Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Titanium Dioxide Nanotubes (TiO2NTs)
2.3. Preparation of Au/TiO2NTs
2.4. Fabrication of Au/TiO2NTs Photoelectrode
2.5. Characterization
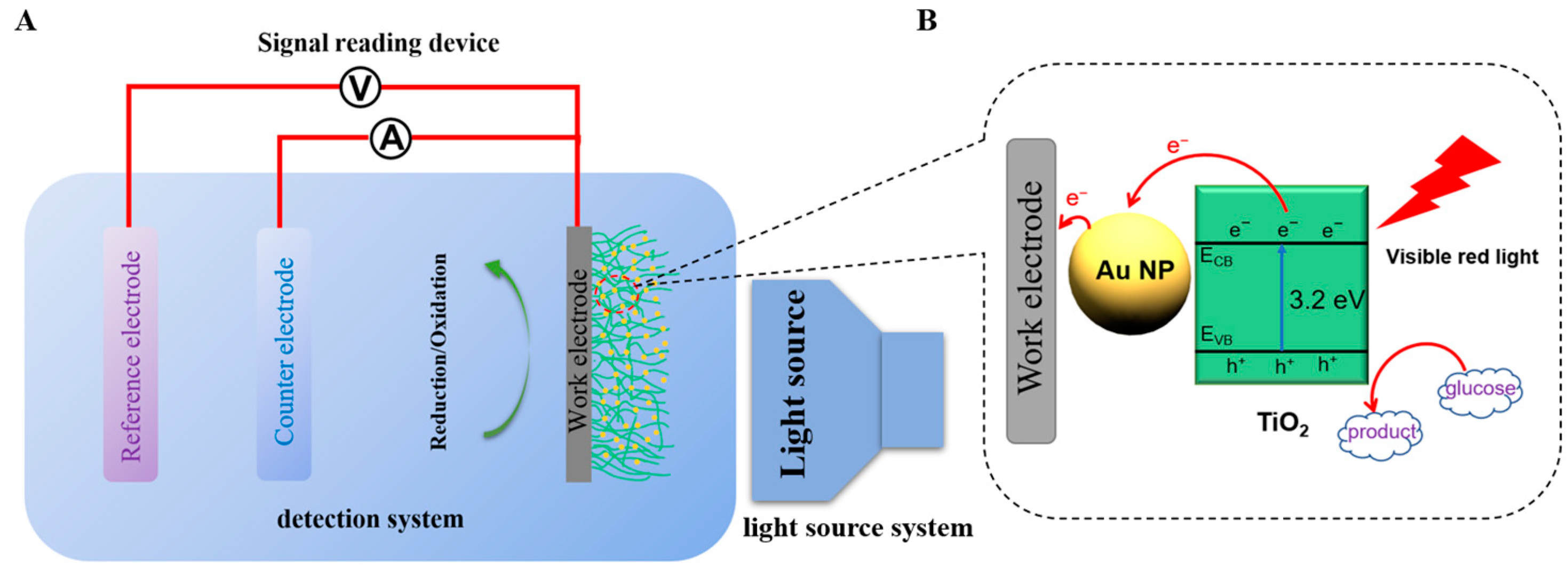
2.6. Photoelectrochemical Measurements
3. Results and Discussion
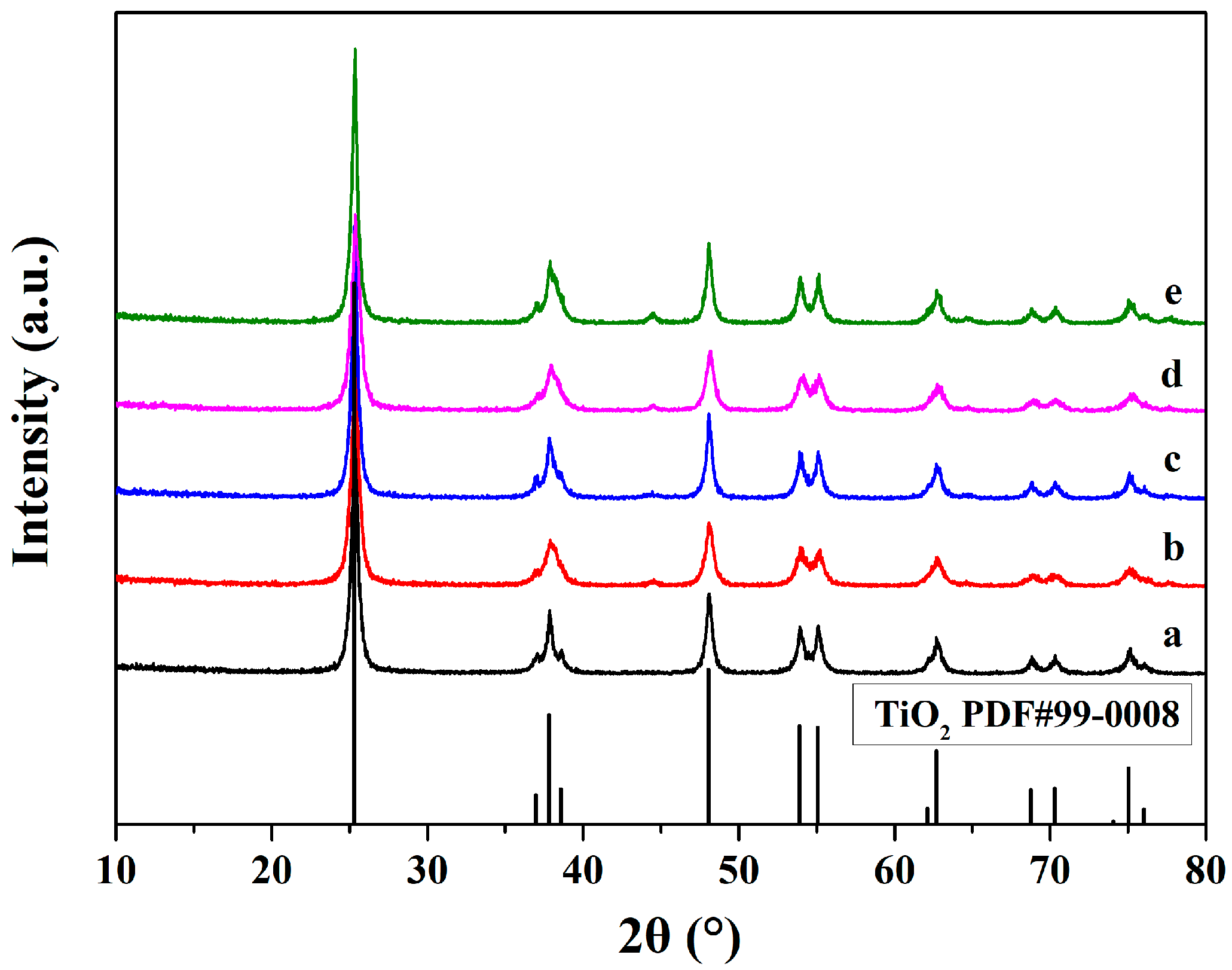
3.1. Structure Characterization by XRD
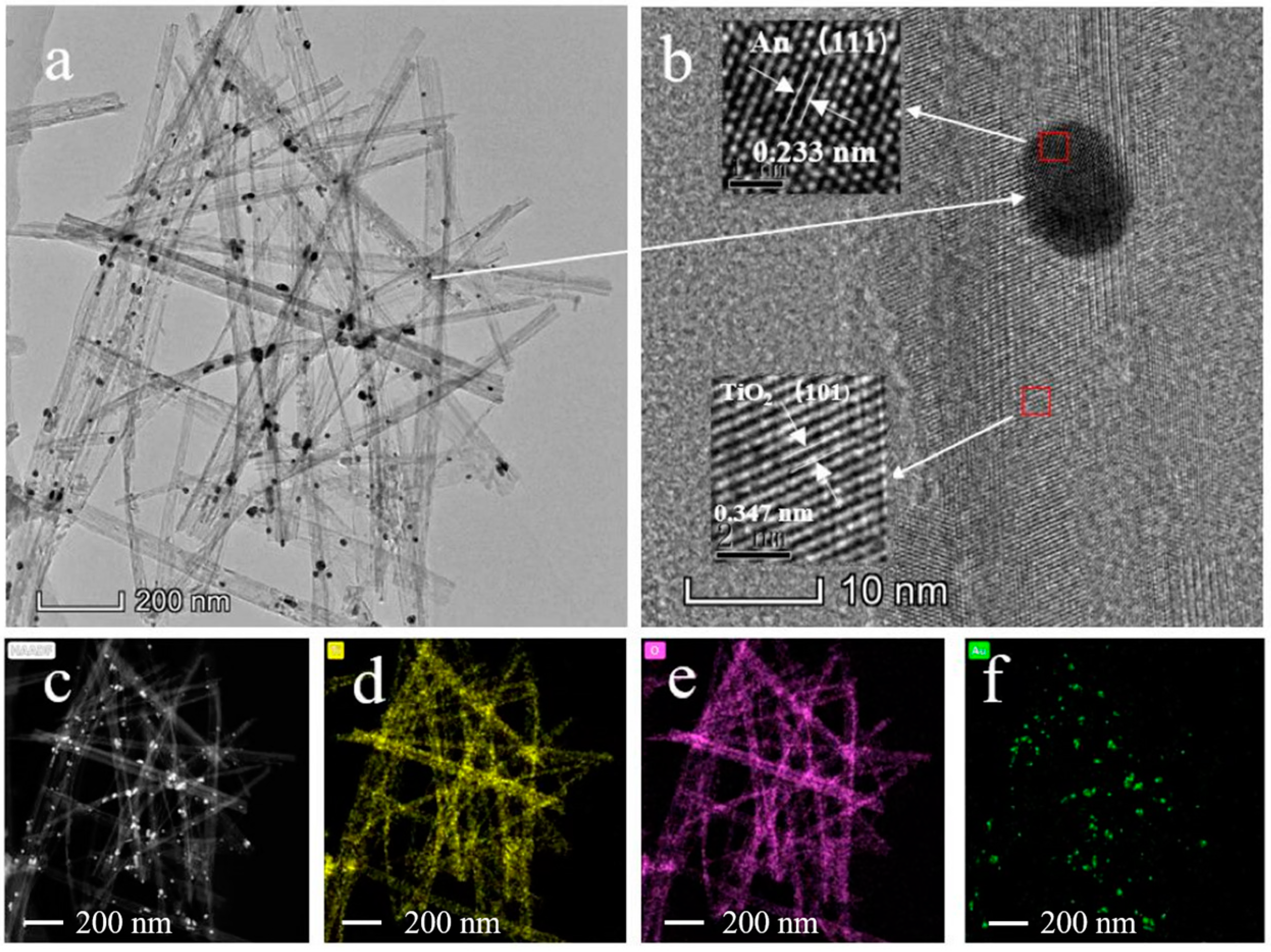
3.2. Morphology Characterization of Samples by TEM
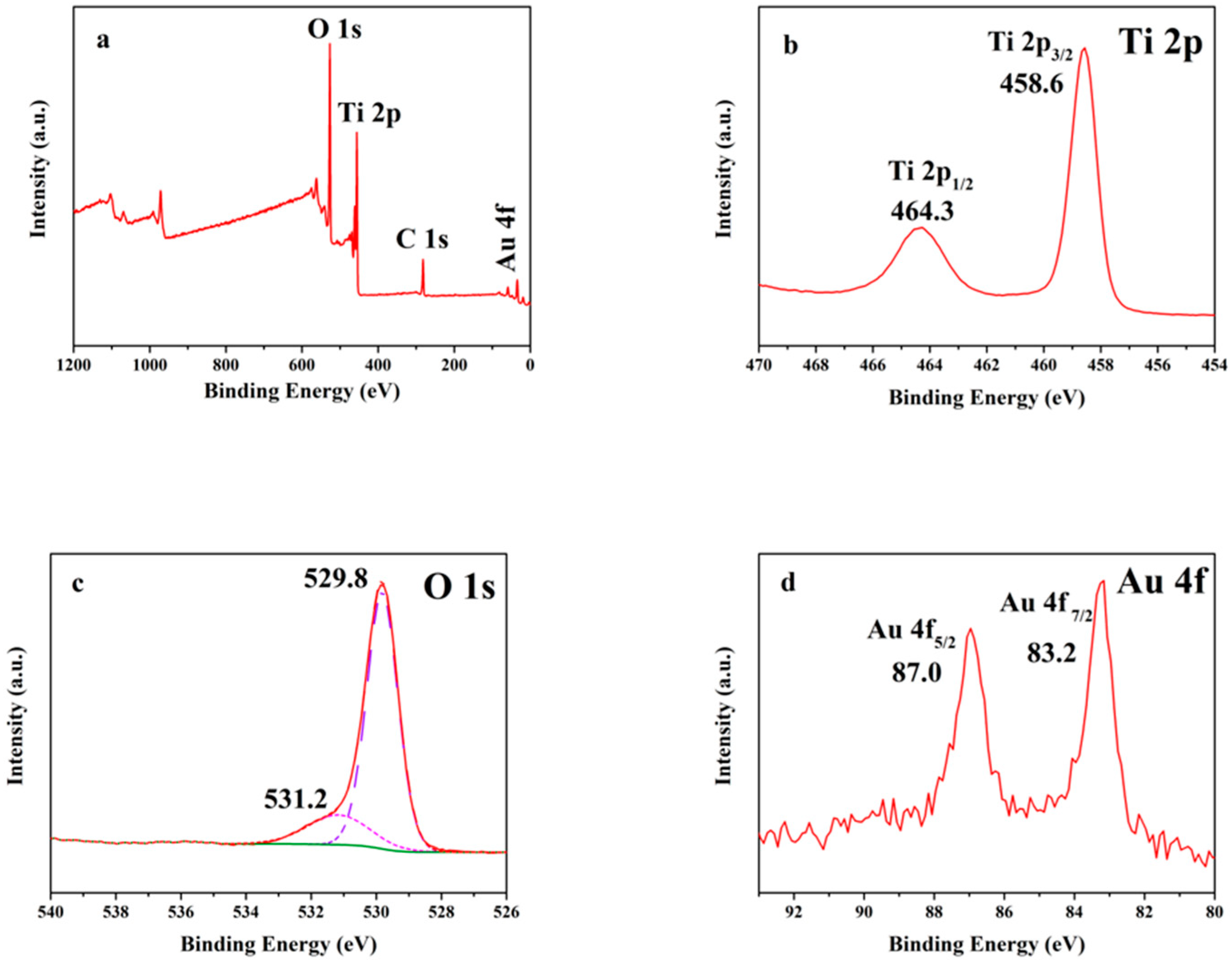
3.3. The Chemical State and Composition Analysis Using XPS
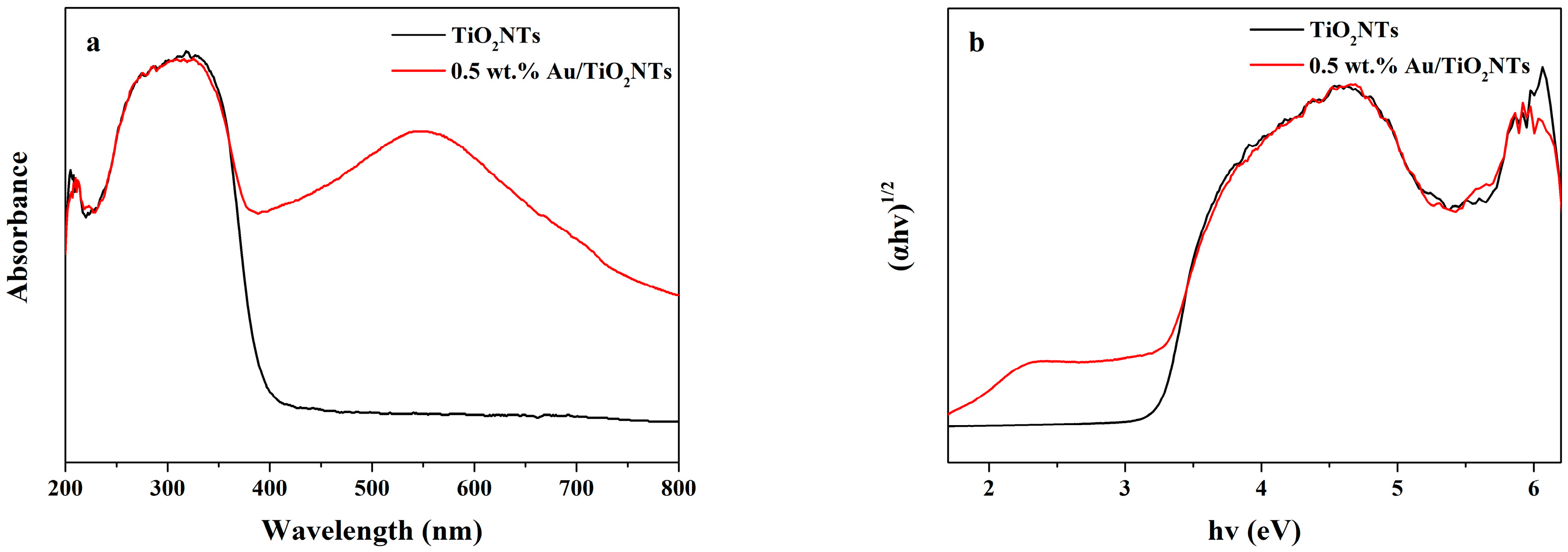
3.4. Optical Absorption Properties Analysis by UV–Vis DRS
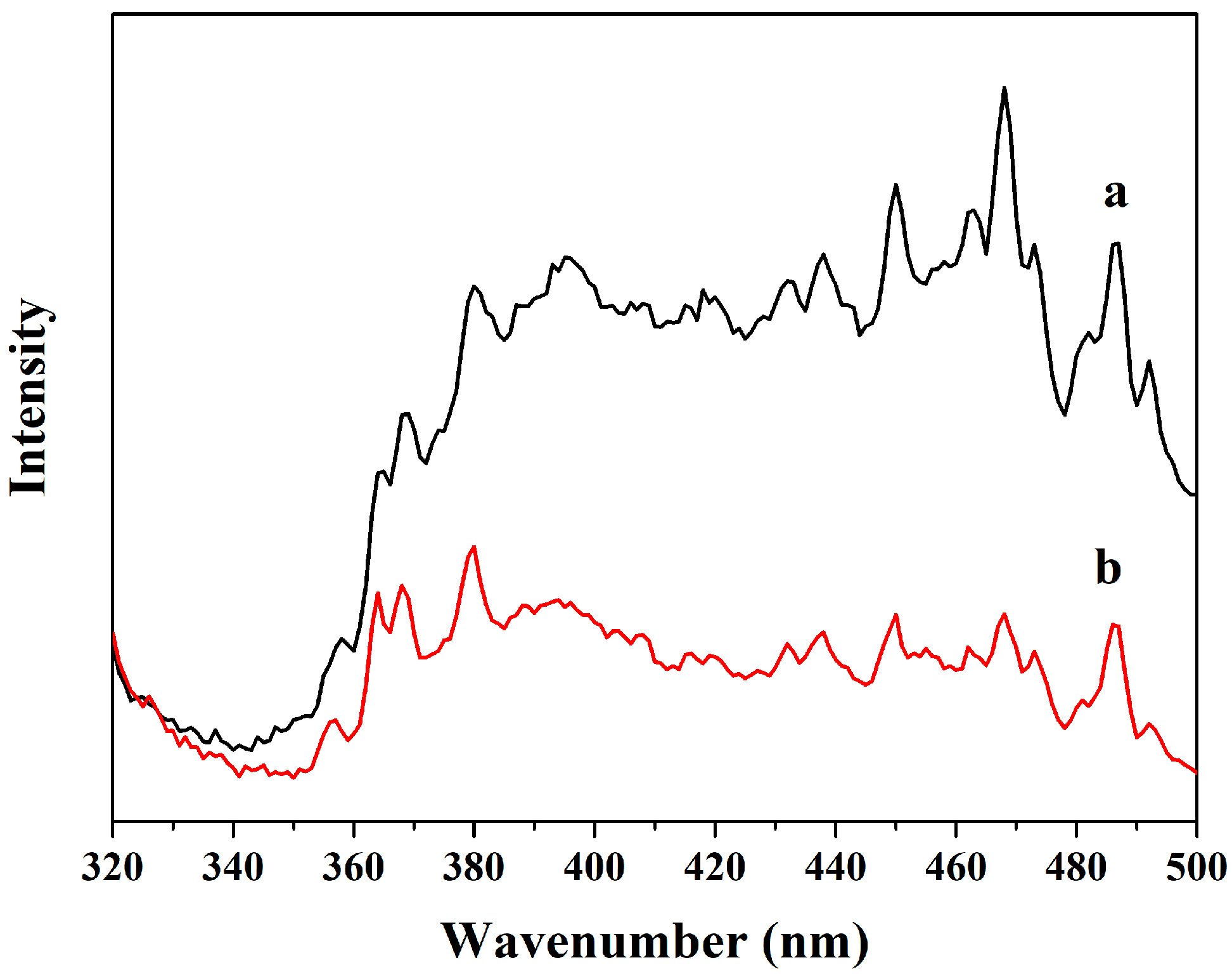
3.5. The Optical Properties Analysis by Photoluminescence (PL) Spectroscopy
3.6. Photoelectrochemical Measurements
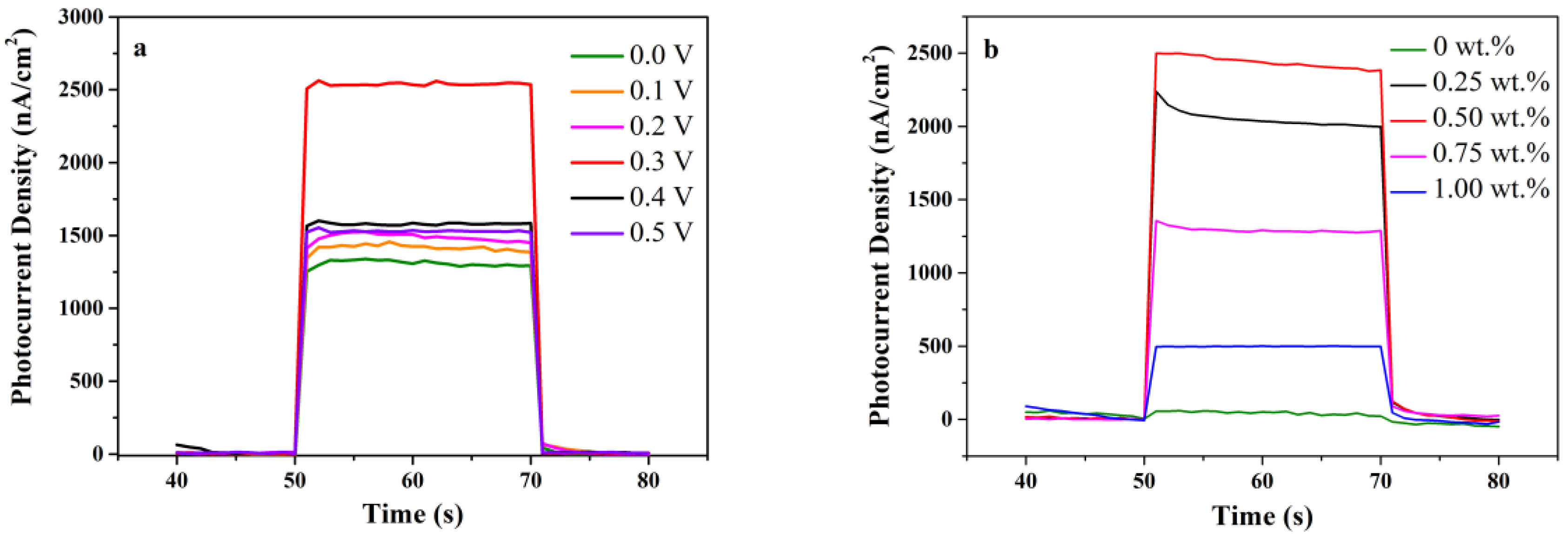
3.6.1. The Influence of Potential and Au Load on the Performance of Biosensing
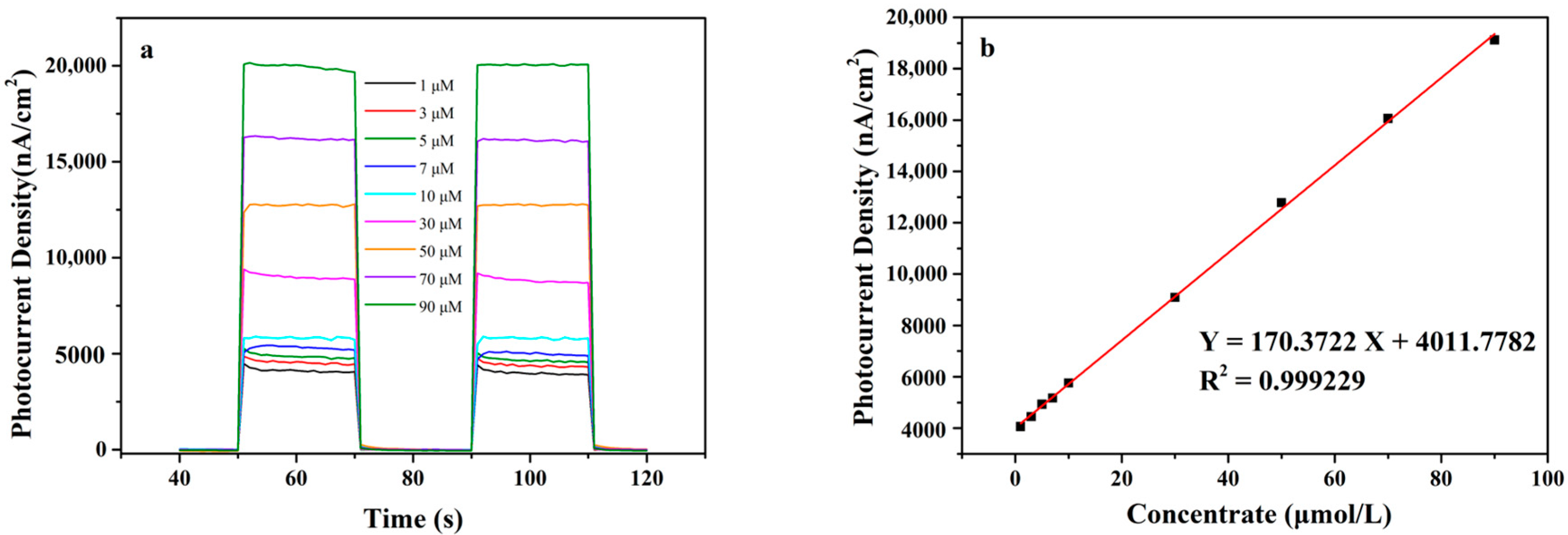
3.6.2. PEC Biosensing Application for Glucose
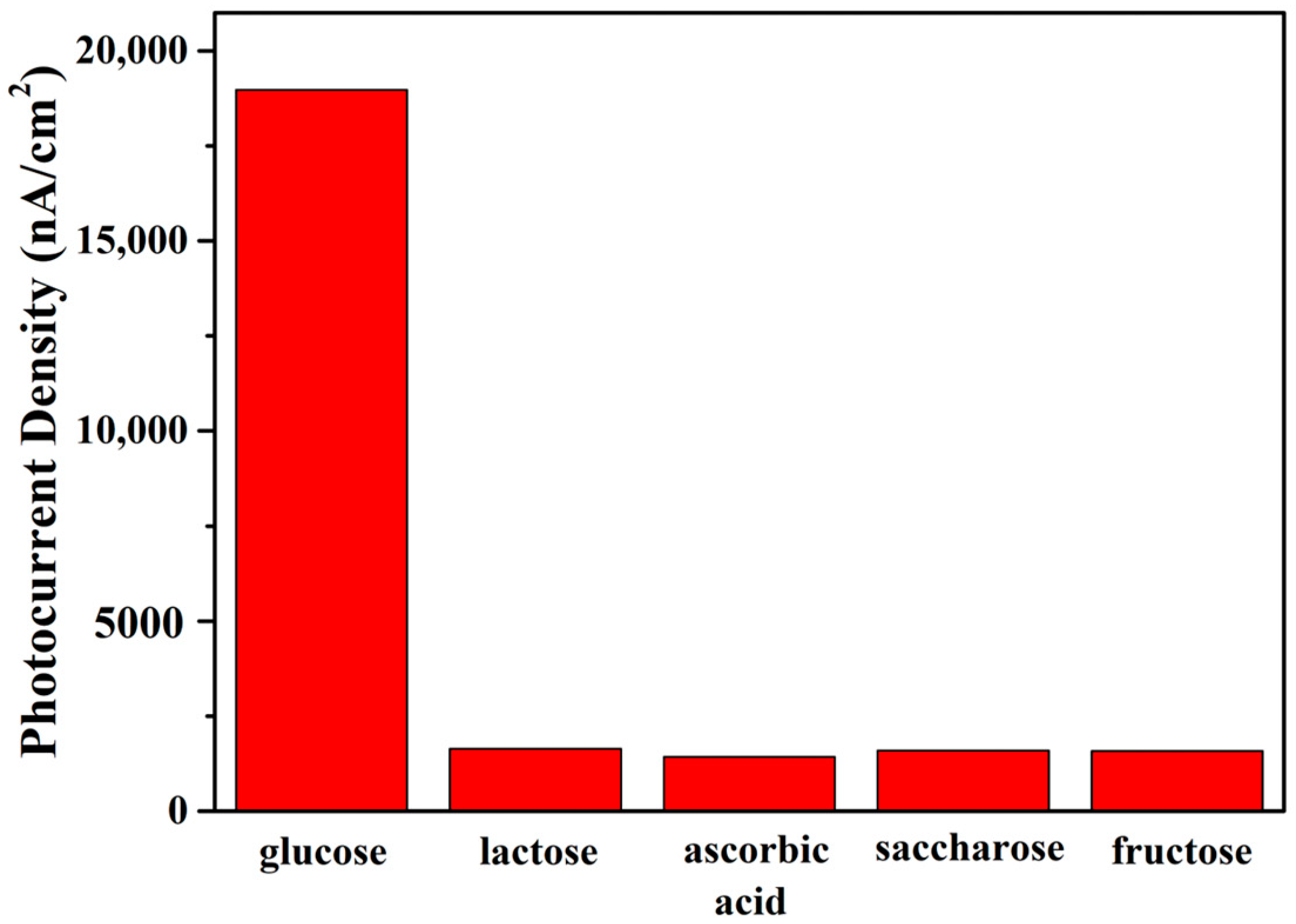
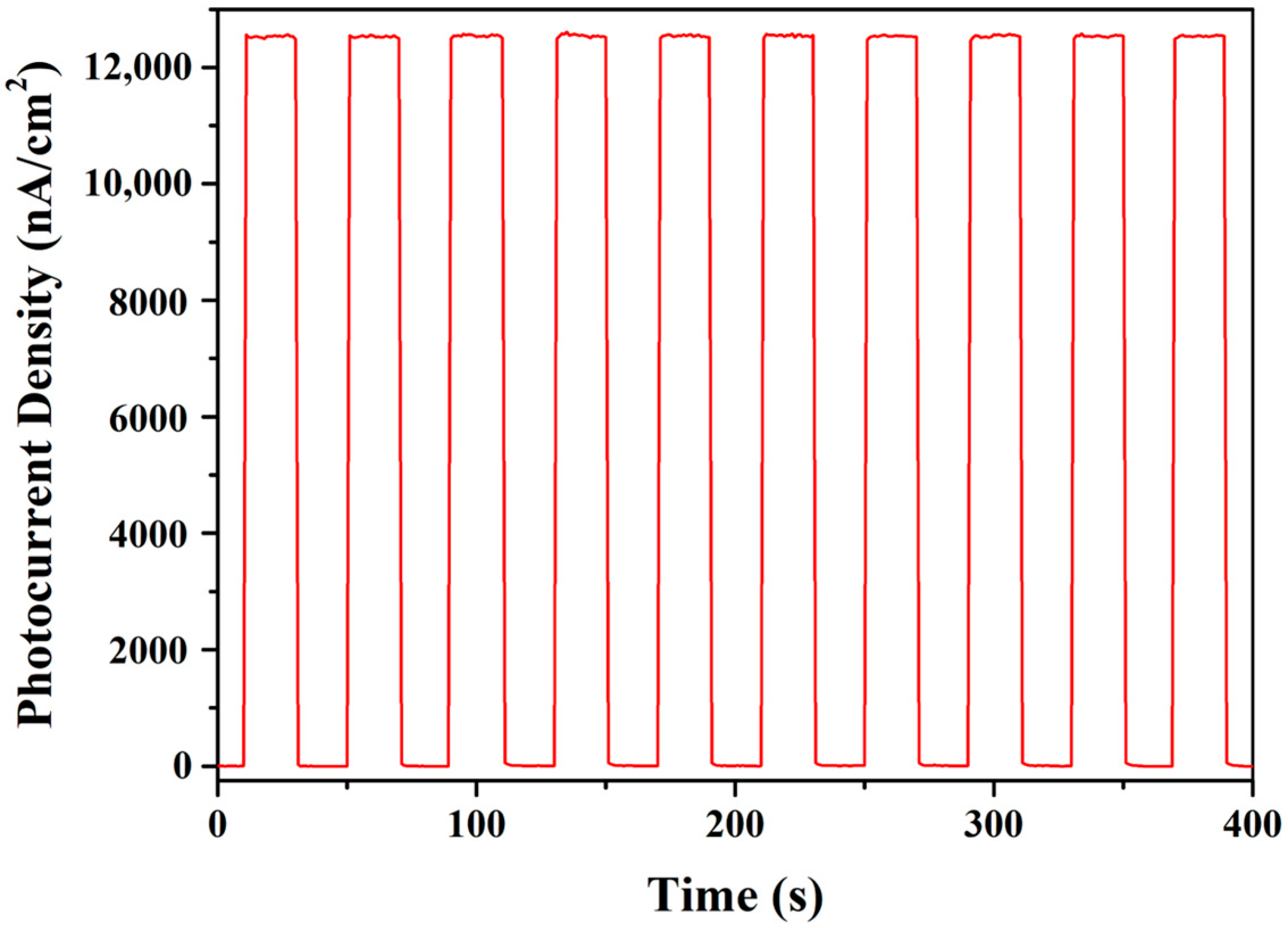
3.6.3. PEC Biosensing Selectivity and Stability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.B.; Jiang, D.S.; Yan, P.C.; Dong, J.T.; Qian, J.C.; Chen, J.P.; Xu, L. Graphitic carbon nitride/α-Fe2O3 heterostructures for sensitive photoelectrochemical non-enzymatic glucose sensor. Inorg. Chem. Commun. 2019, 106, 211–216. [Google Scholar] [CrossRef]
- Çakıroğlu, B.; Özacar, M. A photoelectrochemical biosensor fabricated using hierarchically structured gold nanoparticle and MoS2 on tannic acid templated mesoporous TiO2. Electroanalysis 2020, 32, 166–177. [Google Scholar] [CrossRef]
- He, L.H.; Liu, Q.B.; Zhang, S.J.; Zhang, X.T.; Gong, C.L.; Shu, H.H.; Wang, G.J.; Liu, H.; Wen, S.; Zhang, B.Q. High sensitivity of TiO2 nanorod array electrode for photoelectrochemical glucose sensor and its photo fuel cell application. Electrochem. Commun. 2018, 94, 18–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Liu, D.; Wang, Q.; Li, T.; Wang, Z. Cu2O-BiOI isotype (pp) heterojunction: Boosted visible-light-driven photoelectrochemical activity for non-enzymatic H2O2 sensing. Appl. Surf. Sci. 2020, 521, 146434. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, S.; Zhou, Q.; Tang, D. CoOOH nanosheets coated g-C3N4/CuInS2 nanohybrids for photoelectrochemical biosensor of carcinoembryonic antigen coupling hybridization chain reaction with etching reaction. Sens. Actuators B 2020, 307, 127631. [Google Scholar] [CrossRef]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Yan, P.C.; Jiang, D.S.; Tian, Y.H.; Xu, L.; Qian, J.C.; Li, H.N.; Xia, J.X.; Li, H.M. A sensitive signal-on photoelectrochemical sensor for tetracycline determination using visible-light-driven flower-like CN/BiOBr composites. Biosens. Bioelectron. 2018, 111, 74–81. [Google Scholar] [CrossRef]
- Zhang, S.P.; Xu, G.F.; Gong, L.S.; Dai, H.; Li, Y.L.; Hong, Z.S.; Lin, Y.Y. TiO2-B nanorod based competitive-like non-enzymatic photoelectrochemical sensing platform for noninvasive glucose detection. J. Mater. Chem. B 2015, 3, 7554–7559. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D.P. Current advances in quantum-dots-based photoelectrochemical immunoassays. Chem.—Asian J. 2017, 12, 2780–2789. [Google Scholar] [CrossRef]
- Liras, M.; Barawi, M.; Víctor, A. Hybrid materials based on conjugated polymers and inorganic semiconductors as photocatalysts: From environmental to energy applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhou, Y.Z.; Zheng, G.P.; Huang, Q.Y.; Zheng, X.C.; Liu, P.; Zhang, J.M.; Guan, X.X. Novel assembly and electrochemical properties of anatase TiO2-graphene aerogel 3D hybrids as lithium-ion battery anodes. Chem. Phys. Lett. 2016, 662, 214–220. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, J.; Ju, Y.; Xue, H.G.; Pang, H. Current advances in semiconductor nanomaterial-based photoelectrochemical biosensing. Chem.-A Eur. J. 2018, 24, 14010–14027. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Gui, P.; Zheng, L.N.; Liang, J.S.; Xue, G. Hydrothermal synthesis of sandwich interspersed LaCO3OH/Co3O4/graphene oxide composite and the enhanced catalytic performance for methane combustion. Catal. Today 2019, 327, 134–142. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Wisz, G.; Rybak-Wilusz, E.; Cholewa, M. Numerical analysis and optimization of Cu2O/TiO2, CuO/TiO2, heterojunction solar cells using SCAPS. J. Phys. Conf. Ser. 2018, 1033, 012002. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Fan, G.C.; Jiang, L.P.; Zhu, J.J. Peptide-based photoelectrochemical cytosensor using a hollow-TiO2/EG/ZnIn2S4 cosensitized structure for ultrasensitive detection of early apoptotic cells and drug evaluation. ACS Appl. Mater. Interfaces 2018, 10, 4429–4438. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kong, L.; Sun, X.K.; Feng, J.H.; Chen, Z.W.; Fan, D.W.; Wei, Q. Ultrasensitive photoelectrochemical immunosensor of cardiac troponin I detection based on dual inhibition effect of Ag@Cu2O core-shell submicron-particles on CdS QDs sensitized TiO2 nanosheets. Biosens. Bioelectron. 2018, 117, 340–346. [Google Scholar] [CrossRef]
- Pang, X.H.; Bian, H.J.; Wang, W.J.; Liu, C.; Khan, M.S.; Wang, Q.; Qi, J.N.; Wei, Q.; Du, B. A bio-chemical application of N-GQDs and g-C3N4 QDs sensitized TiO2 nanopillars for the quantitative detection of pcDNA3-HBV. Biosens. Bioelectron. 2017, 91, 456–464. [Google Scholar] [CrossRef]
- Yang, L.W.; Liu, X.Q.; Li, L.L.; Zhang, S.; Zheng, H.J.; Tang, Y.F.; Ju, H.X. A visible light photoelectrochemical sandwich aptasensor for adenosine triphosphate based on MgIn2S4-TiO2 nanoarray heterojunction. Biosens. Bioelectron. 2019, 142, 111487. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.L.; Zhuang, J.Y.; Xu, M.D.; Tang, D.P. In situ generation of electron donor to assist signal amplification on porphyrin-sensitized titanium dioxide nanostructures for ultrasensitive photoelectrochemical immunoassay. ACS Appl. Mater. Interfaces 2015, 7, 23812–23818. [Google Scholar] [CrossRef]
- Liu, X.Q.; Huo, X.H.; Liu, P.P.; Tang, Y.F.; Xu, J.; Liu, X.; Zhou, Y.M. Assembly of MoS2 nanosheet-TiO2 nanorod heterostructure as sensor scaffold for photoelectrochemical biosensing. Electrochim. Acta 2017, 242, 327–336. [Google Scholar] [CrossRef]
- Da, P.M.; Li, W.J.; Lin, X.; Wang, Y.C.; Tang, J.; Zheng, G.F. Surface plasmon resonance enhanced real-time photoelectrochemical protein sensing by gold nanoparticle-decorated TiO2 nanowires. Anal. Chem. 2014, 86, 6633–6639. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.H.; Bian, H.J.; Su, M.H.; Ren, Y.Y.; Qi, J.N.; Ma, H.M.; Wu, D.; Wei, Q. Photoelectrochemical cytosensing of RAW264.7 macrophage cells based on a TiO2 nanoneedls@MoO3 array. Anal. Chem. 2017, 89, 7950–7957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhong, X.; Chen, A.Y.; Liu, J.L.; Li, S.K.; Chai, Y.Q.; Zhuo, Y.; Yuan, R. Novel Ru (bpy)2 (cpaphen)2+/TPrA/TiO2 ternary ECL system: An efficient platform for the detection of glutathione with Mn2+ as substitute target. Anal. Chem. 2019, 91, 3681–3686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.J.; Zhuo, Y.; Chai, Y.Q.; Yuan, R. Highly efficient electrochemiluminescent silver nanoclusters/titanium oxide nanomaterials as a signal probe for ferrocene-driven light switch bioanalysis. Anal. Chem. 2017, 89, 3732–3738. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Liu, X.Q.; Zheng, H.J.; Yang, L.W.; Li, L.L.; Zhang, S.; Zhou, Y.M.; Alwarappan, S. A photoelectrochemical aptasensor for aflatoxin B1 detection based on an energy transfer strategy between Ce-TiO2@MoSe2 and Au nanoparticles. Nanoscale 2019, 11, 9115–9124. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.N.; Chen, S.H.; Li, Y.L.; Dai, H.; Lin, Y.Y. Self-enhanced photocathodic matrix based on poly-dopamine sensitized TiO2 mesocrystals for mycotoxin detection assisted by a dual amplificatory nanotag. New J. Chem. 2017, 41, 3380–3386. [Google Scholar] [CrossRef]
- Dai, H.; Xu, G.F.; Zhang, S.P.; Hong, Z.S.; Lin, Y.Y. A ratiometric biosensor for metallothionein based on a dual heterogeneous electro-chemiluminescent response from a TiO2 mesocrystalline interface. Chem. Commun. 2015, 51, 7697–7700. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Tang, D.P. Nanostructure-based photoelectrochemical sensing platforms for biomedical applications. J. Mater. Chem. B 2020, 8, 2541–2561. [Google Scholar] [CrossRef]
- So, S.; Hwang, I.; Schmuki, P. Hierarchical DSSC structures based on “single walled” TiO2 nanotube arrays reach a back-side illumination solar light conversion efficiency of 8%. Energy Environ. Sci. 2015, 8, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Chen, J.N.; Yang, F.; Peng, T.Y. Self-organized film of ultra-fine TiO2 nanotubes and its application to dye-sensitized solar cells on a flexible Ti-foil substrate. J. Mater. Chem. 2012, 22, 4681–4686. [Google Scholar] [CrossRef]
- Ma, L.L.; Yue, Z.; Huo, G.N.; Zhang, S.S.; Zhu, B.L.; Zhang, S.M.; Huang, W.P. 3D Hydrogen Titanate Nanotubes on Ti Foil: A Carrier for Enzymatic Glucose Biosensor. Sensors 2020, 20, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.H.; Chen, J.; Wang, M.; Sheng, X.; Chen, X.Y.; Feng, X.J.; Mao, S.S. Titanium dioxide nanostructures for photoelectrochemical applications. Prog. Mater. Sci. 2018, 98, 299–385. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Cai, G.N.; Li, M.J.; Tang, D.P. In situ formation of (0 0 1) TiO2/Ti3C2 heterojunctions for enhanced photoelectrochemical detection of dopamine. Electrochem. Commun. 2021, 125, 106987. [Google Scholar] [CrossRef]
- Cai, G.N.; Yu, Z.Z.; Ren, R.R.; Tang, D.P. Exciton–plasmon interaction between AuNPs/graphene nanohybrids and CdS quantum dots/TiO2 for photoelectrochemical aptasensing of prostate-specific antigen. ACS Sens. 2018, 3, 632–639. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.L.; Lv, S.Z.; Zhang, K.Y.; Tang, D.P. Plasmonic enhancement coupling with defect-engineered TiO2–x: A mode for sensitive photoelectrochemical biosensing. Anal. Chem. 2018, 90, 2425–2429. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, W.; Yan, B.D.; Wu, B.Q.; Ma, J.X.; Wang, X.H.; Qiao, B.; Tu, J.C.; Pei, H.; Chen, D.L.; et al. Gold and Platinum Nanoparticle-Functionalized TiO2 Nanotubes for Photoelectrochemical Glucose Sensing. ACS Omega 2022, 7, 2474–2483. [Google Scholar] [CrossRef]
- Cui, L.; Shen, J.Z.; Li, C.C.; Cui, P.P.; Luo, X.L.; Wang, X.L.; Zhang, C.Y. Construction of a dye-sensitized and gold plasmon-enhanced cathodic photoelectrochemical biosensor for methyltransferase activity assay. Anal. Chem. 2021, 93, 10310–10316. [Google Scholar] [CrossRef]
- Matković, A.; Petritz, A.; Schider, G.; Krammer, M.; Kratzer, M.; Karner-Petritz, E.; Fian, A.; Gold, H.; Gärtner, M.; Terfort, A. Interfacial band engineering of MoS2/gold interfaces using pyrimidine-containing self-assembled monolayers: Toward contact-resistance-free bottom-contacts. Adv. Electron. Mater. 2020, 6, 2000110. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.K.; Zhang, L.X.; Wang, Y.; He, W.W. Plasmon excitation facilitating generation of electrons and reactive oxygen species for broad spectrum photocatalytic activity. Appl. Surf. Sci. 2022, 584, 152655. [Google Scholar] [CrossRef]
- Liu, N.; Mesch, M.; Weiss, T.; Hentschel, M.; Giessen, H. Infrared perfect absorber and its application as plasmonic sensor. Nano Lett. 2010, 10, 2342–2348. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Hesselink, L. Solenoidal optical forces from a plasmonic Archimedean spiral. Phys. Rev. A 2019, 100, 013857. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, F.; Xu, Y.Y.; Zhang, J.W.; Zhang, D.Q.; Li, G.S.; Li, H.X. Plasmon-induced photoelectrocatalytic activity of Au nanoparticles enhanced TiO2 nanotube arrays electrodes for environmental remediation. Appl. Catal. B Environ. 2015, 164, 217–224. [Google Scholar] [CrossRef]
- Melvin, A.A.; Illath, K.; Das, T.; Raja, T.; Bhattacharyya, S.; Gopinath, C.S. M–Au/TiO2 (M = Ag, Pd, and Pt) nanophotocatalyst for overall solar water splitting: Role of interfaces. Nanoscale 2015, 7, 13477–13488. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Bensaali, F.; Jaber, F. Novel approach to non-invasive blood glucose monitoring based on transmittance and refraction of visible laser light. IEEE Access 2017, 5, 9163–9174. [Google Scholar] [CrossRef]
- An, H.Q.; Zhu, B.L.; Li, J.X.; Zhou, J.; Wang, S.R.; Zhang, S.M.; Wu, S.H.; Huang, W.P. Synthesis and characterization of thermally stable nanotubular TiO2 and its photocatalytic activity. J. Phys. Chem. C 2008, 112, 18772–18775. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, W.; Han, C.; Guo, J.; Zhang, Q.H.; Xie, H.Y.; Shao, Q.; Sun, Z.G.; Guo, Z.H. Large scaled synthesis of heterostructured electrospun TiO2/SnO2 nanofibers with an enhanced photocatalytic activity. J. Electrochem. Soc. 2017, 164, H651–H656. [Google Scholar] [CrossRef] [Green Version]
- Pustovalova, A.; Boytsova, E.; Aubakirova, D.; Bruns, M.; Tverdokhlebov, S.; Pichugin, V. Formation and structural features of nitrogen-doped titanium dioxide thin films grown by reactive magnetron sputtering. Appl. Surf. Sci. 2020, 534, 147572. [Google Scholar] [CrossRef]
- Wang, H.Q.; Wu, D.Y.; Liu, C.Y.; Guan, J.R.; Li, J.Z.; Huo, P.W.; Liu, X.L.; Wang, Q.; Yan, Y.S. Fabrication of Ag/In2O3/TiO2/HNTs hybrid-structured and plasma effect photocatalysts for enhanced charges transfer and photocatalytic activity. J. Ind. Eng. Chem. 2018, 67, 164–174. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.R. Electrochemically self-doped TiO2 nanotube arrays for supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Han, Q.W.; Zhang, D.Y.; Guo, J.L.; Zhu, B.L.; Huang, W.P.; Zhang, S.M. Improved catalytic performance of Au/α-Fe2O3-like-worm catalyst for low temperature CO oxidation. Nanomaterials 2019, 9, 1118. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Synergistic effect in plasmonic Au/Ag alloy NPs co-coated TiO2 NWs toward visible-light enhanced CO2 photoreduction to fuels. Appl. Catal. B Environ. 2017, 204, 548–560. [Google Scholar] [CrossRef]
- Ge, L.Y.; Hou, R.; Cao, Y.; Tu, J.C.; Wu, Q. Photoelectrochemical enzymatic sensor for glucose based on Au@C/TiO2 nanorod arrays. RSC Adv. 2020, 10, 44225–44231. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.D.; Zhao, X.R.; Chen, D.L.; Cao, Y.; Lv, C.Z.; Tu, J.C.; Wang, X.H.; Wu, Q. Enhanced photoelectrochemical biosensing performance for Au nanoparticle–polyaniline–TiO2 heterojunction composites. RSC Adv. 2020, 10, 43985–43993. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.H.; Janssens, E. Au nanoparticle–decorated ZnO nanorods as fluorescent non-enzymatic glucose probe. Microchim. Acta 2020, 187, 577. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Tan, L.; Liu, H.T.; He, Z.; Tang, A.D. Novel helical TiO2 nanotube arrays modified by Cu2O for enzyme-free glucose oxidation. Biosens. Bioelectron. 2014, 59, 243–250. [Google Scholar] [CrossRef]
- Yang, W.K.; Xu, W.; Wang, Y.D.; Chen, D.L.; Wang, X.H.; Cao, Y.; Wu, Q.; Tu, J.C.; Zhen, C. Photoelectrochemical glucose biosensor based on the heterogeneous facets of nanocrystalline TiO2/Au/glucose oxidase films. ACS Appl. Nano Mater. 2020, 3, 2723–2732. [Google Scholar] [CrossRef]
- Artigues, M.; Abellà, J.; Colominas, S. Analytical parameters of an amperometric glucose biosensor for fast analysis in food samples. Sensors 2017, 17, 2620. [Google Scholar] [CrossRef] [Green Version]

| Configuration of Biosensor | Linear Range (μM) | Sensitivity (μA·mM−1·cm−2) | Detection Limit (μM) | Ref. |
|---|---|---|---|---|
| Au/TiO2 NTs/GCE | 1–90 | 170.37 | 1.3 | This work |
| GODx/Au@C/TiO2/FTO | 100–1600 | 29.76 | 49 | [52] |
| GOx@Au-PANI-TiO2 | 2000–36000 | 0.83 | 20 | [53] |
| ZnO NRs/Au NPs | 10–2000 | 22 | 10 | [54] |
| Cu2O/TiO2 | 3000–9000 | 14.56 | 62 | [55] |
| GOx/TiO2 | 500–3000 | 9.25 | 15 | [56] |
| GOx–Chitosan/TiO2NTAs | 300–1500 | 5.46 | 70 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Duan, W.; Jia, L.; Wang, S.; Guo, Y.; Zhang, G.; Zhu, B.; Huang, W.; Zhang, S. Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes. Nanomaterials 2022, 12, 2058. https://doi.org/10.3390/nano12122058
Liu W, Duan W, Jia L, Wang S, Guo Y, Zhang G, Zhu B, Huang W, Zhang S. Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes. Nanomaterials. 2022; 12(12):2058. https://doi.org/10.3390/nano12122058
Chicago/Turabian StyleLiu, Wanqing, Wei Duan, Liqun Jia, Siyu Wang, Yuan Guo, Guoqing Zhang, Baolin Zhu, Weiping Huang, and Shoumin Zhang. 2022. "Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes" Nanomaterials 12, no. 12: 2058. https://doi.org/10.3390/nano12122058
APA StyleLiu, W., Duan, W., Jia, L., Wang, S., Guo, Y., Zhang, G., Zhu, B., Huang, W., & Zhang, S. (2022). Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes. Nanomaterials, 12(12), 2058. https://doi.org/10.3390/nano12122058





