Synthesis and Characterization of Dendronized Gold Nanoparticles Bearing Charged Peripheral Groups with Antimicrobial Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
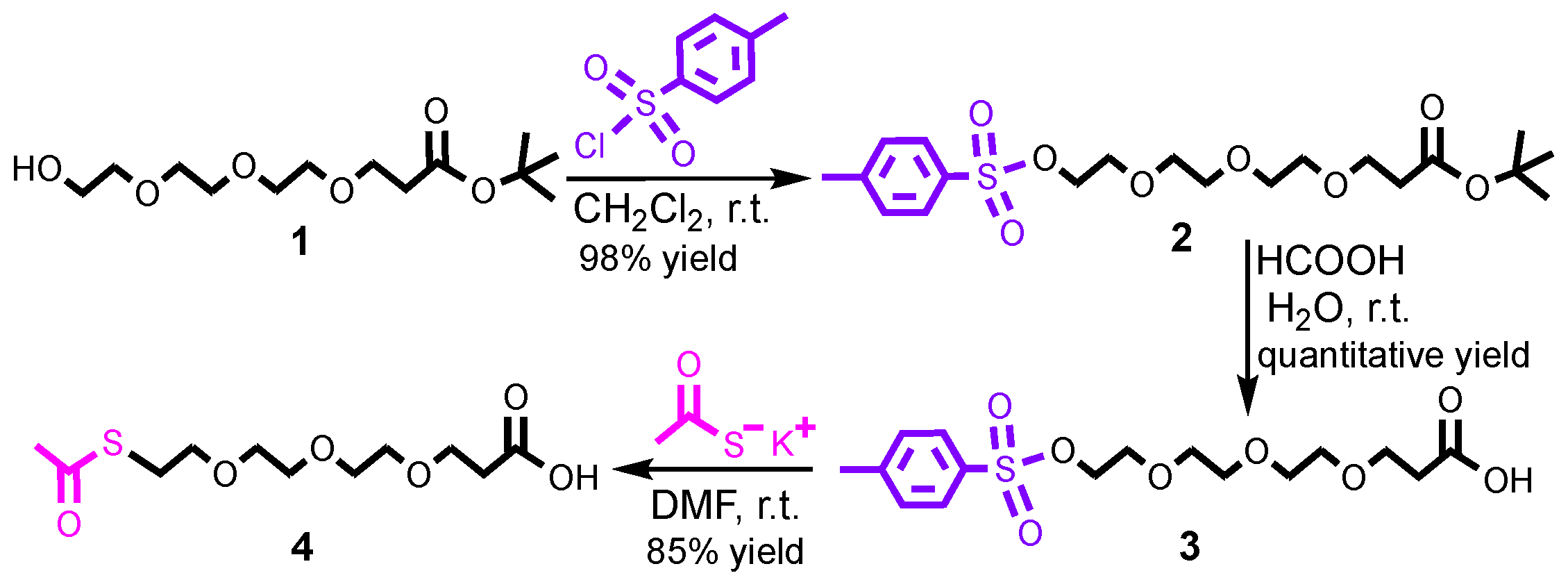
2.2.1. Synthesis of Compound 2
2.2.2. Synthesis of Compound 3
2.2.3. Synthesis of Compound 4
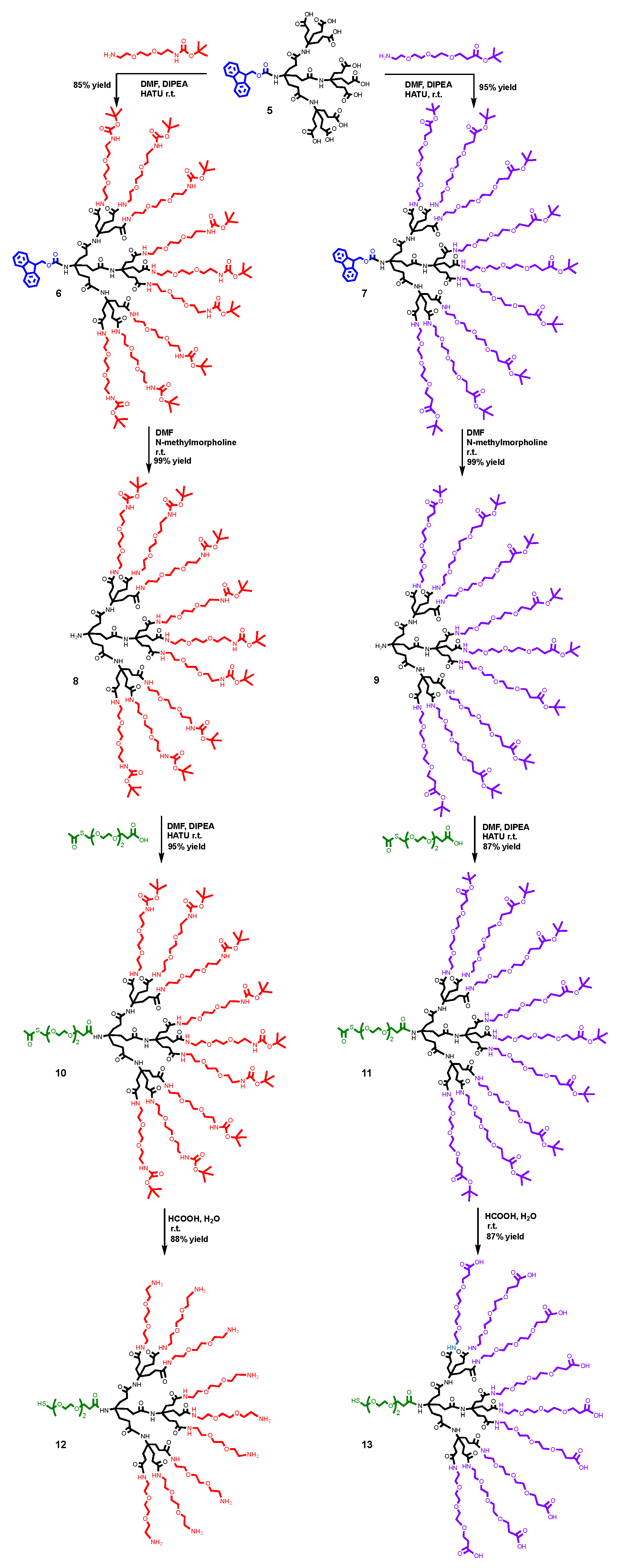
2.2.4. Synthesis of Compound 6
2.2.5. Synthesis of Compound 7
2.2.6. Synthesis of Compound 8
2.2.7. Synthesis of Compound 9
2.2.8. Synthesis of Compound 10
2.2.9. Synthesis of Compound 11
2.2.10. Synthesis of Compound 12
2.2.11. Synthesis of Compound 13
2.3. Nuclear Magnetic Resonance (NMR)
2.4. Mass Spectrometry (MS)
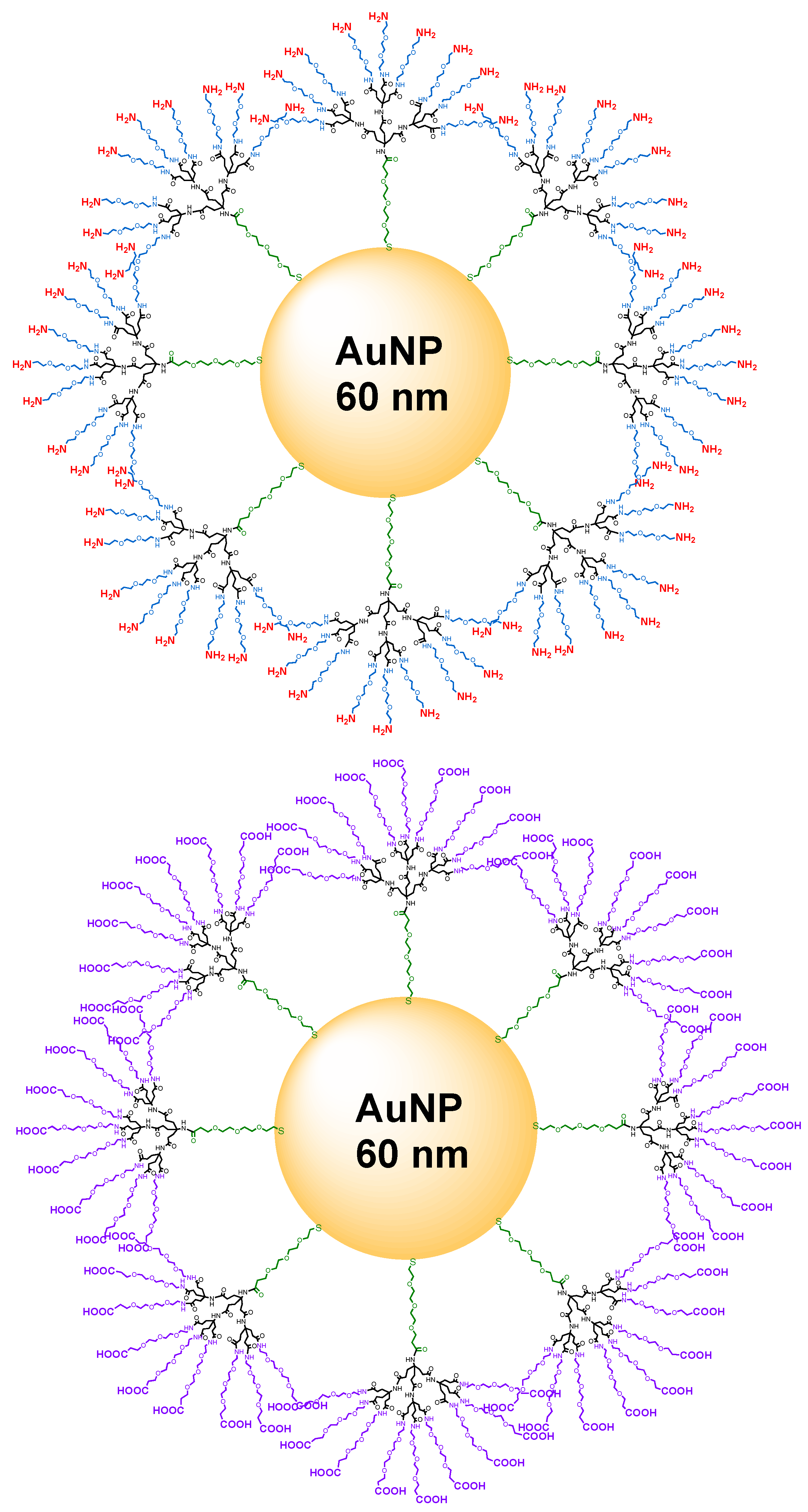
2.5. General Procedure for Functionalization of Gold Nanoparticles
2.6. Dynamic Light Scattering (DLS)
2.7. Zeta Potential
2.8. Absorption Spectroscopy in the UV-Vis
2.9. Scanning Electron Microscopy (SEM)
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 2: Management Strategies and New Agents. Pharm. Ther. 2015, 40, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016; pp. 1–76.
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! an Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [Green Version]
- Eichberg, M.J. Public Funding of Clinical-Stage Antibiotic Development in the United States and European Union. Health Secur. 2015, 13, 156–165. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L.; Surampalli, R.Y.; Hu, Z.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Mumtaz, S.; Li, C.-h.H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar]
- Garg, P.; Attri, P.; Sharma, R.; Chauhan, M.; Chaudhary, G.R. Advances and Perspective on Antimicrobial Nanomaterials for Biomedical Applications. Front. Nanotechnol. 2022, 4, 147–159. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Microbiol. 2021, 19, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Sahoo, R.; Singh, K.; Pati, S.; Mathew, J.; Pandey, A.C.; Kant, R.; Han, I.; Choi, E.-H.; Dwivedi, G.R.; et al. Drug Resistance Reversal Potential of Nanoparticles/Nanocomposites via Antibiotic’s Potentiation in Multi Drug Resistant P. aeruginosa. Nanomaterials 2022, 12, 117. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Shah, M. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. 2014, 19, 1320–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the Next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.-R.; Heo, J.H. Simultaneous Stabilization and Functionalization of Gold Nanoparticles via Biomolecule Conjugation: Progress and Perspectives. ACS Appl. Mater. Interfaces 2021, 13, 42311–42328. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Mahmoud, N.N. Photothermal-Induced Antibacterial Activity of Gold Nanorods Loaded into Polymeric Hydrogel against Pseudomonas aeruginosa Biofilm. Molecules 2019, 24, 2661. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.-H.; Huang, Y.-F.; Zhang, C.-Z.; Niu, J.; Chen, Y.; Chu, Y.; Jiang, Z.-H.; Gao, J.-Q.; Mao, Z.-W. Integration of antimicrobial peptides with gold nanoparticles as unique non-viral vectors for gene delivery to mesenchymal stem cells with antibacterial activity. Biomaterials 2016, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Bagga, P.; Siddiqui, H.H.; Akhtar, J.; Mahmood, T.; Zahera, M.; Khan, M.S. Gold Nanoparticles Conjugated Levofloxacin: For Improved Antibacterial Activity over Levofloxacin Alone. Curr. Drug Deliv. 2017, 14, 1114–1119. [Google Scholar] [CrossRef]
- Payne, J.N.; Waghwani, H.K.; Connor, M.G.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel Synthesis of Kanamycin Conjugated Gold Nanoparticles with Potent Antibacterial Activity. Front. Microbiol. 2016, 7, 607. [Google Scholar] [CrossRef]
- Daduang, J.; Klaynongsruang, S.; Leelayuwat, C.; Limpaiboon, T.; Lulitanond, A.; Boonsiri, P.; Srichan, S.; Soontaranon, S.; Rugmai, S.; Rattanata, N. Gallic acid conjugated with gold nanoparticles: Antibacterial activity and mechanism of action on foodborne pathogens. Int. J. Nanomed. 2016, 11, 3347–3356. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef] [Green Version]
- Dasari, T.P.S.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. 2015, 4, 147–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; Olivares-Trejo, J.d.J. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics 2019, 11, 1265–1276. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef]
- Boisselier, E.; Diallo, A.K.; Salmon, L.; Ornelas, C.; Ruiz, J.; Astruc, D. Encapsulation and Stabilization of Gold Nanoparticles with Click Polyethyleneglycol Dendrimers. J. Am. Chem. Soc. 2010, 132, 2729–2742. [Google Scholar] [CrossRef]
- Cho, T.J.; Zangmeister, R.A.; MacCuspie, R.I.; Patri, A.K.; Hackley, V.A. Newkome-Type Dendron-Stabilized Gold Nanoparticles: Synthesis, Reactivity, and Stability. Chem. Mater. 2011, 23, 2665–2676. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, V.; Bouchet, L.M.; Strumia, M.C. Nanoparticle-cored dendrimers: Functional hybrid nanocomposites as a new platform for drug delivery systems. Nanoscale 2015, 7, 3808–3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C.t. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C. Dendrimers derived from 1→3 branching motifs. Chem. Rev. 2010, 110, 6338–6442. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers; Cambridge University Press: Cambridge, UK, 2012; pp. 250–300. [Google Scholar]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 45–93. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Chuah, J.-A.; Numata, K. Chapter 1. Design Considerations for Properties of Nanocarriers on Disposition and Efficiency of Drug and Gene Delivery; Royal Society of Chemistry (RSC) Publishing: Cambridge, UK, 2016; pp. 1–22. [Google Scholar]
- Daniel, M.C.; Ruiz, J.; Nlate, S.; Blais, J.C.; Astruc, D. Nanoscopic assemblies between supramolecular redox active metallodendrons and gold nanoparticles: Synthesis, characterization, and selective recognition of H2PO4-, HSO4-, and adenosine-5′-triphosphate (ATP(2-)) anions. J. Am. Chem. Soc. 2003, 125, 2617–2628. [Google Scholar] [CrossRef]
- Peña-González, C.E.; García-Broncano, P.; Ottaviani, M.F.; Cangiotti, M.; Fattori, A.; Hierro-Oliva, M.; González-Martín, M.L.; Pérez-Serrano, J.; Gómez, R.; Muñoz-Fernández, M.Á.; et al. Dendronized Anionic Gold Nanoparticles: Synthesis, Characterization, and Antiviral Activity. Chem. A Eur. J. 2016, 22, 2987–2999. [Google Scholar] [CrossRef]
- Ornelas, C. Brief Timelapse on Dendrimer Chemistry: Advances, Limitations, and Expectations. Macromol. Chem. Phys. 2016, 217, 148–179. [Google Scholar] [CrossRef]
- Bertuzzi, D.L.; Perli, G.; Braga, C.B.; Ornelas, C. Synthesis, characterization, and anticancer activity of folate γ-ferrocenyl conjugates. New J. Chem. 2020, 44, 4694–4703. [Google Scholar] [CrossRef]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic Amphiphilic Dendrimers as Antibacterial Agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Leroueil, P.R.; Majoros, I.J.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. The Binding Avidity of a Nanoparticle-Based Multivalent Targeted Drug Delivery Platform. Chem. Biol. 2007, 14, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Van Dongen, M.A.; Silpe, J.E.; Dougherty, C.A.; Kanduluru, A.K.; Choi, S.K.; Orr, B.G.; Low, P.S.; Banaszak Holl, M.M. Avidity Mechanism of Dendrimer–Folic Acid Conjugates. Mol. Pharm. 2014, 11, 1696–1706. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kumar, P.V.; Koushik, O.S. Dendrimeric Biocides—A Tool for Effective Antimicrobial Therapy. J. Nanomed. Nanotechnol. 2016, 7, 1–19. [Google Scholar] [CrossRef]
- Perli, G.; Wang, Q.; Braga, C.B.; Bertuzzi, D.L.; Fontana, L.A.; Soares, M.C.P.C.P.; Ruiz, J.; Megiatto, J.D.; Astruc, D.; Ornelas, C.; et al. Self-Assembly of a Triazolylferrocenyl Dendrimer in Water Yields Nontraditional Intrinsic Green Fluorescent Vesosomes for Nanotheranostic Applications. J. Am. Chem. Soc. 2021, 143, 12948–12954. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Kaeppeli, A.; Siriwardena, T.; Darbre, T.; Perron, K.; Jafari, P.; Reymond, J.-L.; Pioletti, D.P.; Applegate, L.A. Anti-Microbial Dendrimers against Multidrug-Resistant P. aeruginosa Enhance the Angiogenic Effect of Biological Burn-wound Bandages. Sci. Rep. 2016, 6, 22020. [Google Scholar] [CrossRef]
- Holmes, A.M.; Heylings, J.R.; Wan, K.-W.; Moss, G.P. Antimicrobial efficacy and mechanism of action of poly(amidoamine) (PAMAM) dendrimers against opportunistic pathogens. Int. J. Antimicrob. Agents 2019, 53, 500–507. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I. Chapter 20—Dendrimer as antimicrobial agents. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 363–384. [Google Scholar]
- Kaufman, E.A.; Tarallo, R.; Elacqua, E.; Carberry, T.P.; Weck, M. Synthesis of Well-Defined Bifunctional Newkome-Type Dendrimers. Macromolecules 2017, 50, 4897–4905. [Google Scholar] [CrossRef]
- Buhr, E.; Senftleben, N.; Klein, T.; Bergmann, D.; Gnieser, D.; Frase, C.G.; Bosse, H. Characterization of nanoparticles by scanning electron microscopy in transmission mode. Meas. Sci. Technol. 2009, 20, 100–1010. [Google Scholar] [CrossRef]
- Shan, Y.; Luo, T.; Peng, C.; Sheng, R.; Cao, A.; Cao, X.; Shen, M.; Guo, R.; Tomas, H.; Shi, X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials 2012, 33, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Rajchakit, U.; Sarojini, V. Recent Developments in Antimicrobial-Peptide-Conjugated Gold Nanoparticles. Bioconjugate Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef]
- Hunter, R. Zeta Potential in Colloid Science: Principles and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 1981; pp. 93–117. [Google Scholar]
- Ohshima, H. Encyclopedia of Colloid and Interface Science; Zeta Potential; Springer: Berlin/Heidelberg, Germany, 2013; pp. 148–207. [Google Scholar]
- Perli, G.; Pessoa, A.C.S.N.; Balbino, T.A.; de la Torre, L.G. Ionic strength for tailoring the synthesis of monomodal stealth cationic liposomes in microfluidic devices. Colloids Surf. B Biointerfaces 2019, 179, 233–241. [Google Scholar] [CrossRef]
- Ottewil, R.H.L.; Rowell, R.L. Colloid Science. In Zeta Potential in Colloid Science; Elsevier: Amsterdam, The Netherlands, 1981; pp. 95–120. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
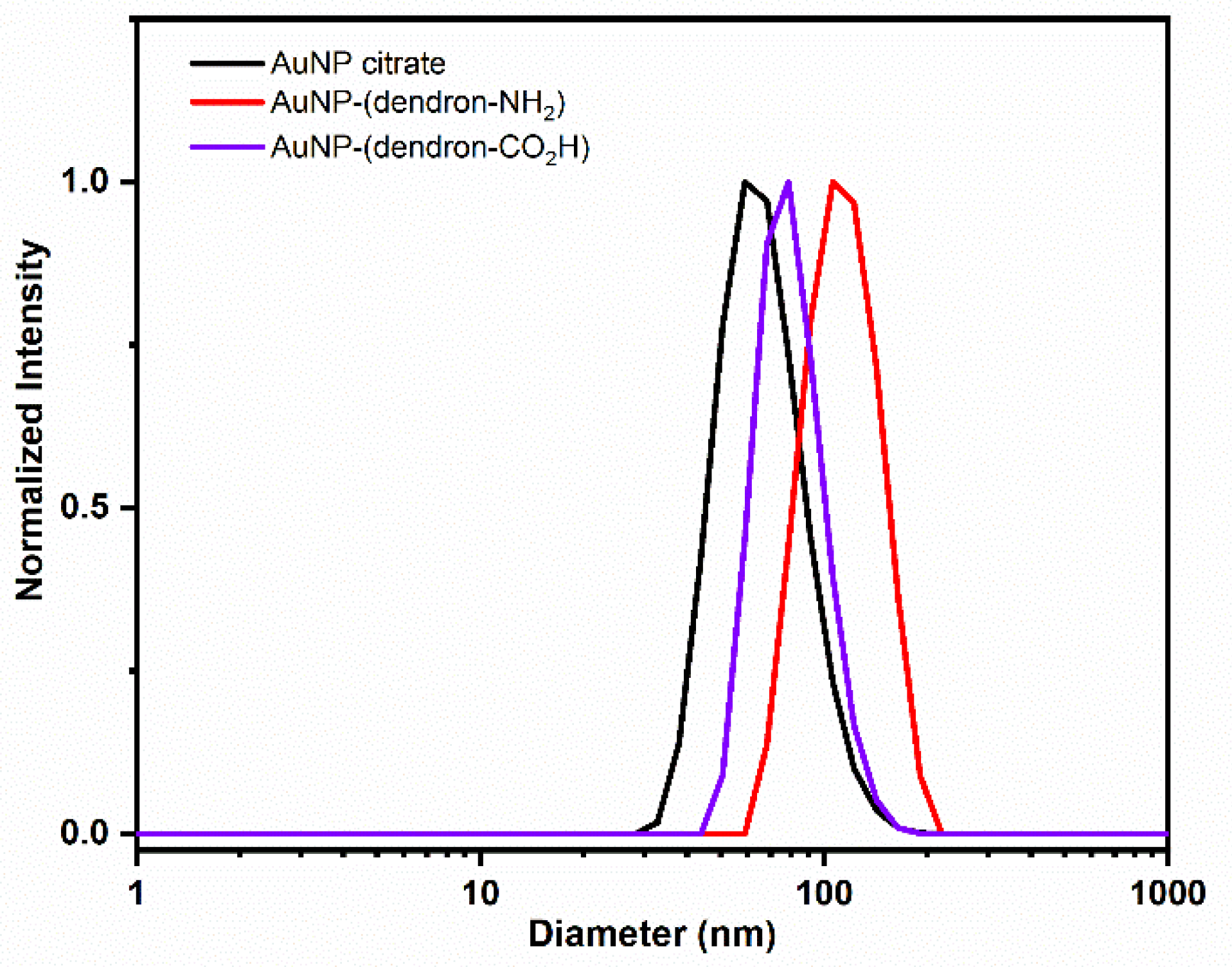
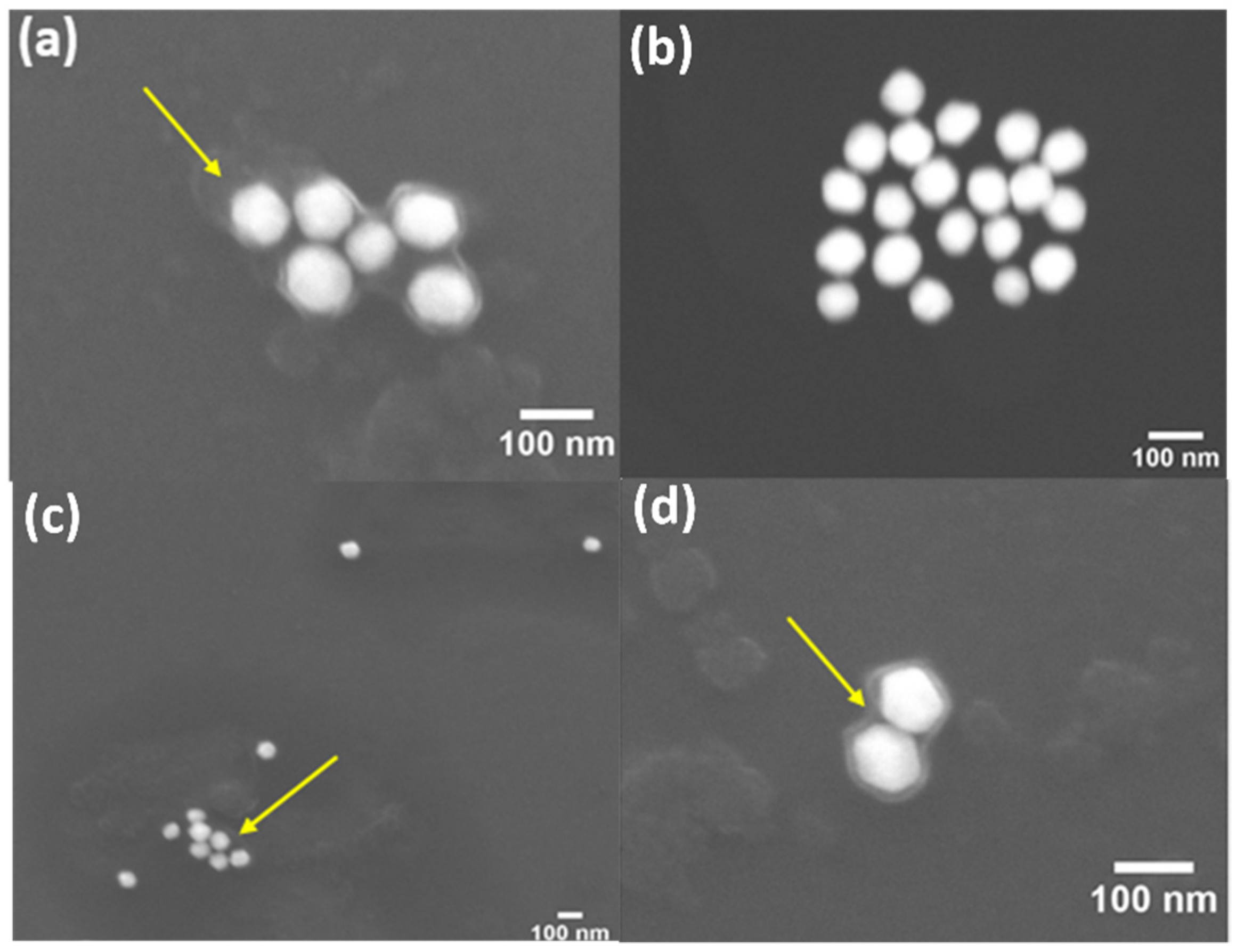
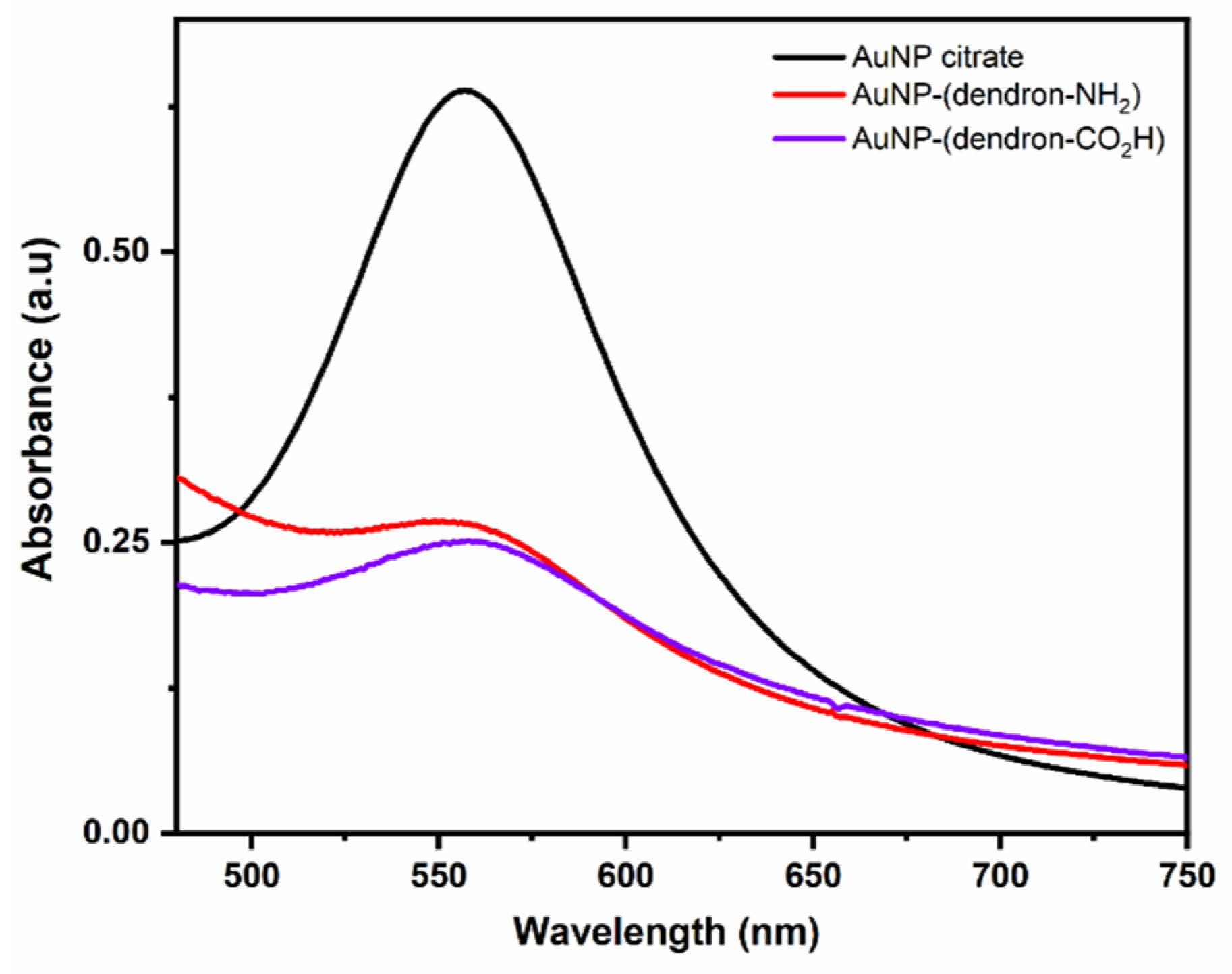
| Zeta Potential (mV ± SD) | Diameter (nm ± SD) | PDI (PDI ± SD) | |
|---|---|---|---|
| AuNP–citrate | −49 ± 9 | 60 ± 2 | 0.06 ± 0.01 |
| AuNP–(dendron-NH2) | 27 ± 6 | 109 ± 1 | 0.19 ± 0.09 |
| AuNP–(dendron-CO2H) | −31 ± 8 | 80 ± 1 | 0.05 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perli, G.; Bertuzzi, D.L.; Souto, D.E.P.; Ramos, M.D.; Braga, C.B.; Aguiar, S.B.; Ornelas, C. Synthesis and Characterization of Dendronized Gold Nanoparticles Bearing Charged Peripheral Groups with Antimicrobial Potential. Nanomaterials 2022, 12, 2610. https://doi.org/10.3390/nano12152610
Perli G, Bertuzzi DL, Souto DEP, Ramos MD, Braga CB, Aguiar SB, Ornelas C. Synthesis and Characterization of Dendronized Gold Nanoparticles Bearing Charged Peripheral Groups with Antimicrobial Potential. Nanomaterials. 2022; 12(15):2610. https://doi.org/10.3390/nano12152610
Chicago/Turabian StylePerli, Gabriel, Diego L. Bertuzzi, Dênio E. P. Souto, Miguel D. Ramos, Carolyne B. Braga, Samile B. Aguiar, and Catia Ornelas. 2022. "Synthesis and Characterization of Dendronized Gold Nanoparticles Bearing Charged Peripheral Groups with Antimicrobial Potential" Nanomaterials 12, no. 15: 2610. https://doi.org/10.3390/nano12152610
APA StylePerli, G., Bertuzzi, D. L., Souto, D. E. P., Ramos, M. D., Braga, C. B., Aguiar, S. B., & Ornelas, C. (2022). Synthesis and Characterization of Dendronized Gold Nanoparticles Bearing Charged Peripheral Groups with Antimicrobial Potential. Nanomaterials, 12(15), 2610. https://doi.org/10.3390/nano12152610





