Quasi-Solid-State Lithium-Sulfur Batteries Assembled by Composite Polymer Electrolyte and Nitrogen Doped Porous Carbon Fiber Composite Cathode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
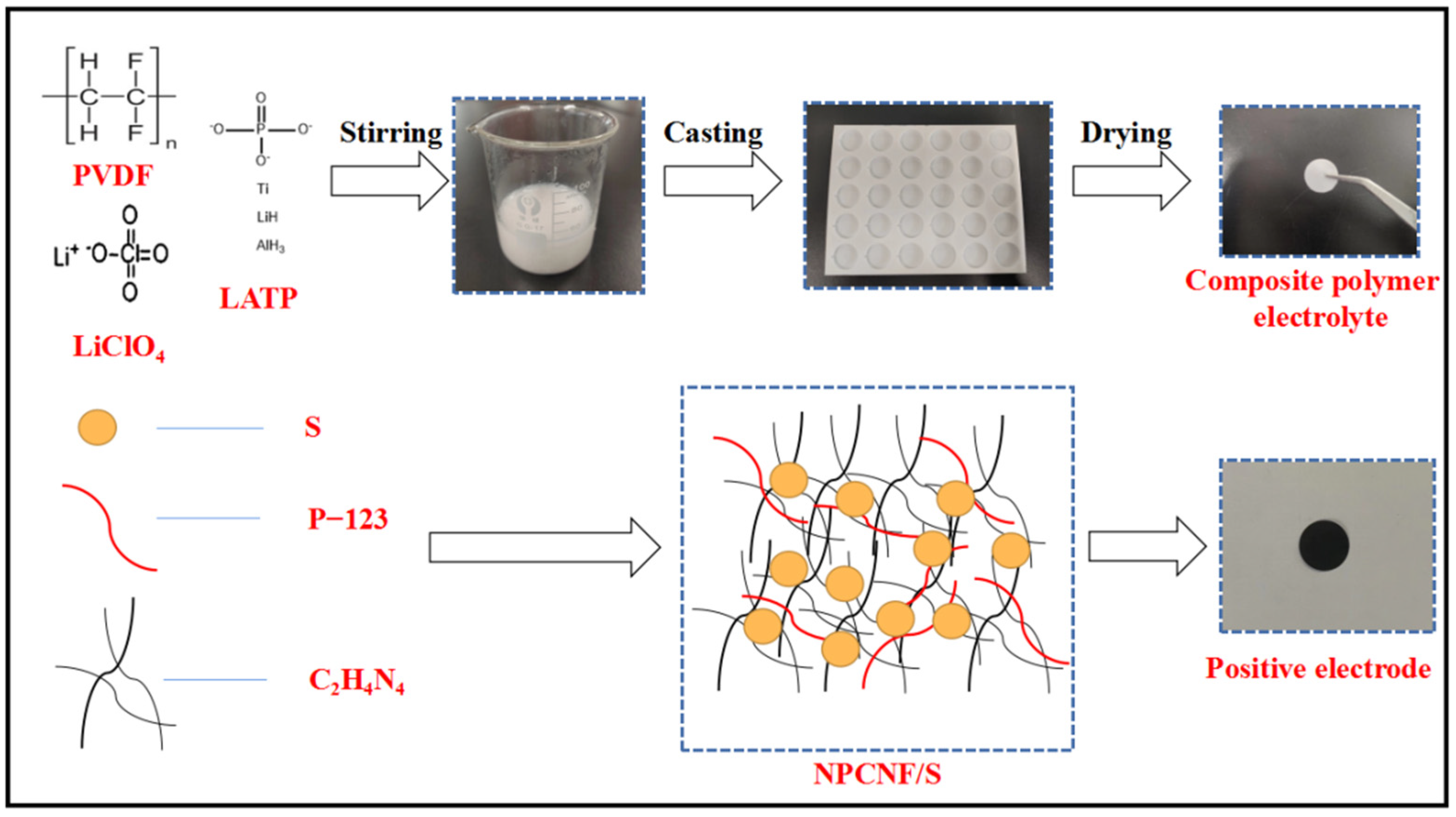
2.2. Preparation of the NPCNF/S Composite
2.3. Preparation of the Composite Solid Electrolyte Membrane (CPEs)
2.4. Battery Assembly
2.5. Characterization
2.6. Electrochemical Measurements
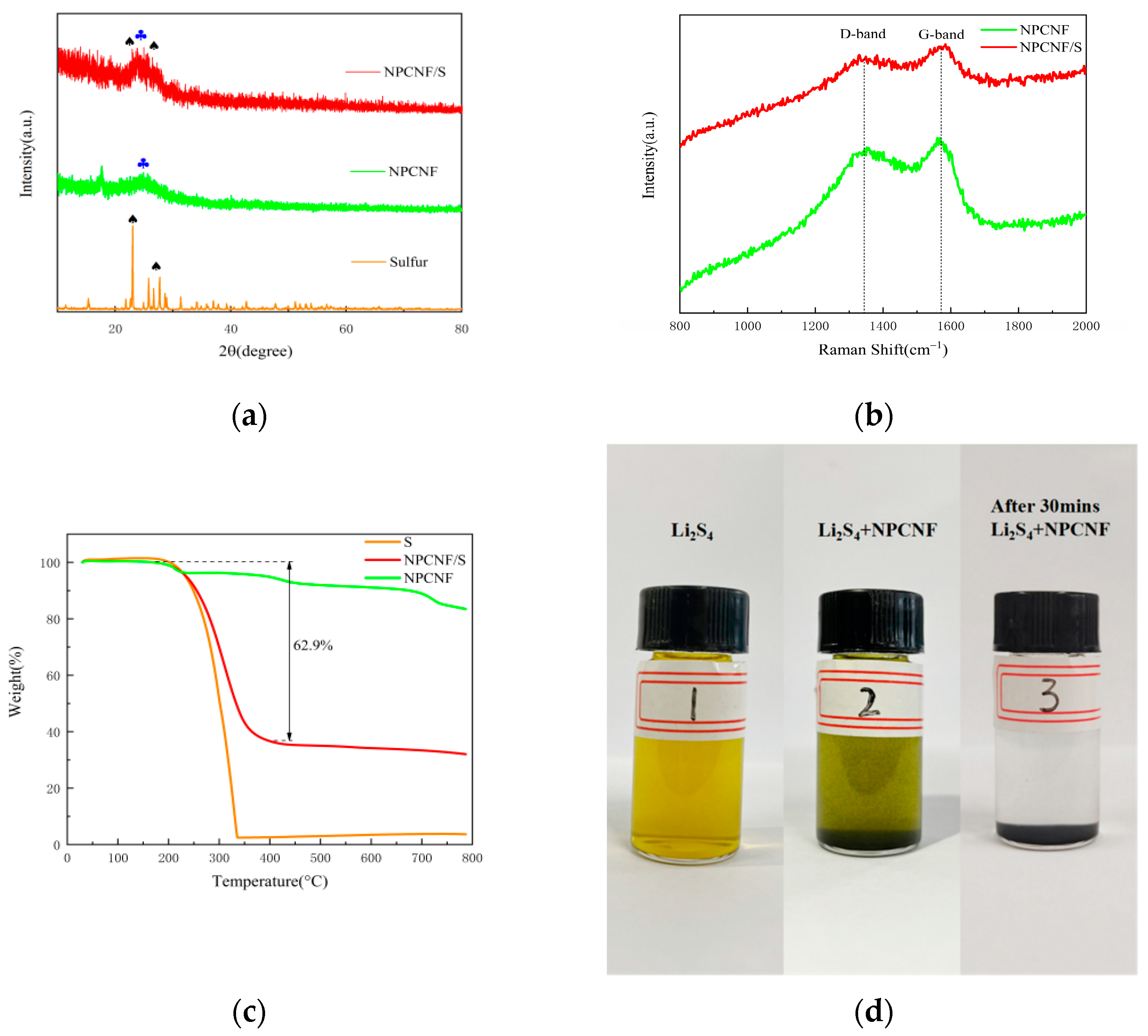
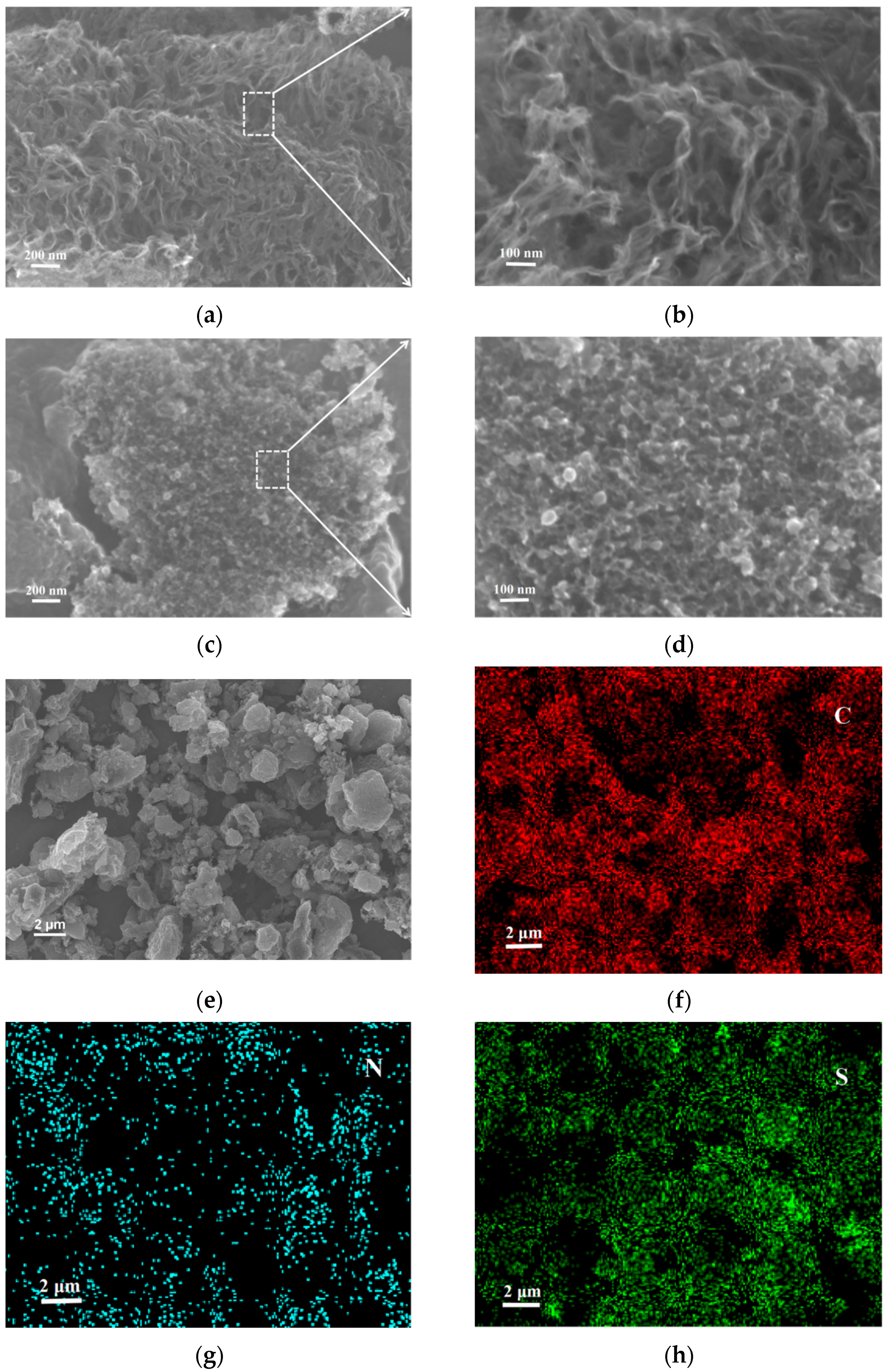
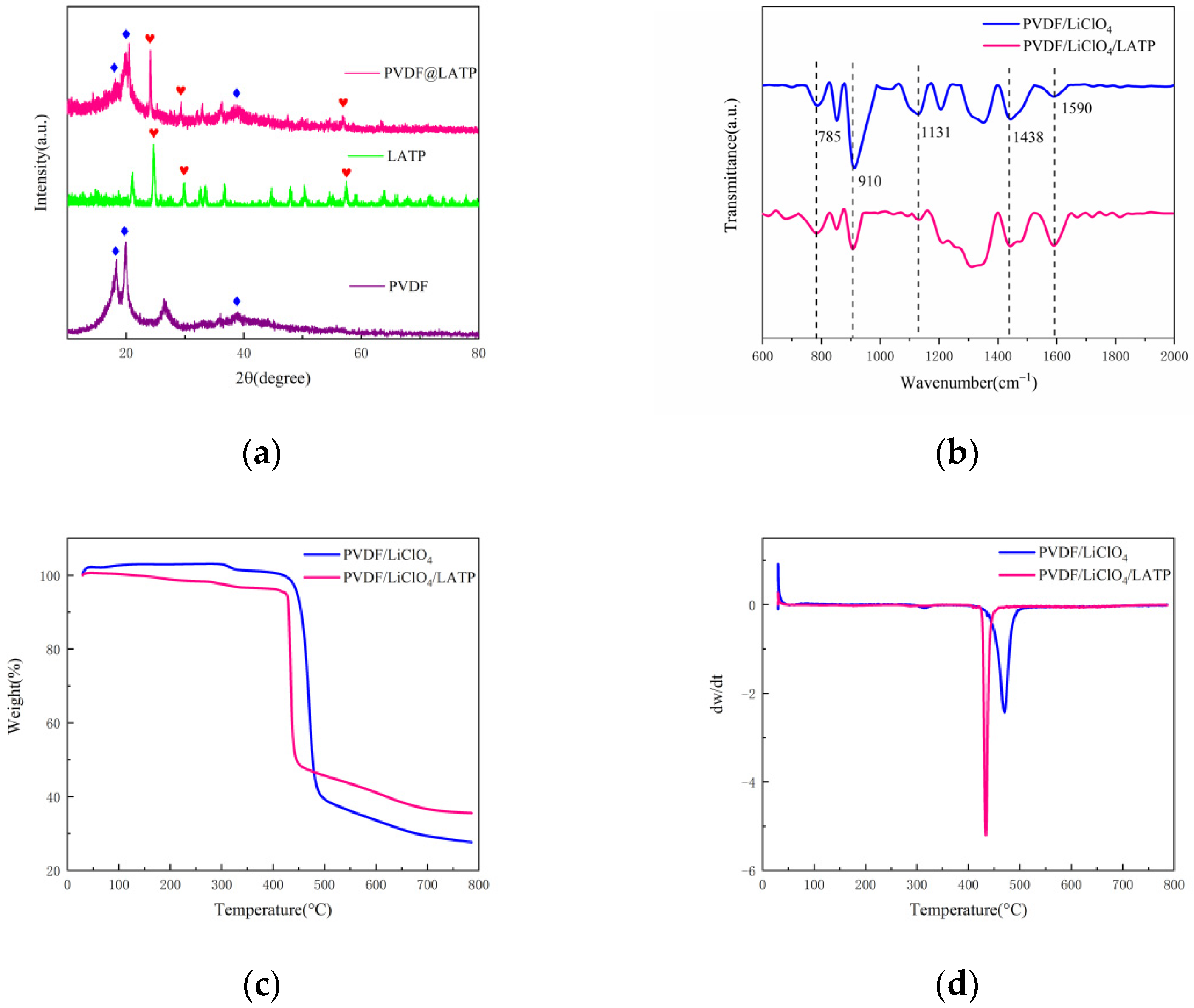
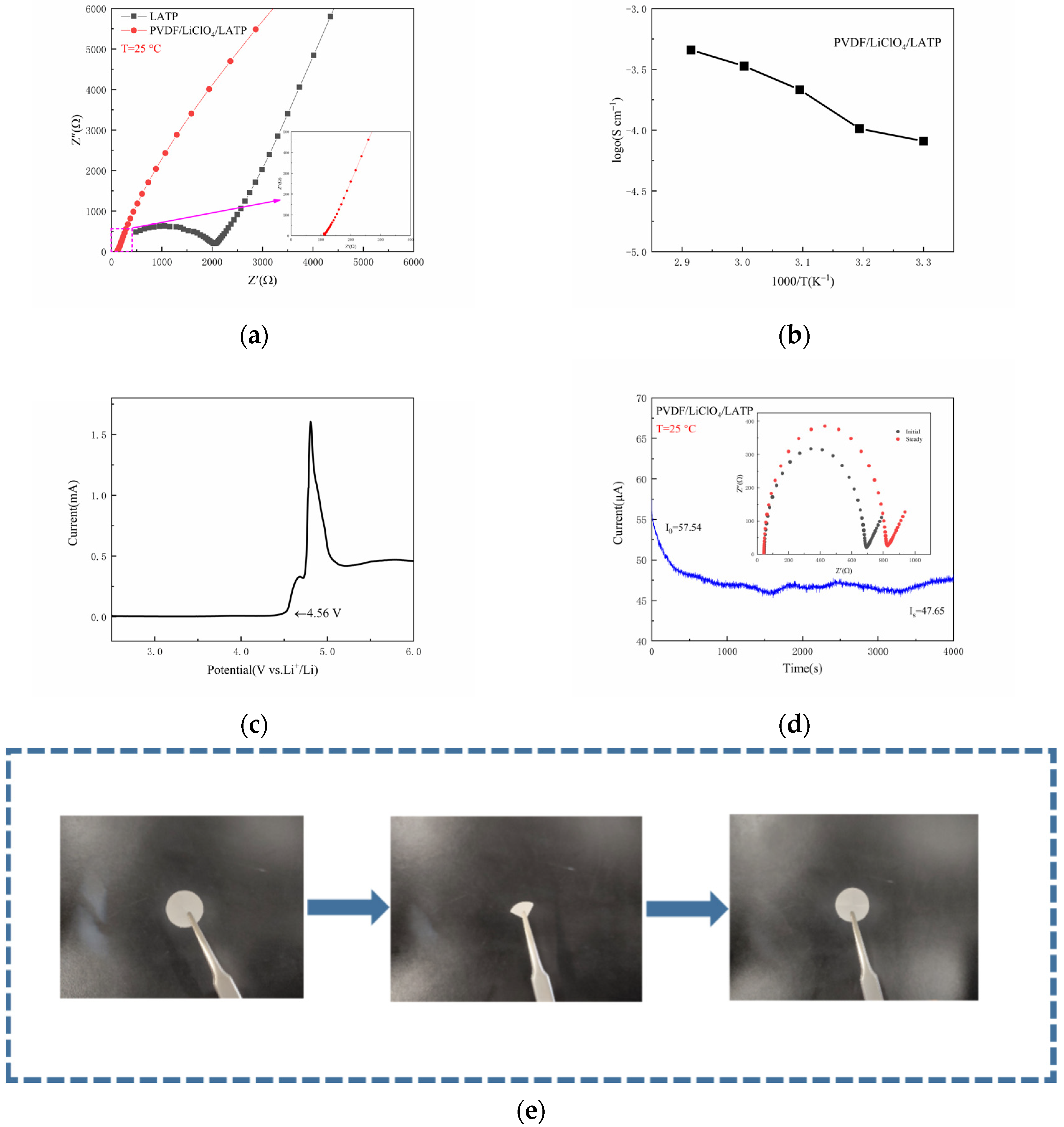
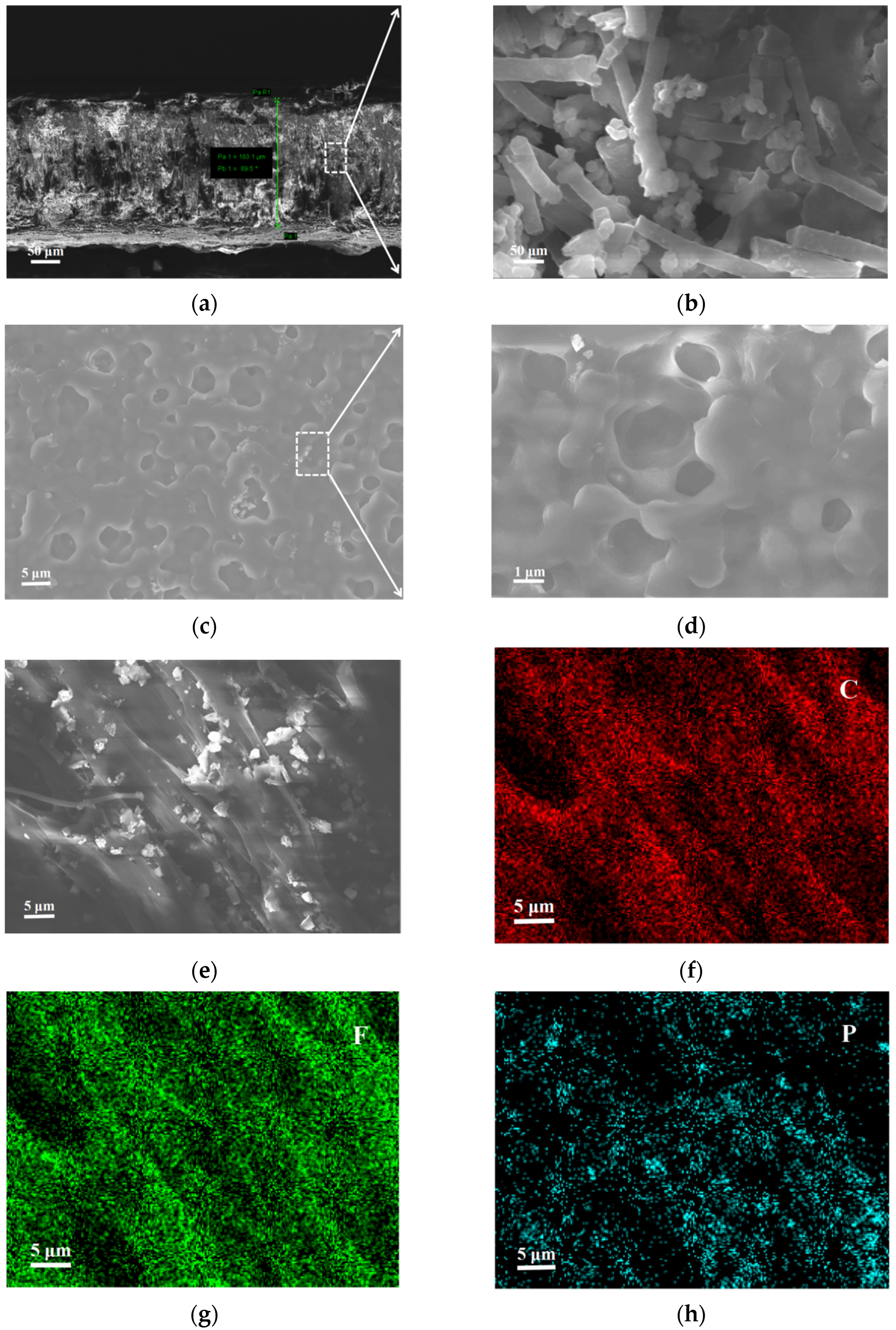
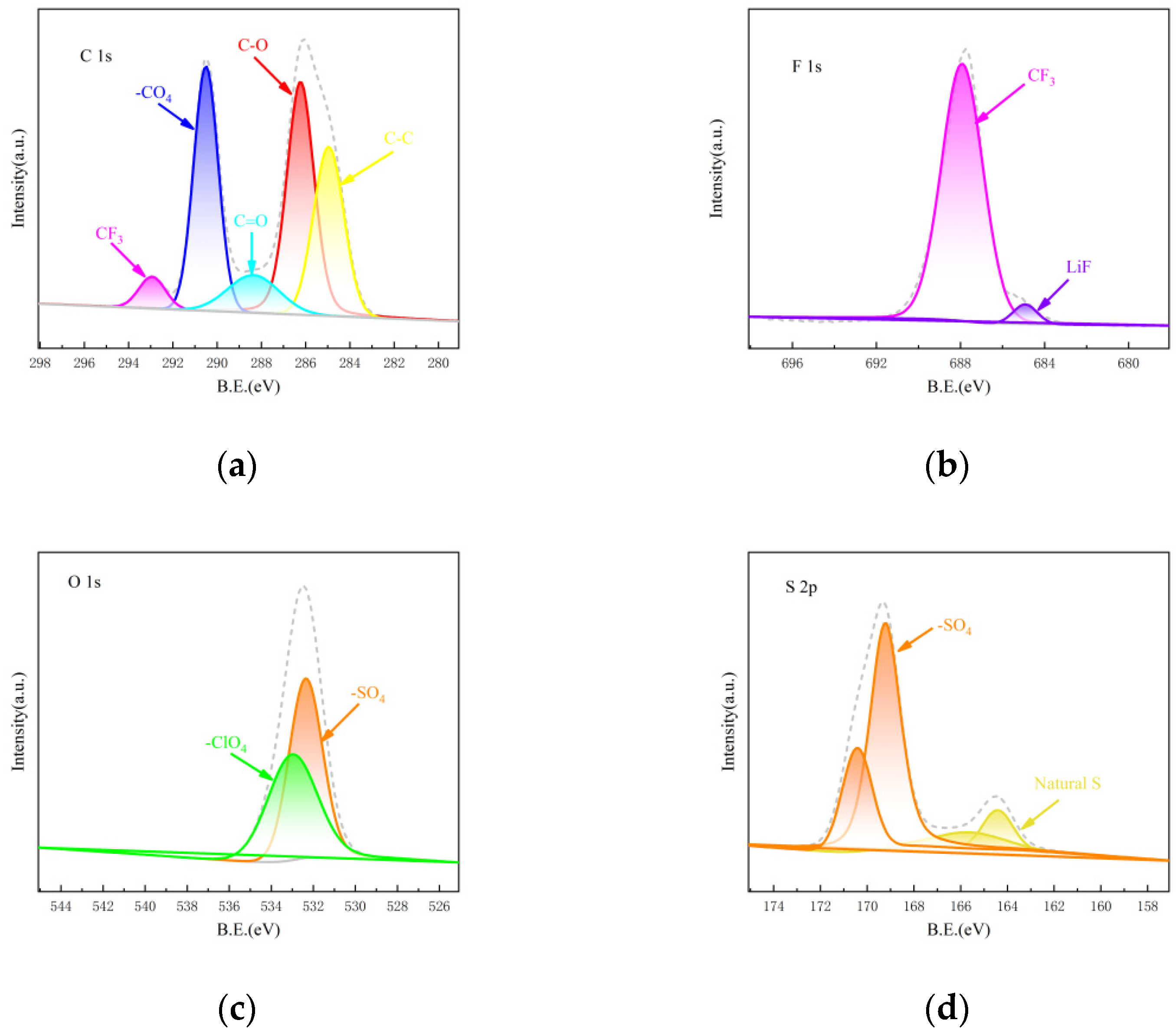
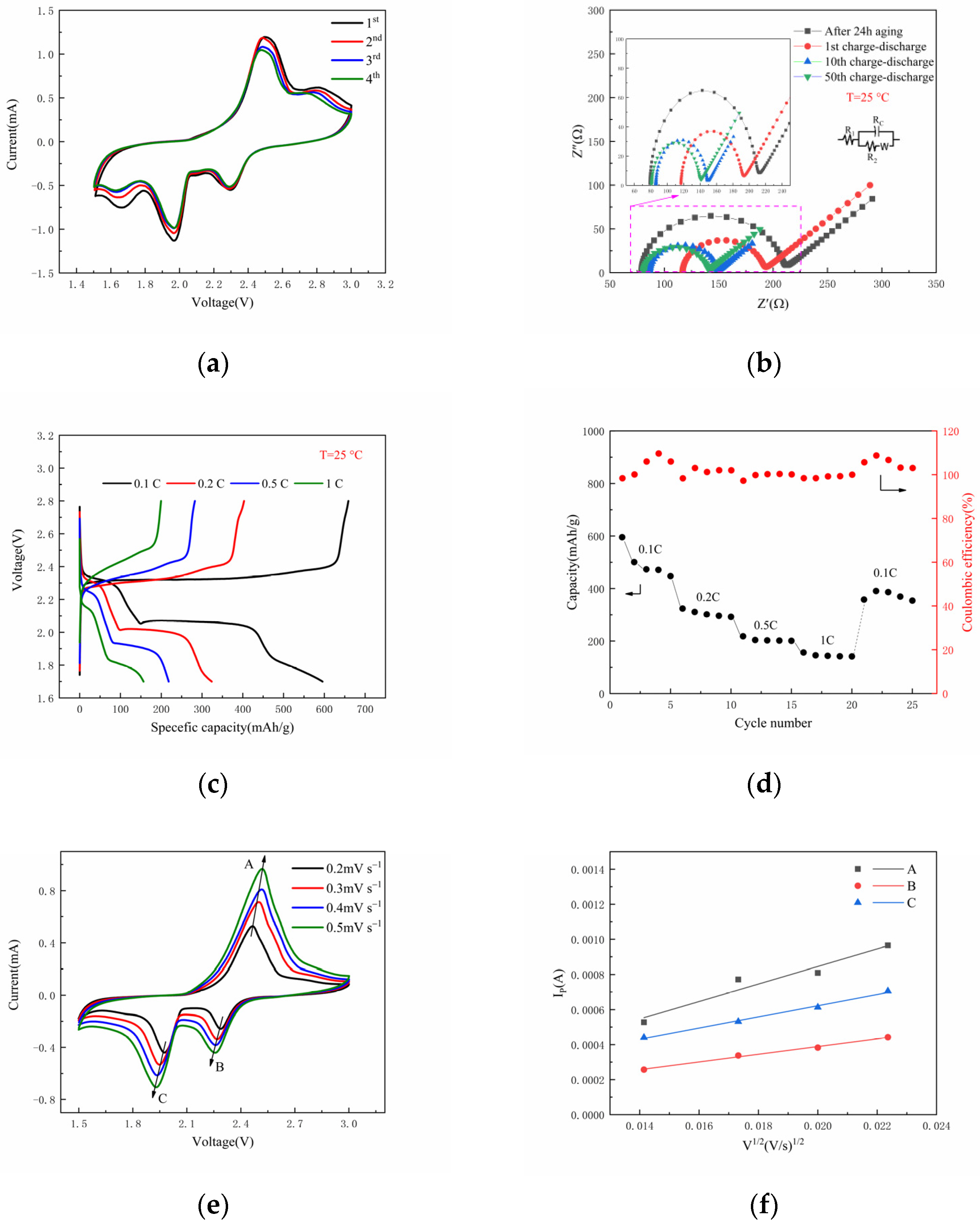
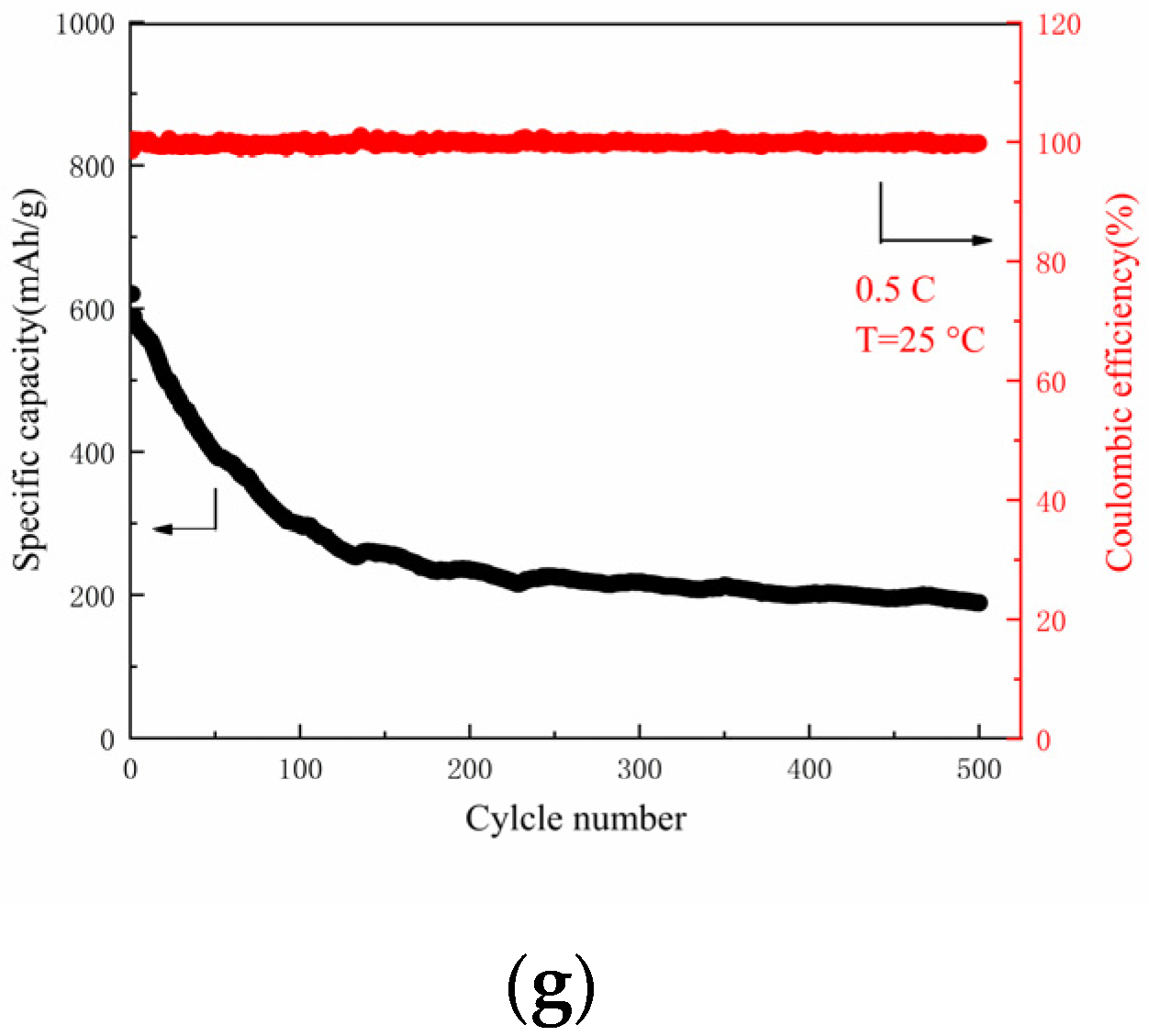
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Pei, F.; Cui, J.; Fang, X.; Zheng, N. Recent advances in hollow porous carbon materials for lithium-sulfur batteries. Small 2019, 15, 1804786. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Deng, N.P.; Yan, J.; Li, Z.H.; Kang, W.M.; Cheng, B.W. The recent research status quo and the prospect of electrolytes for lithium sulfur batteries. Chem. Eng. J. 2019, 369, 874–897. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhou, P.; Hao, S. Rechargeable solid-state Li-Air and Li-S batteries: Materials, construction, and challenges. Adv. Energy Mater. 2018, 8, 1701602. [Google Scholar] [CrossRef]
- Urbonaite, S.; Poux, T.; Petr, N. Progress towards commercially viable Li-S battery cells. Adv. Energy Mater. 2015, 5, 1500118. [Google Scholar] [CrossRef]
- Skilton, R.A.; Bourne, R.A.; Amara, Z.; Horvath, R.; Jin, J.; Scully, M.J.; Streng, E.; Tang, S.L.Y.; Summers, P.A.; Wang, J. Remote-controlled experiments with cloud chemistry. Nat. Chem. 2014, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Chen, X.; Xia, Y.; Huang, H.; Gan, Y.; Wu, R.; Chen, F.; Zhang, W. Highly mesoporous carbon foams synthesized by a facile, cost-effective and template-free Pechini method for advanced lithium-sulfur batteries. J. Mater. Chem. A 2013, 1, 3295–3301. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Mangani, L.R.; Villevieille, C. Mechanical vs. chemical stability of sulphide-based solid-state batteries. Which one is the biggest challenge to tackle? Overview of solid-state batteries and hybrid solid state batteries. J. Mater. Chem. A 2020, 8, 10150–10167. [Google Scholar] [CrossRef]
- Lei, D.; Shi, K.; Ye, H.; Wan, Z.; Wang, Y.; Shen, L.; Li, B.; Yang, Q.H.; Kang, F.; He, Y.B. Solid-state electrolytes: Progress and perspective of solid-state lithium-sulfur batteries. Adv. Funct. Mater. 2018, 28, 1870272. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.O.; Kwon, S.W.; Kim, T.J.; Lee, E.H.; Jeong, S.H.; Jung, Y. Effect of carbon black materials on the electrochemical properties of sulfur-based composite cathode for lithium-sulfur cells. J. Nanosci. Nanotechnol. 2013, 13, 7870–7874. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.L. Nitrogen-doped carbons in Li-S batteries: Materials design and electrochemical mechanism. Front. Energy Res. 2014, 2, 49. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Lee, M.; Kim, Y.; Lim, H.K.; Chae, J.; Hwang, G.S.; Lee, S. Agent molecule modulated low-temperature activation of solid-state lithium-ion transport for polymer electrolytes. J. Power Sources 2021, 505, 229917–229924. [Google Scholar] [CrossRef]
- Chen, X.; He, W.; Ding, L.X.; Wang, S.; Wang, H. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 2019, 12, 938–944. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Zuo, C.; Yang, M.; Wang, Z.; Jiang, K.; Li, S.; Luo, W.; He, D.; Liu, C.; Xie, X.; Xue, Z. Cyclophosphazene-based hybrid polymer electrolyte via epoxyamine reaction for high-performance all-solid-state lithium-ion batteries. J. Mater. Chem. A 2019, 7, 18871–18879. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Liang, Y.; Dong, H.; Hao, F.; Wang, A.; Zhu, Y.; Cui, X.; Yao, Y. Hyperbranched PEO-based hyperstar solid polymer electrolytes with simultaneous improvement of ion transport and mechanical strength. ACS Appl. Energy Mater. 2019, 2, 1608–1615. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, N.; Sun, Z.; Wu, W.; Kou, W.; Cui, G.; Zhao, K.; Wang, J. Thin laminar composite solid electrolyte with high ionic conductivity and mechanical strength towards advanced all-solid-state lithium-sulfur battery. J. Mater. Chem. A 2020, 8, 23344–23353. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Correia, D.M.; Gonçalves, R.; de Zea Bermudez, V.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Enhanced ionic conductivity in poly(vinylidene fluoride) electrospun separator membranes blended with different ionic liquids for lithium ion batteries. J. Colloid Interface Sci. 2021, 582, 376–386. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Gosselink, D.; Chen, P. Synthesis of poly(ethylene-oxide)/nanoclay solid polymer electrolyte for all solid-state lithium/sulfur battery. Ionics 2015, 21, 381–385. [Google Scholar] [CrossRef]
- McGrogan, F.P.; Swamy, T.; Bishop, S.R.; Eggleton, E.; Porz, L.; Chen, X.; Chiang, Y.M.; Van Vliet, K.J. Compliant yet brittle mechanical behavior of Li2S-P2S5 lithium-ion-conducting solid electrolyte. Adv. Energy Mater. 2017, 7, 1602011. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bai, Z.; Li, Y.; Ma, L.; Dai, A.; Wang, X.; Luo, D.; Wu, T.; Liu, P.; Yang, L.; et al. Electrochemically primed functional redox mediator generator from the decomposition of solid state electrolyte. Nat. Commun. 2019, 10, 1890–1898. [Google Scholar] [CrossRef]
- Li, M.; Frerichs, J.E.; Kolek, M.; Sun, W.; Zhou, D.; Huang, C.J.; Hwang, B.J.; Hansen, M.R.; Winter, M.; Bieker, P. Solid-state lithium-sulfur battery enabled by Thio-LiSICON/Polymer composite electrolyte and sulfurized polyacrylonitrile cathode. Adv. Funct. Mater. 2020, 30, 1910123. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Sun, Q.; Li, M.; Adair, K.; Yu, C.; Li, J.; Li, W.; Fu, J.; Li, X.; Li, R.; et al. Insight into cathode surface to boost the performance of solid-state batteries. Energy Storage Mater. 2021, 35, 661–668. [Google Scholar] [CrossRef]
- Schell, K.G.; Bucharsky, E.C.; Lemke, F.; Hoffmann, M.J. Effect of calcination conditions on lithium conductivity in Li1.3Ti1.7Al0.3(PO4)3 prepared by sol-gel route. Ionics 2016, 23, 821–827. [Google Scholar] [CrossRef]
- Han, J.P.; Zhang, B.; Wang, L.Y.; Zhu, H.L.; Qi, Y.X.; Yin, L.; Li, N.; Lun, N.; Bai, Y.J. Li1.3Al0.3Ti1.7(PO4)3 behaving as a fast ionic conductor and bridge to boost the electrochemical performance of Li4Ti5O12. ACS Sustain. Chem. Eng. 2018, 6, 7273–7282. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Gao, H.; Xue, L.; Xin, S.; Goodenough, J.B. A high-energy-density potassium battery with a polymer-gel electrolyte and a polyaniline cathode. Angew. Chem. Int. Ed. Engl. 2018, 130, 5449–5453. [Google Scholar] [CrossRef]
- Fu, C.; Ma, Y.; Zuo, P.; Zhao, W.; Gao, Y.; Tang, W.; Yin, G.; Wang, J. In-situ thermal polymerization boosts succinonitrile-based composite solid-state electrolyte for high performance Li-metal battery. J. Power Sources 2021, 496, 229861. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, Y.; An, T.; Wang, X.; Zhou, H.; Fan, T. 3d interconnected crumpled porous carbon sheets modified with high-level nitrogen doping for high performance lithium sulfur battery. Carbon 2019, 154, 58–66. [Google Scholar] [CrossRef]
- Xu, Z.L.; Kim, J.K.; Kang, K. Carbon nanomaterials for advanced lithium sulfur batteries. Nano Today 2018, 19, 84–107. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using ftir. Rsc Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Li, L.; Yang, X. Solid-state polymer electrolyte solves the transfer of lithium ions between the solid–solid interface of the electrode and the electrolyte in lithium–sulfur and lithium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 5101–5112. [Google Scholar] [CrossRef]
- Baskaran, R.; Selvasekarapandian, S.; Kuwata, N.; Kawamura, J.; Hattori, T. Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ion. 2006, 177, 2679–2682. [Google Scholar] [CrossRef]
- Cho, Y.G.; Hwang, C.; Cheong, D.S.; Kim, Y.S.; Song, H.K. Gel/solid polymer electrolytes characterized by in situ gelation or polymerization for electrochemical energy systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef]
- Kim, D.H.; Hwang, S.; Cho, J.J.; Yu, S.; Kim, S.; Jeon, J.; Ahn, K.H.; Lee, C.; Song, H.K.; Lee, H. Toward fast operation of lithium batteries: Ion activity as the factor to determine the concentration polarization. ACS Energy Lett. 2019, 4, 1265–1270. [Google Scholar] [CrossRef]
- Bag, S.; Zhou, C.; Kim, P.J.; Pol, V.G.; Thangadurai, V. Lif modified stable flexible PVDF-garnet hybrid electrolyte for high performance all-solid-state li-s batteries. Energy Storage Mater. 2019, 24, S2405–S8297. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Huang, S.; Jiang, Y.; Chen, Z. Preparation and electrochemical study of PVDF-HFP/LATP/g-C3N4 composite polymer electrolyte membrane. Inorg. Chem. Commun. 2021, 131, 108793–108799. [Google Scholar] [CrossRef]
- Yue, Q.; Shi, S.; Hong, L.; Jr, L. Defect thermodynamics and diffusion mechanisms in Li2CO3 and implications for the solid electrolyte interphase in Li-ion batteries. J. Phys. Chem. B 2013, 117, 8579–8593. [Google Scholar]
- Bai, Y.; Li, L.; Li, Y.; Chen, G.; Zhao, H.; Wang, Z.; Li, H.; Wu, F.; Wu, C. Reversible and irreversible heat generation of nca/si–c pouch cell during electrochemical energy-storage process. Energy Storage Mater. 2019, 16, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Feng, W.; Ying, B.; Li, Y.; Chen, G.; Wang, Z.; Wu, C. Regulating li deposition by constructing lif-rich host for dendrite-free lithium metal anode. Energy Storage Mater. 2018, 16, 411–418. [Google Scholar] [CrossRef]
- Chao, X.; Bing, S.; Gustafsson, T.; Edstrm, K.; Hahlin, M. Interface layer formation in solid polymer electrolyte lithium batteries: An xps study. J. Mater. Chem. A 2014, 2, 7256–7264. [Google Scholar]
- Fang, R.; Xu, H.; Xu, B.; Li, X.; Li, Y.; Goodenough, J.B. Reaction mechanism optimization of solid-state Li-S batteries with a PEO-based electrolyte. Adv. Funct. Mater. 2020, 31, 2001812. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium−sulfur batteries. Nat. Commun. 2015, 6, 5682–5689. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, K.; Sun, Z.; Hu, G.; Xiao, R.; Cheng, H.M.; Li, F. Structure-related electrochemical performance of organosulfur compounds for lithium-sulfur batteries. Energy Environ. Sci. 2020, 13, 1076–1095. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Zhang, Y.; Ning, Y.; Huang, D.; Lan, L.; Li, S. Quasi-Solid-State Lithium-Sulfur Batteries Assembled by Composite Polymer Electrolyte and Nitrogen Doped Porous Carbon Fiber Composite Cathode. Nanomaterials 2022, 12, 2614. https://doi.org/10.3390/nano12152614
Liang X, Zhang Y, Ning Y, Huang D, Lan L, Li S. Quasi-Solid-State Lithium-Sulfur Batteries Assembled by Composite Polymer Electrolyte and Nitrogen Doped Porous Carbon Fiber Composite Cathode. Nanomaterials. 2022; 12(15):2614. https://doi.org/10.3390/nano12152614
Chicago/Turabian StyleLiang, Xinghua, Yu Zhang, Yujuan Ning, Dongxue Huang, Linxiao Lan, and Siying Li. 2022. "Quasi-Solid-State Lithium-Sulfur Batteries Assembled by Composite Polymer Electrolyte and Nitrogen Doped Porous Carbon Fiber Composite Cathode" Nanomaterials 12, no. 15: 2614. https://doi.org/10.3390/nano12152614
APA StyleLiang, X., Zhang, Y., Ning, Y., Huang, D., Lan, L., & Li, S. (2022). Quasi-Solid-State Lithium-Sulfur Batteries Assembled by Composite Polymer Electrolyte and Nitrogen Doped Porous Carbon Fiber Composite Cathode. Nanomaterials, 12(15), 2614. https://doi.org/10.3390/nano12152614




