 1.1 4.2 ± 0.4 |
| E5 (3 wt%) | Reverse Forward | 11.76 ± 0.30 7.52 ± 0.30 | 0.83 ± 0.02 0.75 ± 0.02 | 50.62 ± 1.90 25.41 ± 0.77 | 5.16 ± 0.31 1.50 ± 0.12 | 297.2 ± 28.6 271.0 ± 27.4 | 11.7 ± 1.7 5.4 ± 0.8 |
| Scan Direction | Jsc, mA cm−2 | Voc, V | FF, % | PCE, % | Integrated Jsc, mA cm−2 |
|---|---|---|---|---|---|
| Reverse | 16.6 | 0.958 | 62.9 | 10.006 | 10.76 |
| Forward | 12.3 | 0.925 | 25.4 | 2.906 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, M.S.; Sadirkhanov, Z.T.; Kakimov, A.G.; Parkhomenko, H.P.; Ng, A.; Jumabekov, A.N. Solution-Processed SnO2 Quantum Dots for the Electron Transport Layer of Flexible and Printed Perovskite Solar Cells. Nanomaterials 2022, 12, 2615. https://doi.org/10.3390/nano12152615
Kiani MS, Sadirkhanov ZT, Kakimov AG, Parkhomenko HP, Ng A, Jumabekov AN. Solution-Processed SnO2 Quantum Dots for the Electron Transport Layer of Flexible and Printed Perovskite Solar Cells. Nanomaterials. 2022; 12(15):2615. https://doi.org/10.3390/nano12152615
Chicago/Turabian StyleKiani, Muhammad Salman, Zhandos T. Sadirkhanov, Alibek G. Kakimov, Hryhorii P. Parkhomenko, Annie Ng, and Askhat N. Jumabekov. 2022. "Solution-Processed SnO2 Quantum Dots for the Electron Transport Layer of Flexible and Printed Perovskite Solar Cells" Nanomaterials 12, no. 15: 2615. https://doi.org/10.3390/nano12152615
APA StyleKiani, M. S., Sadirkhanov, Z. T., Kakimov, A. G., Parkhomenko, H. P., Ng, A., & Jumabekov, A. N. (2022). Solution-Processed SnO2 Quantum Dots for the Electron Transport Layer of Flexible and Printed Perovskite Solar Cells. Nanomaterials, 12(15), 2615. https://doi.org/10.3390/nano12152615








