Facile Synthesis of 4,4′-biphenyl Dicarboxylic Acid-Based Nickel Metal Organic Frameworks with a Tunable Pore Size towards High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization Techniques and Electrochemical Measurements
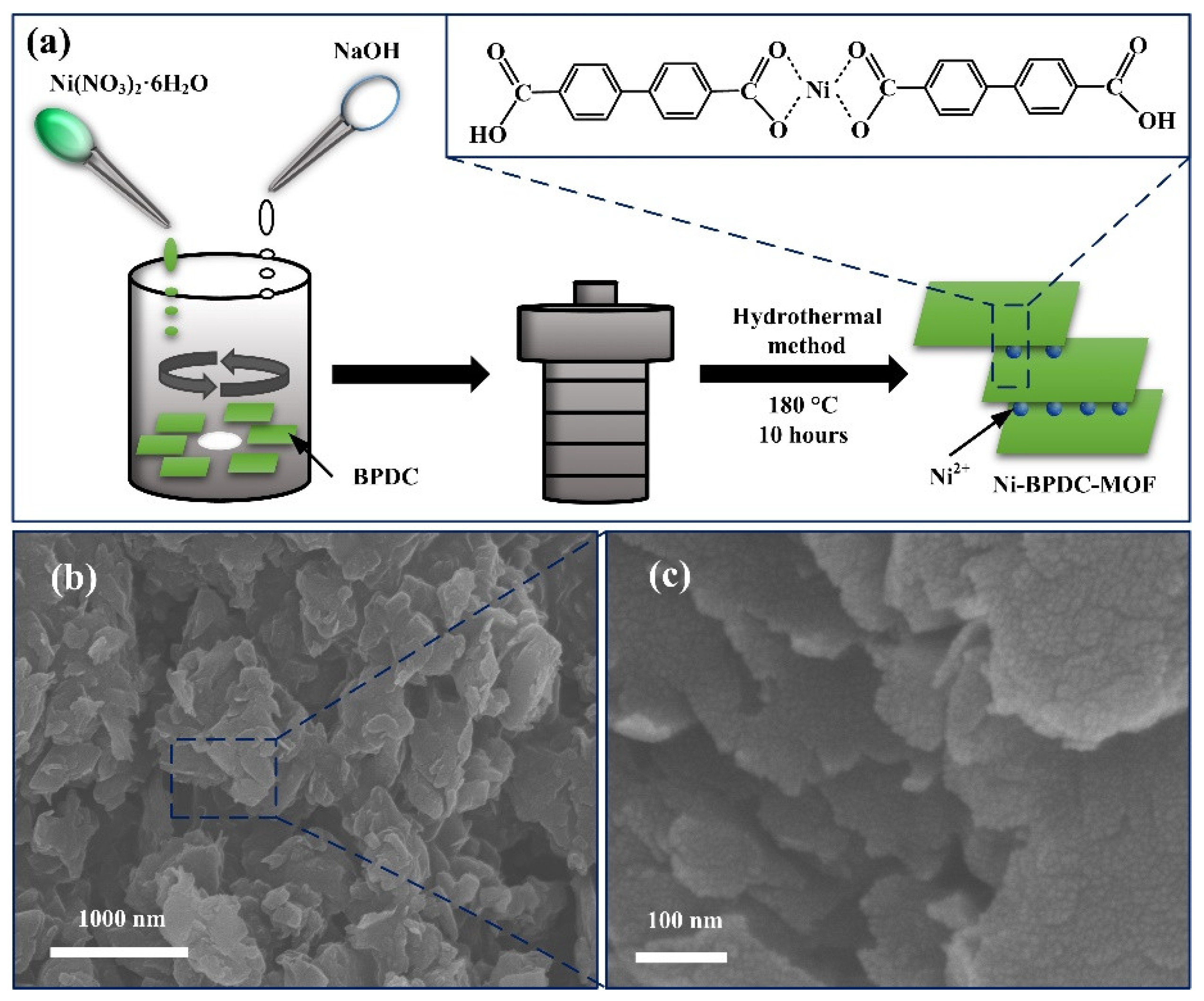
2.2. Synthesis Methods
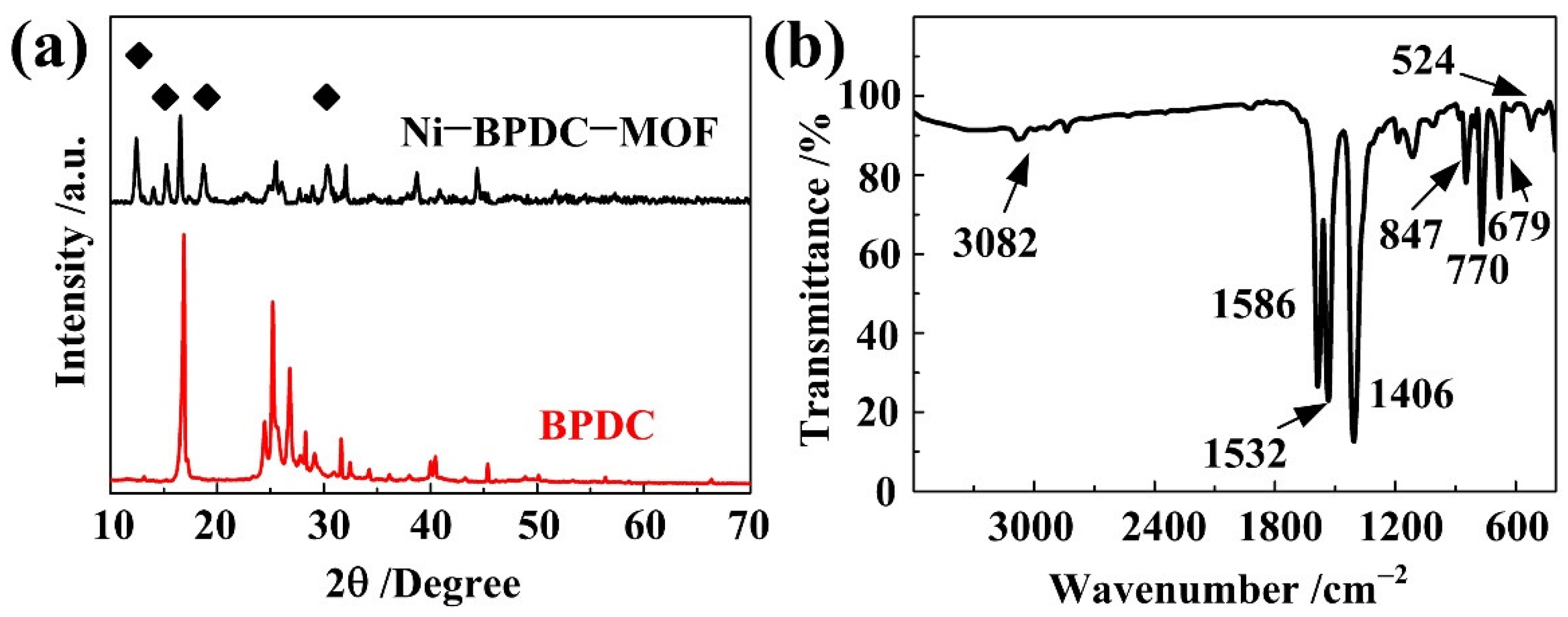
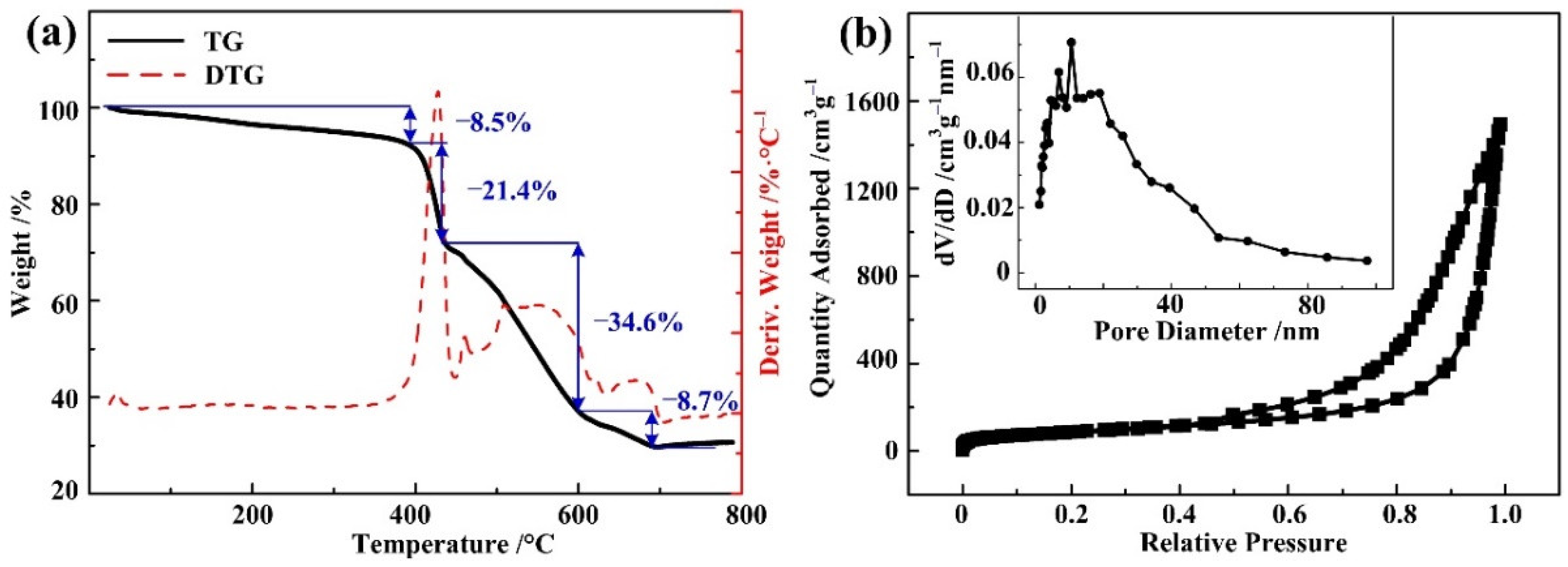
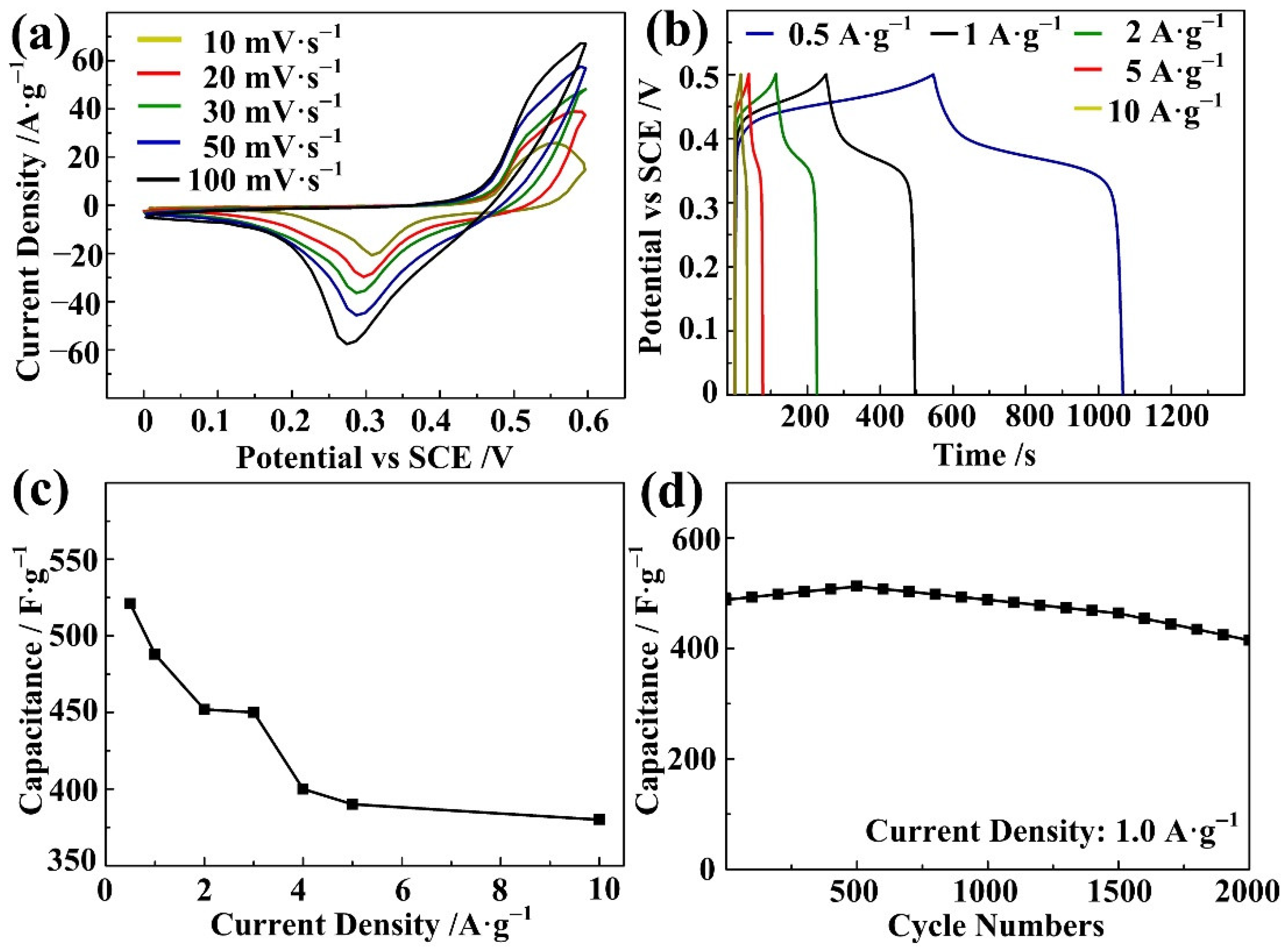
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal-organic frameworks for energy storage: Batteries and supercapacitors. Coordin. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Naoi, K.; Naoi, W.; Aoyagi, S.; Miyamoto, J.; Kamino, T. New generation “nanohybrid supercapacitor”. Accounts Chem. Res. 2014, 46, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Feng, F.; Xie, Y. Design of vanadium oxide structures with controllable electrical properties for energy applications. Chem. Soc. Rev. 2013, 42, 5157–5183. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, Y.; Zhu, J.; Gan, X.; Qin, F.; Tang, T.; Luo, W.; Guo, N.; Liu, Z.; Wang, L.; et al. Ultrafast pore-tailoring of dense microporous carbon for high volumetric performance asupercapacitors in organic electrolyte. Carbon 2022, 191, 19–27. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.; O’Keeffe, M.; Yaghi, O. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Ke, F.; Wu, Y.; Deng, H. Metal-organic frameworks for lithium ion batteries and supercapacitors. J. Solid. State. Electr. 2015, 223, 109–121. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, Y.; Pang, H. Exposing {001} crystal plane on hexagonal Ni-MOF with surface-grown cross-linked mesh-structures for electrochemical energy storage. Small 2019, 15, 1902463. [Google Scholar] [CrossRef]
- Yan, Y.; Gu, P.; Zheng, S.; Zheng, M.; Pang, H.; Xue, H. Facile synthesis of an accordion-like Ni-MOF superstructure for high-performance flexible supercapacitors. J. Mater. Chem. A 2016, 4, 19078–19085. [Google Scholar] [CrossRef]
- Maina, J.W.; Gonzalo, C.P.; Kong, L.; Schutz, J.; Hill, M.; Dumee, L.F. Metal organic framework based catalysts for CO2 conversion. Mater. Horiz. 2017, 4, 345–361. [Google Scholar] [CrossRef]
- Li, L.; Jung, H.S.; Lee, J.W.; Kang, Y.T. Review on applications of metal-organic frameworks for CO2 capture and the performance enhancement mechanisms. Renew. Sust. Energ. Rev. 2022, 162, 112441. [Google Scholar] [CrossRef]
- Furukawa, H.; Nakeum, K.; Yong, G.; Aratani, N.; Choi, S.; Yazaydin, A.; Snurr, R.; O’Keeffe, M.; Yaghi, O. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Lim, W.; Oh, T.; Suh, M. A highly porous metal–organic framework: Structural transformations of a guest-free MOF depending on activation method and temperature. Chem. Eur. J. 2011, 17, 7251–7260. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Yu, C.; Duan, H.; Yang, B.; Meng, X.; Li, Z. Six isomorphous window-beam MOFs: Explore the effects of metal ions on MOF-derived carbon for supercapacitors. Chem. Eur. J. 2018, 24, 16160–16169. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, F.; Zhu, D.; Duan, H.; Lv, Y.; Li, L.; Gan, L. Ultramicroporous carbon nanoparticles derived from metaleorganic framework nanoparticles for high-performance supercapacitors. Mater. Chem. Phys. 2018, 211, 234–241. [Google Scholar] [CrossRef]
- Rawool, C.; Karna, S.; Srivastava, A. Enhancing the supercapacitive performance of Nickel based metal organic framework-carbon nanofibers composite by changing the ligands. Electrochim. Acta 2019, 294, 345–356. [Google Scholar] [CrossRef]
- He, F.; Yang, N.; Li, K.; Wang, X.; Cong, S.; Zhang, L.; Xiong, S.; Zhou, A. Hydrothermal synthesis of Ni-based metal organic frameworks/graphene oxide composites as supercapacitor electrode materials. J. Mater. Res. 2020, 35, 1439–1450. [Google Scholar] [CrossRef]
- Rahmanifar, M.S.; Hesari, H.; Noori, A.; Masoomi, M.Y.; Morsali, A.; Mousavi, M.F. A dual Ni/Co-MOF-reduced graphene oxide nanocomposite as a high performance supercapacitor electrode material. Electrochim. Acta 2018, 275, 76–86. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Y.; Lu, R.; Guan, L.; Peng, X.; Sha, J. Anodic electrodeposition of a porous nickel oxide–hydroxide film on passivated nickel foam for supercapacitors. J. Mater. Chem. A 2014, 2, 7161–7164. [Google Scholar] [CrossRef]
- Lee, S.; Shinde, D.; Kim, E.; Lee, W.; Oh, I.; Shrestha, N.; Lee, J.; Han, S. Supercapacitive property of metal–organic-frameworks with different pore dimensions and morphology. Micropor. Mesopor. Mater. 2013, 171, 53–57. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Yang, N.; He, F.; Chu, J.; Gong, M.; Wu, B.; Zhang, R.; Xiong, S. Hydrothermal synthesis of NiCo-based bimetal-organic frameworks as electrode materials for supercapacitors. J. Solid State Chem. 2019, 270, 370–378. [Google Scholar] [CrossRef]
- Wang, X.; Yang, N.; Li, Q.; He, F.; Yang, Y.; Cong, S.; Li, K.; Xiong, S.; Zhou, A. Hydrothermal synthesis of humate-layer-based bimetal organic framework composites as high rate-capability and enery-density electrode materials for supercapacitors. ChemistrySelect 2020, 5, 2794–2804. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Xue, Y.; Wang, C.; Cao, J.; Chen, Z. Facile synthesis of novel metal-organic nickel hydroxide nanorods for high performance supercapacitor. Electrochim. Acta 2016, 211, 595–602. [Google Scholar] [CrossRef]
- Inoue, S.; Fujihara, S. Liquid-liquid biphasic synthesis of layered Zinc hydroxides intercalated with long-chain carboxylate ions and their conversion into ZnO nanostructures. Inorg. Chem. 2011, 50, 3605–3612. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ahn, T.; Soundararajan, D.; Ko, J.; Kim, J. Non-aqueous approach to the preparation of reduced graphene oxide/α-Ni(OH)2 hybrid composites and their high capacitance behavior. Chem. Commun. 2011, 22, 6305–6307. [Google Scholar] [CrossRef]
- Yan, H.; Bai, J.; Wang, J.; Zhang, X.; Wang, B.; Liu, Q.; Liu, L. Graphene homogeneously anchored with Ni(OH)2 nanoparticles as advanced supercapacitor electrodes. CrystEngComm 2013, 15, 10007–10015. [Google Scholar] [CrossRef]
- Zhang, W.; Hirai, Y.; Tsuchiya, T.; Osamu, T. Effect of substrate bias voltage on tensile properties of single crystal silicon microstructure fully coated with plasma CVD diamond-like carbon film. Appl. Surf. Sci. 2018, 443, 48–54. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Dang, K.; Zhang, Q.; Shen, Z.; Zhan, S. Conjugated π electrons of MOFs drive charge separation at heterostructures interface for enhanced photoelectrochemical water oxidation. Small 2021, 17, 2100367. [Google Scholar] [CrossRef]
- Waki, I.; Fujioka, H.; Ono, K.; Oshima, M.; Miki, H.; Fukizawa, A. The effect of surface cleaning by wet treatments and ultra high vacuum annealing for Ohmic contact formation of P-type GaN. Jpn. J. Appl. Phys. 2000, 39, 4451–4455. [Google Scholar] [CrossRef]
- Salam, H.; Nassar, H.N.; Khidr, A.; Zaki, T. Antimicrobial activities of green synthesized Ag nanoparticles@Ni-MOF nanosheets. J. Inorg. Organomet. P. 2018, 28, 2791–2798. [Google Scholar] [CrossRef]
- Li, G.; Hou, J.J.; Zhang, W.; Li, P.; Liu, G.; Wang, Y.; Wang, K. Graphene-bridged WO3/MoS2 Z-scheme photocatalyst for enhanced photodegradation under visible light irradiation. Mater. Chem. Phys. 2020, 246, 122827. [Google Scholar] [CrossRef]
- Du, R.; Wu, Y.; Yang, Y.; Zhai, T.; Zhou, T.; Shang, Q.; Zhu, L.; Shang, C.; Guo, Z. Porosity engineering of MOF-based materials for electrochemical energy storage. Adv. Energy Mater. 2021, 11, 2100154. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, X.; Chen, X.; Zhang, Z. Synergistic effect of novel redox additives of p-nitroaniline and dimethylglyoxime for highly improving the supercapacitor performances. Phys. Chem. Chem. Phys. 2016, 18, 2718–2729. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wei, T.; Qiao, W.; Shao, B.; Zhao, Q.; Zhang, L.; Fan, Z. Rapid microwave-assisted synthesis of graphene nanosheet/Co3O4 composite for supercapacitors. Electrochim. Acta 2010, 55, 6973–6978. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, Z.; Liu, J.; Sun, X. Stable ultrahigh specific capacitance of NiO nanorod arrays. Nano Res. 2011, 4, 658–665. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, F.; Wang, J.; Gao, S.; Ding, X.; Wei, M. ZnV2O4-CMK nanocomposite as an anode material for rechargeable lithium-ion batteries. J. Mater. Chem. 2012, 22, 14284–14288. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Xiong, Y.; Cheng, D.; Zeng, Y.; Bu, Y. Fabrication of 3D Co-doped Ni-based MOF hierarchical micro-flowers as a high-performance electrode material for supercapacitors. Appl. Surf. Sci. 2019, 483, 1158–1165. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Liu, S. Porous carbon nanosheets derived from Al-based MOFs for supercapacitors. Micropor. Mesopor. Mat. 2016, 236, 94–99. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, S.; Zhao, H.; Wang, Y.; Zhang, X. Hierarchically porous carbons derived from nonporous metal-organic frameworks: Synthesis and influence of struts. Electrochim. Acta 2015, 180, 651–657. [Google Scholar] [CrossRef]
- Yin, W.; Zhang, G.; Wang, X.; Pang, H. One–dimensional metal–organic frameworks for electrochemical applications. Adv. Colloid Interface Sci. 2021, 298, 102562. [Google Scholar] [CrossRef] [PubMed]


| Sample | Surface Area (m2·g−1) | Average Diameter (nm) | Electrolyte | Scan Rate (mV·s−1) | Current Density (A·g−1) | Rct (Ω) | Capacitance (F·g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| C-BPDC | 1137 | 4.2 | 6M KOH | 1 | — | 0.34 | 170 | [40] |
| Al-BPDC | 415.2 | 18.5 | 6M KOH | — | 0.25 | 3.25 | 119 | [39] |
| C-BPDC | 843 | 0.53 | 6M KOH | — | 1 | 1.23 | 256 | [41] |
| Ni-BPDC | 347 | 32.7 | 6M KOH | — | 1 | 1.10 | 328 | [16] |
| Ni-BPDC | — | — | 6M KOH | — | 1 | 0.65 | 432 | [17] |
| Ni-BPDC | 311.99 | 29.16 | 3M KOH | — | 1 | 0.49 | 488 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yin, H.; Yu, Z.; Jia, X.; Liang, J.; Li, G.; Li, Y.; Wang, K. Facile Synthesis of 4,4′-biphenyl Dicarboxylic Acid-Based Nickel Metal Organic Frameworks with a Tunable Pore Size towards High-Performance Supercapacitors. Nanomaterials 2022, 12, 2062. https://doi.org/10.3390/nano12122062
Zhang W, Yin H, Yu Z, Jia X, Liang J, Li G, Li Y, Wang K. Facile Synthesis of 4,4′-biphenyl Dicarboxylic Acid-Based Nickel Metal Organic Frameworks with a Tunable Pore Size towards High-Performance Supercapacitors. Nanomaterials. 2022; 12(12):2062. https://doi.org/10.3390/nano12122062
Chicago/Turabian StyleZhang, Wenlei, Hongwei Yin, Zhichao Yu, Xiaoxia Jia, Jianguo Liang, Gang Li, Yan Li, and Kaiying Wang. 2022. "Facile Synthesis of 4,4′-biphenyl Dicarboxylic Acid-Based Nickel Metal Organic Frameworks with a Tunable Pore Size towards High-Performance Supercapacitors" Nanomaterials 12, no. 12: 2062. https://doi.org/10.3390/nano12122062
APA StyleZhang, W., Yin, H., Yu, Z., Jia, X., Liang, J., Li, G., Li, Y., & Wang, K. (2022). Facile Synthesis of 4,4′-biphenyl Dicarboxylic Acid-Based Nickel Metal Organic Frameworks with a Tunable Pore Size towards High-Performance Supercapacitors. Nanomaterials, 12(12), 2062. https://doi.org/10.3390/nano12122062






