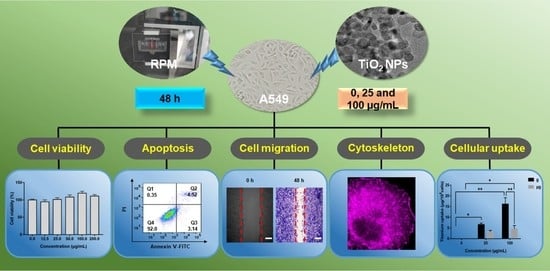
Effects of Titanium Dioxide Nanoparticles on Cell Growth and Migration of A549 Cells under Simulated Microgravity
Abstract
1. Introduction
2. Materials and Methods
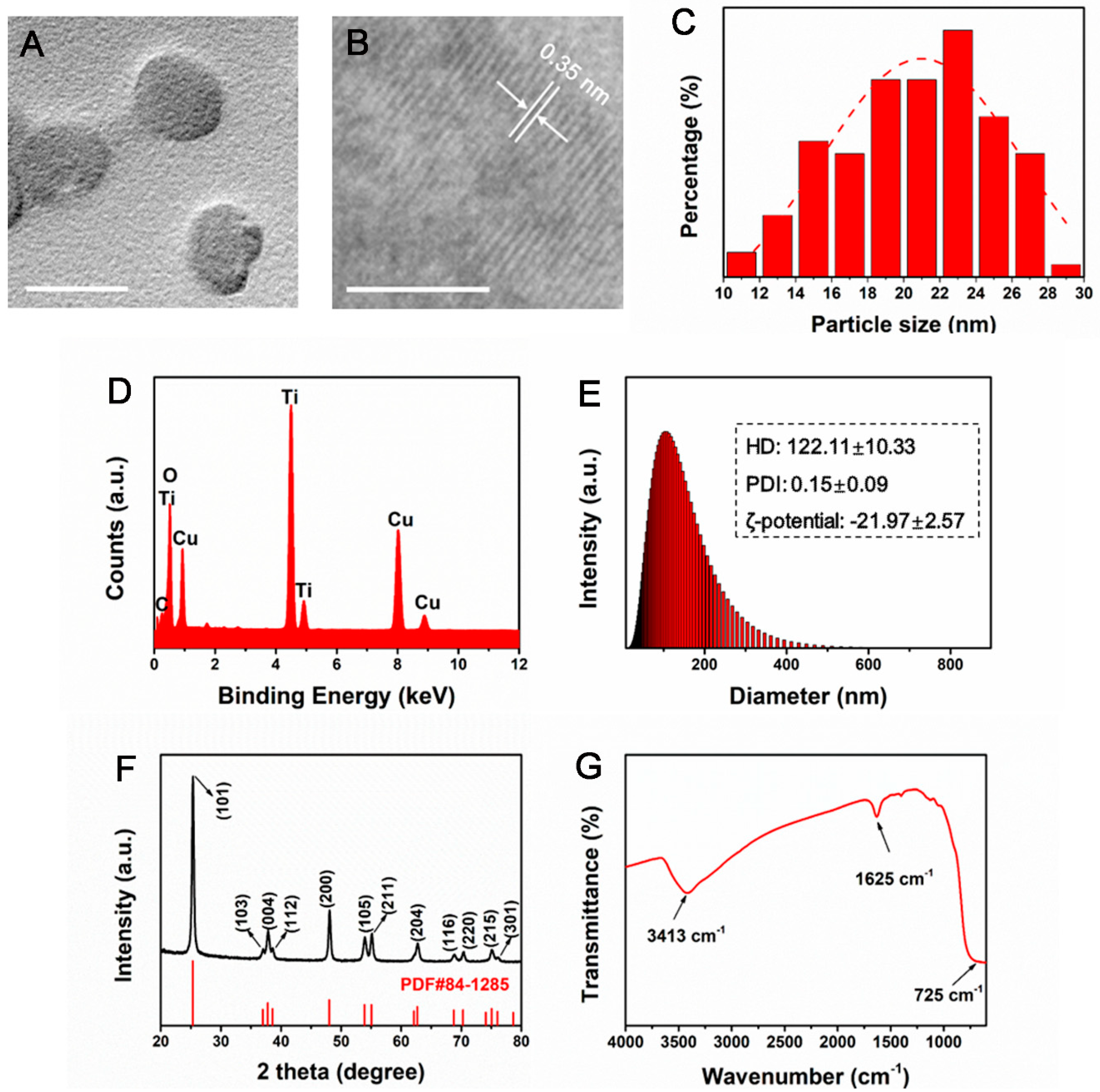
2.1. Nanoparticle Characterization
2.2. Cell Culture
2.3. Simulated Microgravity and Cell Exposure
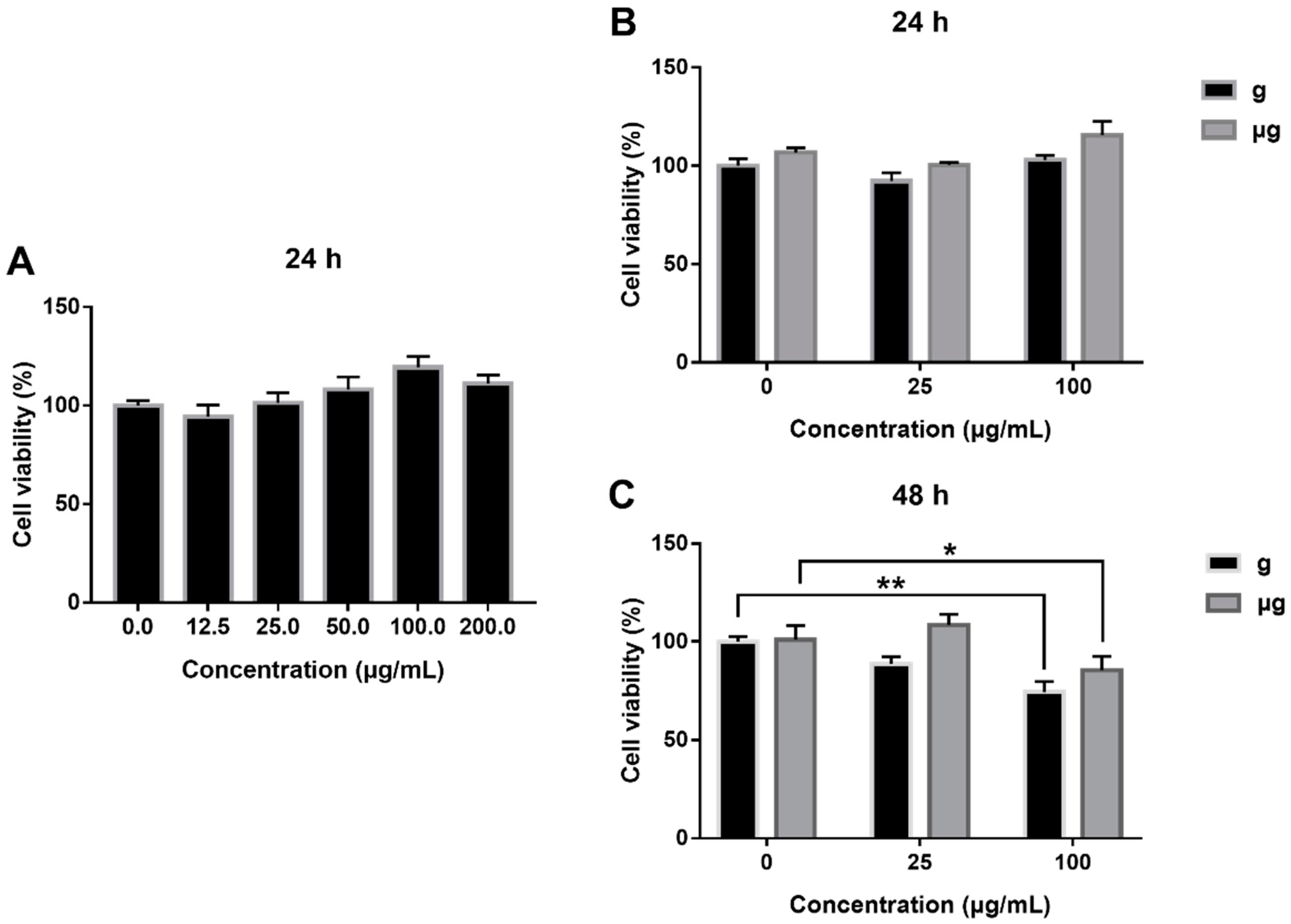
2.4. Cell Viability Assay
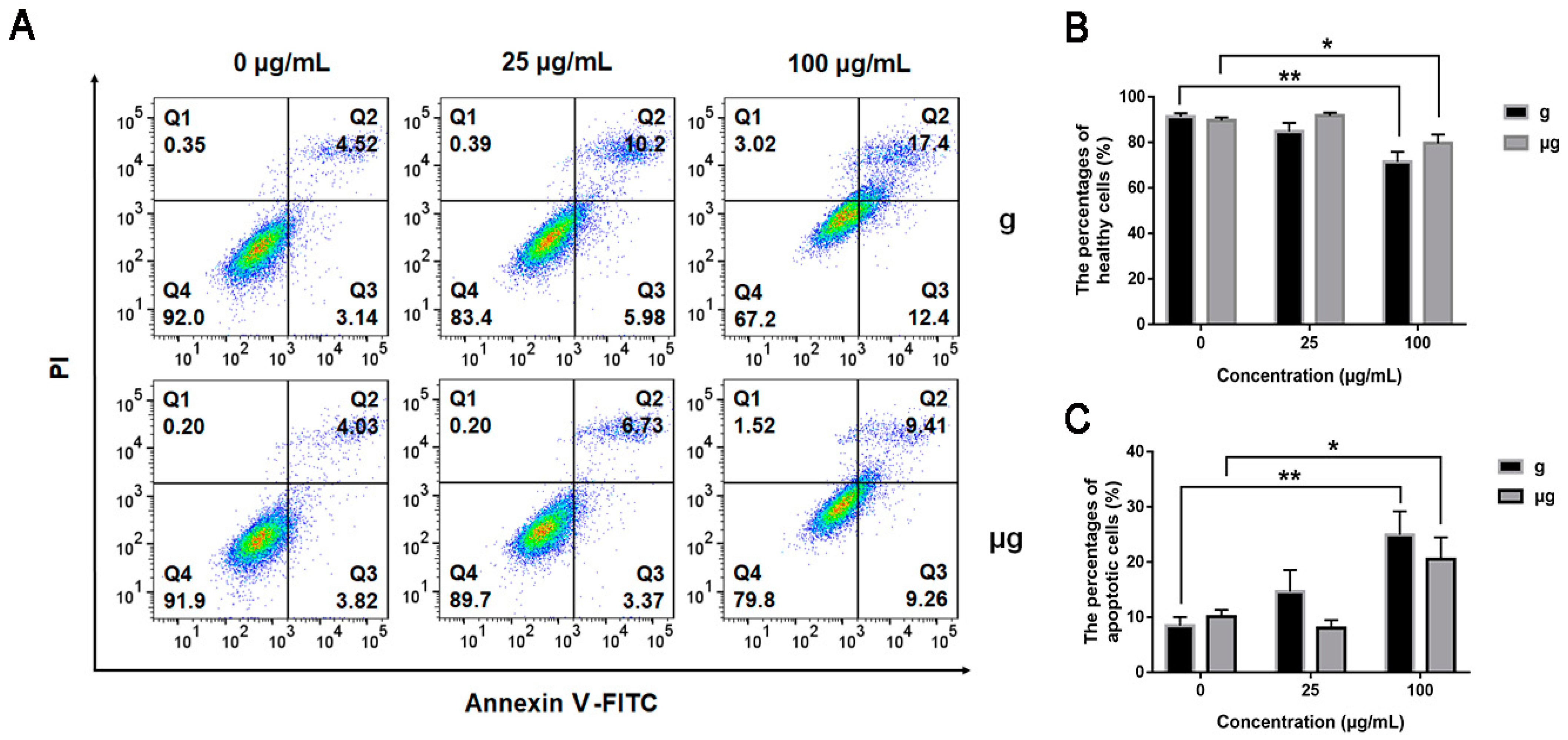
2.5. Annexin V-FITC/PI Double Staining
2.6. F-actin Staining
2.7. Wound Healing Assay
2.8. Immunofluorescence Analysis
2.9. Western Blot Analysis
2.10. Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES)
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of TiO2 NPs
3.2. Cytotoxicity Evaluation of TiO2 NPs Exposure under Simulated Microgravity and Normal Gravity
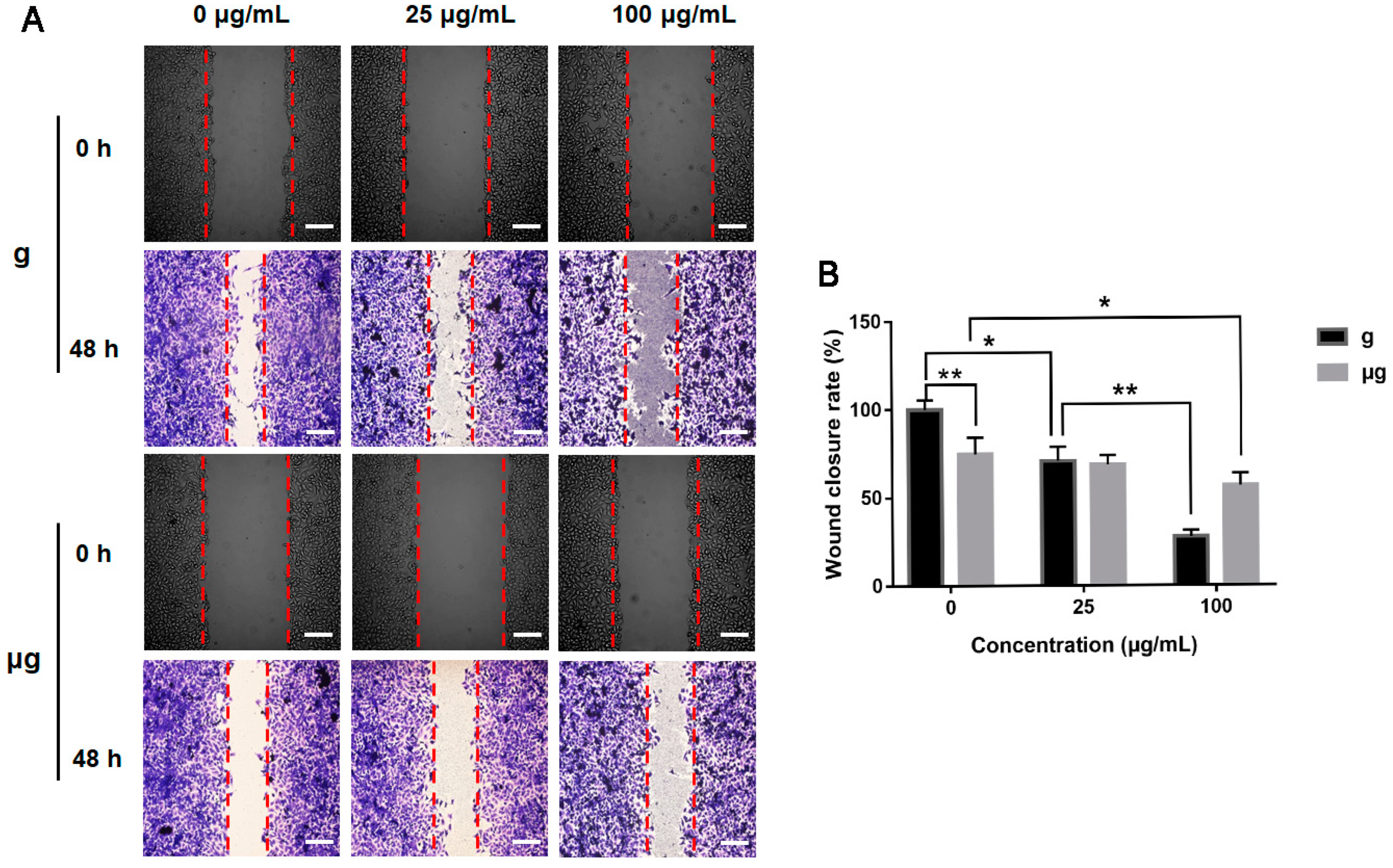
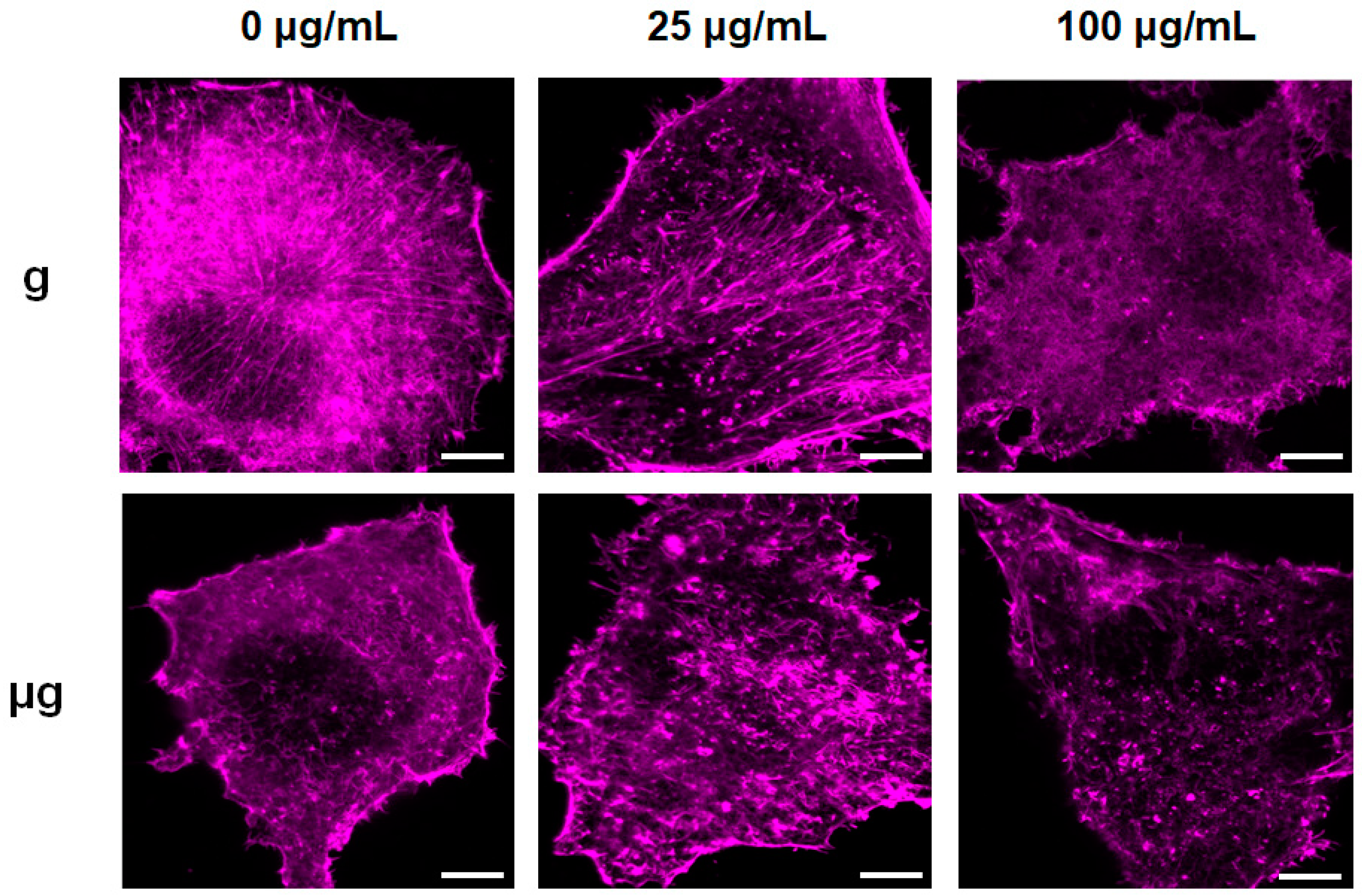
3.3. Effects of TiO2 NPs Exposure on Cell Migration and Cytoskeleton under Normal Gravity and Simulated Microgravity
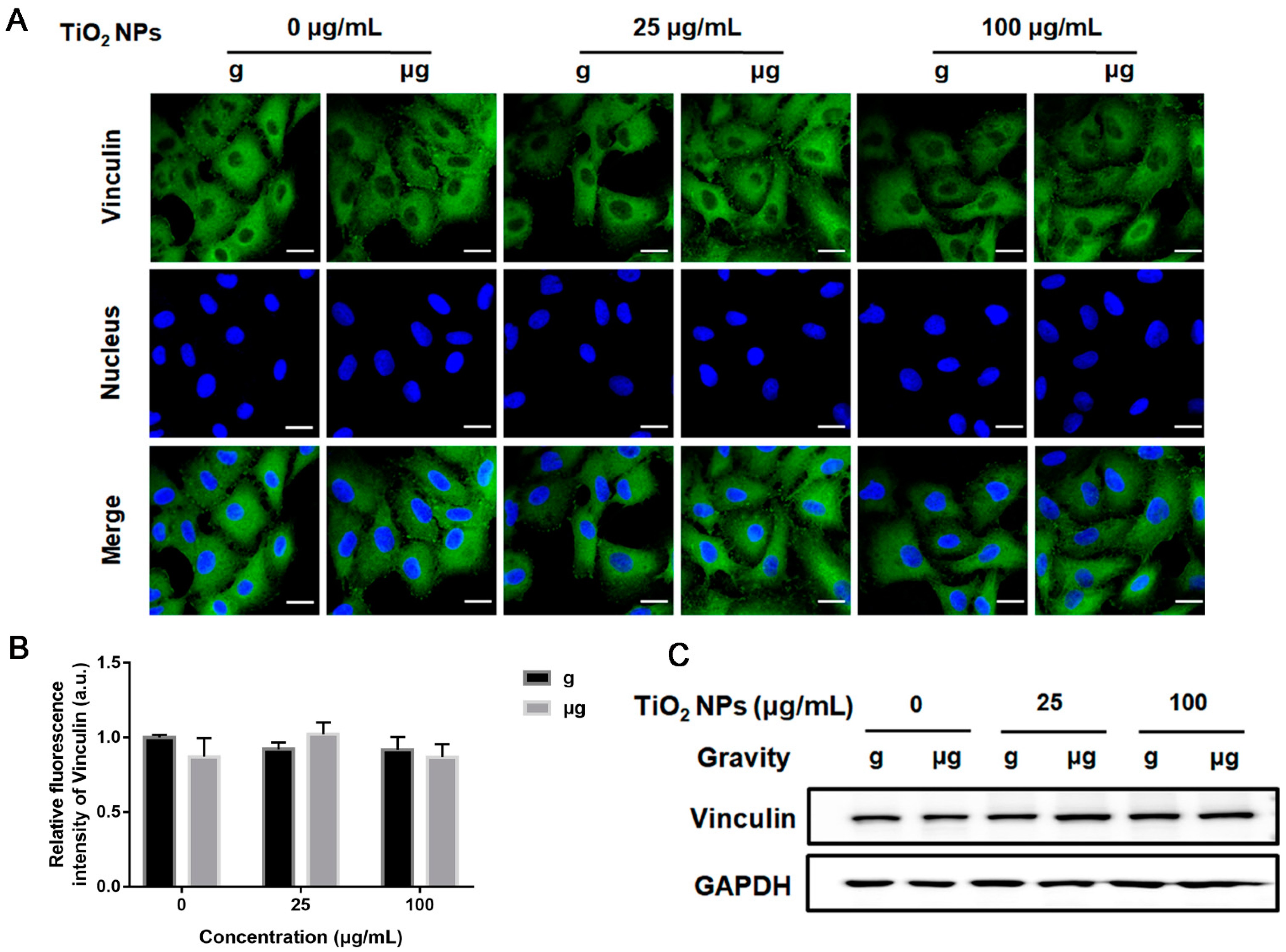
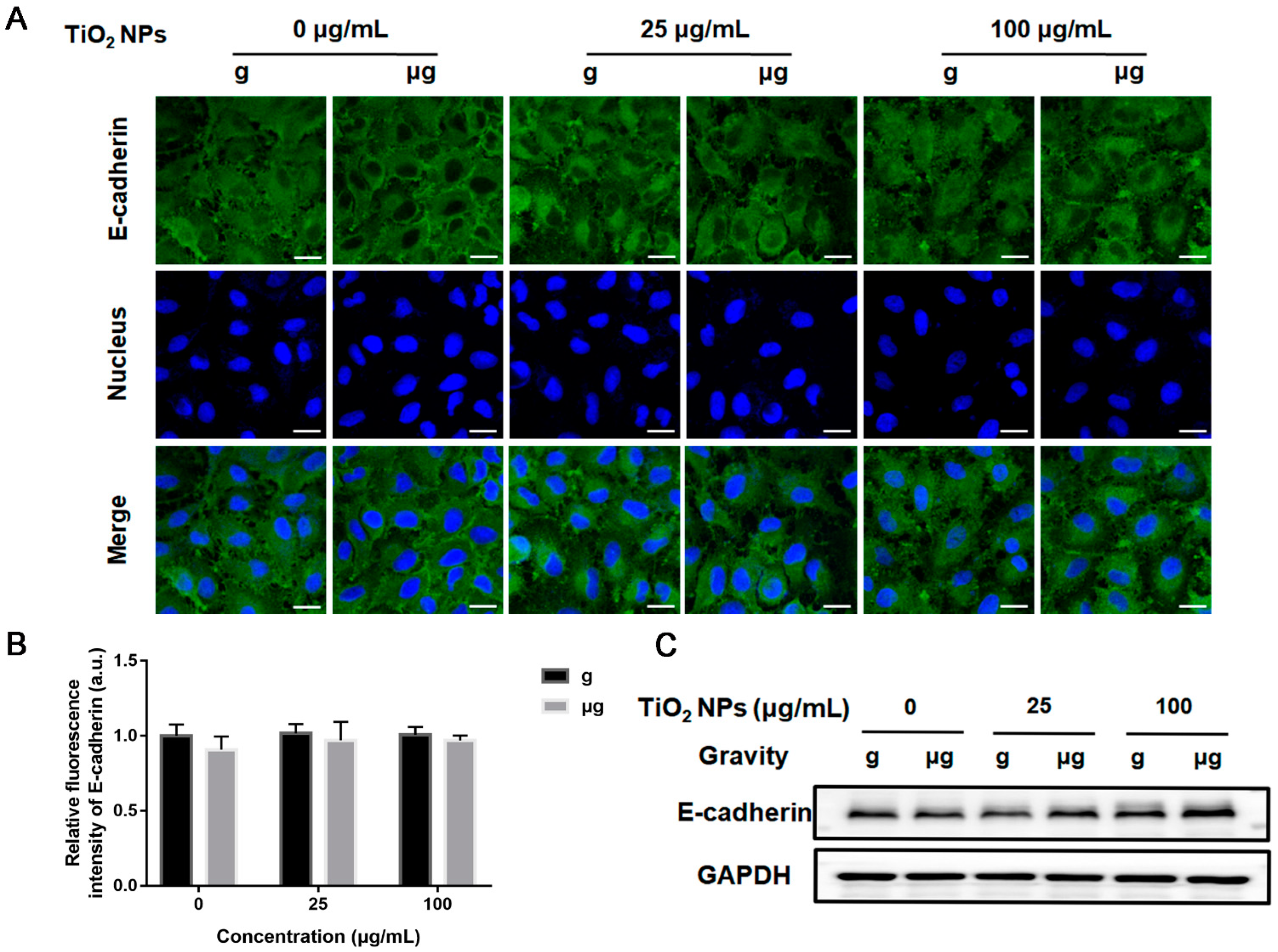
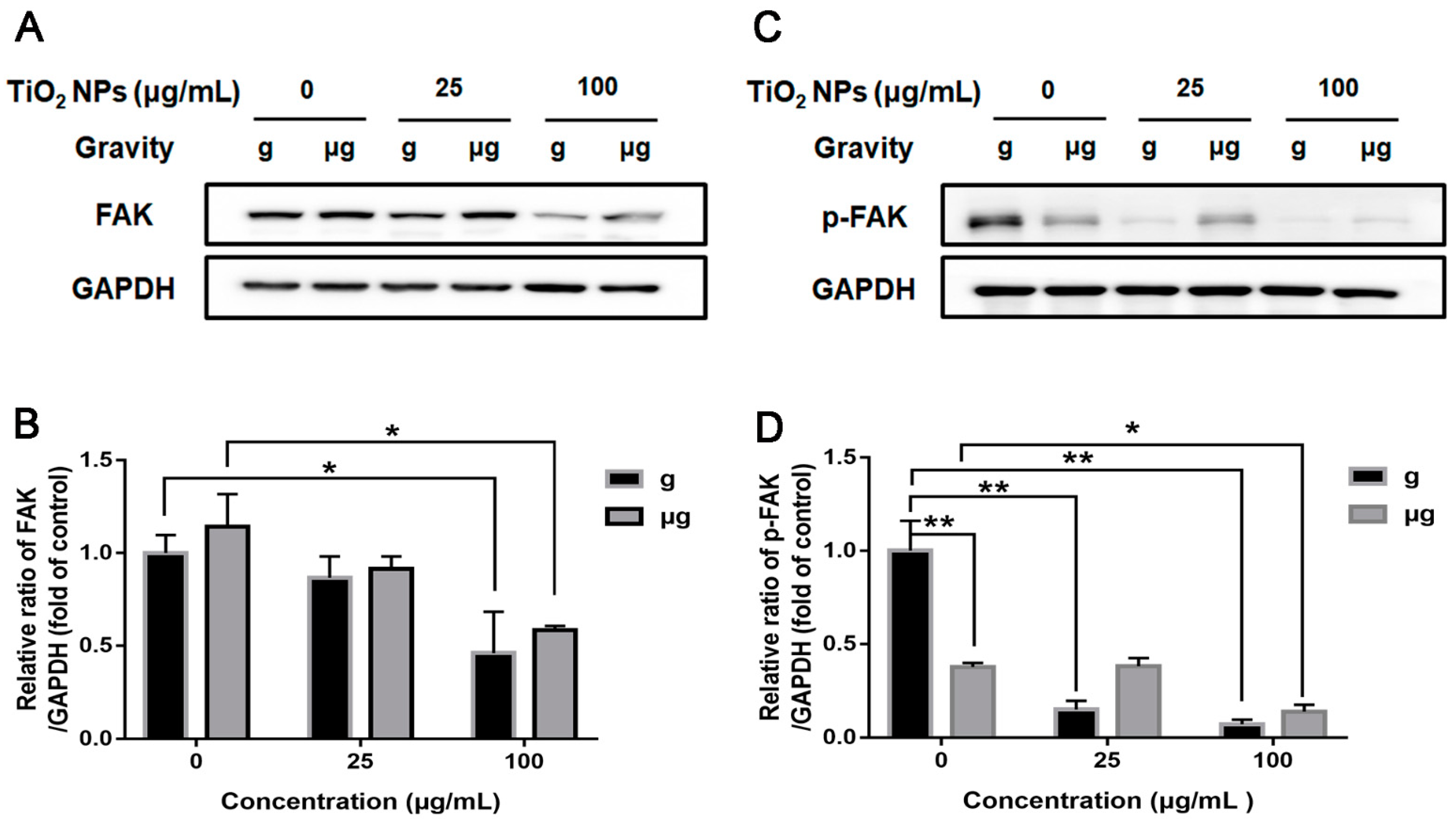
3.4. Effects of TiO2 NPs Exposure on Cell Adhesion-Associated Molecules under Normal Gravity and Simulated Microgravity
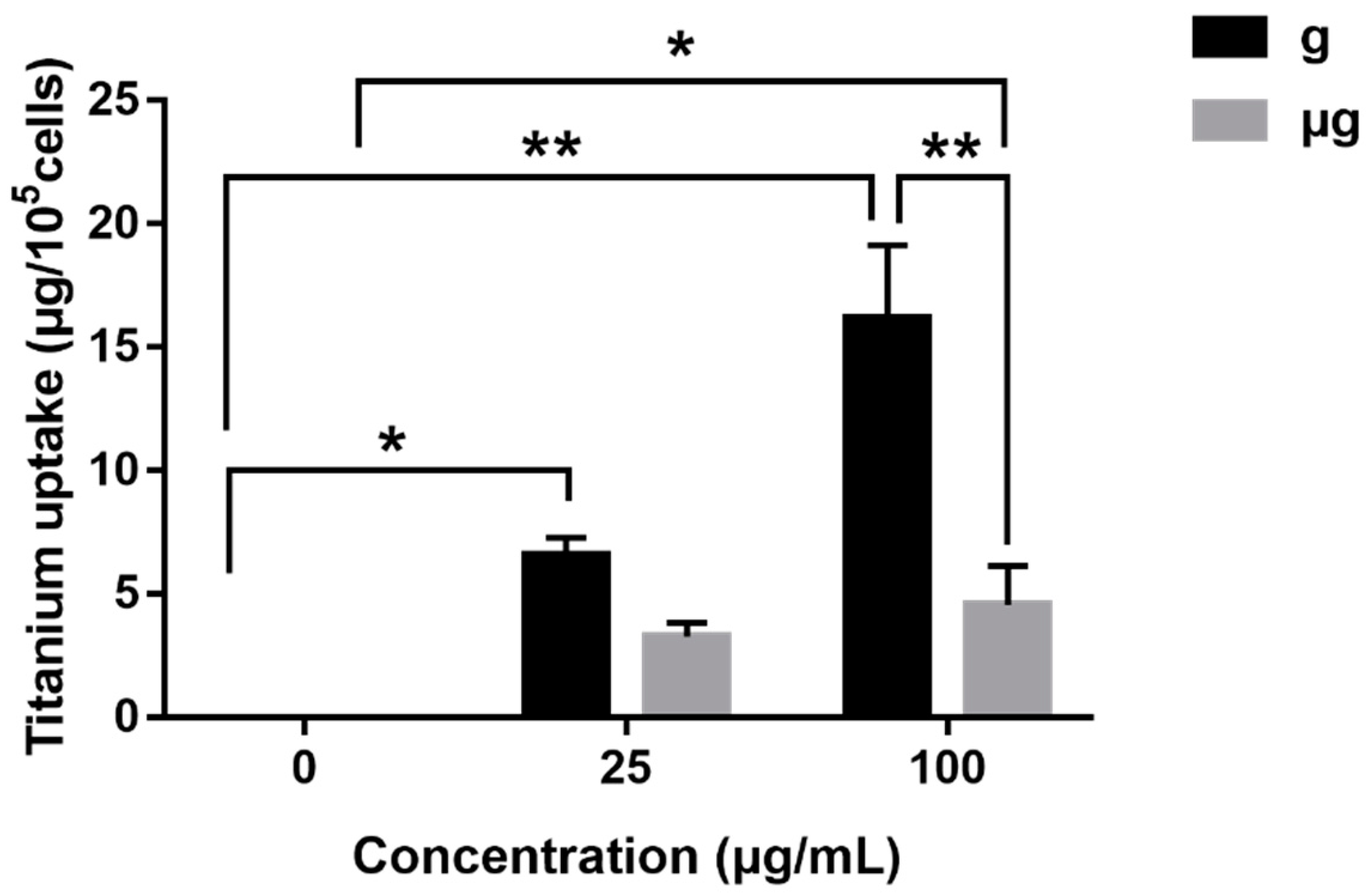
3.5. ICP-OES Determination of Cellular Uptake of TiO2 NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Luo, Z.; Li, N.; Lee, J.Y.; Xie, J.; Lu, J. Amphiphilic polymeric nanocarriers with luminescent gold nanoclusters for concurrent bioimaging and controlled drug release. Adv. Funct. Mater. 2013, 23, 4324–4331. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, L.; Xu, T.; Liu, H.; Liu, X.; Meng, J.; Yang, G.; Jiang, L.; Wang, S. Programmable fractal nanostructured interfaces for specific recognition and electrochemical release of cancer cells. Adv. Mater. 2013, 25, 3566–3570. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, I.; Xu, S.; Teel, G.; Mariotti, D.; Walker, M.L.R.; Keidar, M. Recent progress and perspectives of space electric propulsion systems based on smart nanomaterials. Nat. Commun. 2018, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, J.; Zhu, J.; Cui, W. Studies on the preparation and thermal stability of SiO2 aerogels doped with fibrous bructite and TiO2 opacifier. Asian J. Chem. 2013, 25, 877–879. [Google Scholar] [CrossRef]
- Alagarsamy, S.V.; Ravichandran, M. Investigations on tribological behaviour of AA7075-TiO2 composites under dry sliding conditions. Ind. Lubr. Tribol. 2019, 71, 1064–1071. [Google Scholar]
- Lata, S.; Pandey, A.; Labhansh; Sharma, A.; Meena, K.; Rana, R.; Lal, R. An experimental study and analysis of the mechanical properties of titanium dioxide reinforced aluminum (AA 5051) composite. Mater. Today Proc. 2018, 5, 6090–6097. [Google Scholar] [CrossRef]
- Zhang, F. Research and Design of Double Tuning Fork Temperature and Humidity Sensor; Harbin University of Science and Technology: Harbin, China, 2021. [Google Scholar]
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef]
- Aubert, A.E.; Beckers, F.; Verheyden, B. Cardiovascular function and basics of physiology in microgravity. Acta Cardiol. 2005, 60, 129–151. [Google Scholar] [CrossRef]
- Tuday, E.C.; Berkowitz, D.E. Microgravity and cardiac atrophy: No sex discrimination. J. Appl. Physiol. 2007, 103, 1–2. [Google Scholar] [CrossRef]
- Hughson, R.L.; Helm, A.; Durante, M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2018, 15, 167–180. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. Microgravity and immune responsiveness: Implications for space travel. Nutrition 2002, 18, 889–898. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Z.; Li, W.; Xue, Y.; Xu, H.; Li, J.; Shang, P. Glucocorticoid: A potential role in microgravity-induced bone loss. Acta Astronaut. 2017, 140, 206–212. [Google Scholar] [CrossRef]
- Tanaka, K.; Nishimura, N.; Kawai, Y. Adaptation to microgravity, deconditioning, and countermeasures. J. Physiol. Sci. 2017, 67, 271–281. [Google Scholar] [CrossRef]
- Prisk, G.K.; Fine, J.M.; Cooper, T.K.; West, J.B. Vital capacity, respiratory muscle strength, and pulmonary gas exchange during long-duration exposure to microgravity. J. Appl. Physiol. 2006, 101, 439–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prisk, G.K. Microgravity and the respiratory system. Eur. Respir. J. 2014, 43, 1459–1471. [Google Scholar] [CrossRef]
- Oluwafemi, F.A.; Abdelbaki, R.; Lai, J.C.-Y.; Mora-Almanza, J.G.; Afolayan, E.M. A review of astronaut mental health in manned missions: Potential interventions for cognitive and mental health challenges. Life Sci. Space Res. 2021, 28, 26–31. [Google Scholar] [CrossRef]
- Lämmerzahl, C.; Steinberg, T. Facilities to Alter Weight. In Generation and Applications of Extra-Terrestrial Environments on Earth; Beysens, D.A., van Loon, J.J.W.A., Eds.; River Publishers: Alborg, Denmark, 2015; pp. 45–91. [Google Scholar]
- Sun, Y.; Kuang, Y.; Zuo, Z. The emerging role of macrophages in immune system dysfunction under real and simulated microgravity conditions. Int. J. Mol. Sci. 2021, 22, 2333. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Walther, I.; Furrer, R.; Anderegg, R.; Sekler, J.; Egli, M. A novel microgravity simulator applicable for three-dimensional cell culturing. Microgravity Sci. Technol. 2014, 26, 77–88. [Google Scholar] [CrossRef]
- Hoson, T.; Kamisaka, S.; Masuda, Y.; Yamashita, M.; Buchen, B. Evaluation of the three-dimensional clinostat as a simulator of weightlessness. Planta 1997, 203, S187–S197. [Google Scholar] [CrossRef]
- Wuest, S.L.; Stéphane, R.; Sascha, K.; Daniela, G.; Marcel, E. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. BioMed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef]
- Brungs, S.; Egli, M.; Wuest, S.L.; Christianen, P.; Van Loon, J.J.W.A.; Anh, T.; Hemmersbach, R. Facilities for simulation of microgravity in the ESA ground-based facility programme. Microgravity Sci. Technol. 2016, 28, 191–203. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Cialdai, F.; Monici, M.; Morbidelli, L. The impact of microgravity and hypergravity on endothelial cells. BioMed Res. Int. 2015, 2015, 434803. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, J.-H.; Han, D.G.; Kang, H.-W.; Lee, S.-H.; Lee, J.-I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef]
- Thiel, C.S.; Tauber, S.; Lauber, B.; Polzer, J.; Ullrich, O. Rapid morphological and cytoskeletal response to microgravity in human primary macrophages. Int. J. Mol. Sci. 2019, 20, 2402. [Google Scholar] [CrossRef]
- Jha, R.; Wu, Q.; Singh, M.; Preininger, M.K.; Han, P.; Ding, G.; Cho, H.C.; Jo, H.; Maher, K.O.; Wagner, M.B. Simulated microgravity and 3D culture enhance induction, viability, proliferation and differentiation of cardiac progenitors from human pluripotent stem cells. Sci. Rep. 2016, 6, 30956. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Dong, D.; Lv, H.; Shang, P. Iron overload as a high risk factor for microgravity-induced bone loss. Acta Astronaut. 2019, 164, 407–414. [Google Scholar] [CrossRef]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis induction and alteration of cell adherence in human lung cancer cells under simulated microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef]
- Pan, Y.K.; Li, C.F.; Gao, Y.; Wang, Y.C.; Sun, X.Q. Effect of miR-27b-5p on apoptosis of human vascular endothelial cells induced by simulated microgravity. Apoptosis 2020, 25, 73–91. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Villa, A.; Versari, S.; Maier, J.A.M.; Bradamante, S. Cell behavior in simulated microgravity: A comparison of results obtained with RWV and RPM. Gravit. Space Biol. Bull. 2005, 18, 89–90. [Google Scholar]
- Hatton, J.P.; Gaubert, F.; Lewis, M.L.; Darsel, Y.; Ohlmann, P.; Cazenave, J.-P.; Schmitt, D. The kinetics of translocation and cellular quantity of protein kinase C in human leukocytes are modified during spaceflight. FASEB J. 1999, 13, S23–S33. [Google Scholar] [CrossRef]
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2021, 18, 1489–1502. [Google Scholar] [CrossRef]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Krüger, M. Influence of microgravity on apoptosis in cells, tissues, and other systems in vivo and in vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef]
- Rijken, P.J.; Groot, R.P.D.; Kruijer, W.; Laat, S.W.D.; Verkleij, A.J.; Boonstra, J. Identification of specific gravity sensitive signal transduction pathways in human A431 carcinoma cells. Adv. Space Res. 1992, 12, 145–152. [Google Scholar] [CrossRef]
- Hughes-Fulford, M.; Lewis, M.L. Effects of microgravity on osteoblast growth activation. Exp. Cell Res. 1996, 224, 103–109. [Google Scholar] [CrossRef]
- Meloni, M.A.; Galleri, G.; Pani, G.; Saba, A.; Pippia, P.; Cogoli-Greuter, M. Space flight affects motility and cytoskeletal structures in human monocyte cell line J-111. Cytoskeleton 2011, 68, 125–137. [Google Scholar] [CrossRef]
- Bradbury, P.; Wu, H.; Choi, J.U.; Rowan, A.E.; Zhang, H.; Poole, K.; Lauko, J.; Chou, J. Modeling the impact of microgravity at the cellular level: Implications for human disease. Front. Cell Dev. Biol. 2020, 8, 96. [Google Scholar] [CrossRef]
- Strauch, S.M.; Grimm, D.; Corydon, T.J.; Krüger, M.; Richter, P. Current knowledge about the impact of microgravity on the proteome. Expert Rev. Proteom. 2018, 16, 5–16. [Google Scholar] [CrossRef]
- Carlsson, S.I.M.; Bertilaccio, M.T.S.; Ballabio, E.; Maier, J.A.M. Endothelial stress by gravitational unloading: Effects on cell growth and cytoskeletal organization. Biochim. Biophys. Acta 2003, 1642, 173–179. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, J.; Du, T.; Wang, Y.; Wang, Z. Modeled microgravity causes changes in the cytoskeleton and focal adhesions, and decreases in migration in malignant human MCF-7 cells. Protoplasma 2009, 238, 23–33. [Google Scholar] [CrossRef]
- Pache, C.; Kühn, J.; Westphal, K.; Toy, M.F.; Parent, J.M.; Büchi, O.; Franco-Obregón, A.; Depeursinge, C.; Egli, M. Digital holographic microscopy real-time monitoring of cytoarchitectural alterations during simulated microgravity. J. Biomed. Opt. 2010, 15, 026021. [Google Scholar]
- Moes, M.J.A.; Gielen, J.C.; Boonstra, R.-J.; van Loon, J.J.W.A.; Christianen, P.C.M.; Boonstra, J. Simulation of microgravity by magnetic levitation and random positioning: Effect on human A431 cell morphology. Microgravity Sci. Technol. 2011, 23, 249–261. [Google Scholar] [CrossRef]
- Masiello, M.G.; Cucina, A.; Proietti, S.; Palombo, A.; Coluccia, P.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Morini, V.; Bizzarri, M. Phenotypic switch induced by simulated microgravity on MDA-MB-231 breast cancer cells. BioMed Res. Int. 2014, 2014, 652434. [Google Scholar] [CrossRef]
- Sciola, L.; Cogoli-Greuter, M.; Cogoli, A.; Spano, A.; Pippia, P. Influence of microgravity on mitogen binding and cytoskeleton in Jurkat cells. Adv. Space Res. 1999, 24, 801–805. [Google Scholar] [CrossRef]
- Yang, F.; Li, Y.; Ding, B.; Nie, J.; Wang, H.; Zhang, X.; Wang, C.; Ling, S.; Ni, C.; Dai, Z.; et al. Reduced function and disassembled microtubules of cultured cardiomyocytes in spaceflight. Chin. Sci. Bull. 2008, 53, 1185–1192. [Google Scholar] [CrossRef]
- Genchi, G.G.; Degl’Innocenti, A.; Salgarella, A.; Pezzini, I.; Marino, A.; Menciassi, A.; Piccirillo, S.; Balsamo, M.; Ciofani, G. Modulation of gene expression in rat muscle cells following treatment with nanoceria in different gravity regimes. Nanomedicine 2018, 13, 2821–2833. [Google Scholar] [CrossRef]
- Genchi, G.G.; Degl’Innocenti, A.; Martinelli, C.; Battaglini, M.; Pasquale, D.D.; Prato, M.; Marras, S.; Pugliese, G.; Drago, F.; Mariani, A.; et al. Cerium oxide nanoparticle administration to skeletal muscle cells under different gravity and radiation conditions. ACS Appl. Mater. Interfaces 2021, 13, 40200–40213. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Qu, L.; Kan, G.; Zhang, T.; Shen, J.; Li, Y.; Yang, J.; Niu, Y.; Xiao, Z. Reactive mesoporous silica nanoparticles loaded with limonene for improving physical and mental health of mice at simulated microgravity condition. Bioact. Mater. 2020, 5, 1127–1137. [Google Scholar] [CrossRef]
- Cristofaro, F.; Pani, G.; Pascucci, B.; Mariani, A.; Balsamo, M.; Donati, A.; Mascetti, G.; Rea, G.; Rizzo, A.M.; Visai, L. The NATO project: Nanoparticle-based countermeasures for microgravity-induced osteoporosis. Sci. Rep. 2019, 9, 17141. [Google Scholar] [CrossRef]
- Gaminian, H.; Montazer, M. Enhanced self-cleaning properties on polyester fabric under visible light through single-step synthesis of cuprous oxide doped nano-TiO2. Photochem. Photobiol. 2015, 91, 1078–1087. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Cao, G.; Zhang, C.; Xia, L.; Xu, W.; Xiao, S. Atomic layer deposition TiO2/Al2O3 nanolayer of dyed polyamide/aramid blend fabric for high intensity UV light protection. Polym. Eng. Sci. 2015, 55, 1296–1302. [Google Scholar] [CrossRef]
- Montazer, M.; Seifollahzadeh, S. Enhanced self-cleaning, antibacterial and UV protection properties of nano TiO2 treated textile through enzymatic pretreatment. Photochem. Photobiol. 2011, 87, 877–883. [Google Scholar] [CrossRef]
- Halappanavar, S.; Jackson, P.; Williams, A.; Jensen, K.A.; Hougaard, K.S.; Vogel, U.; Yauk, C.L.; Wallin, H. Pulmonary response to surface-coated nanotitanium dioxide particles includes induction of acute phase response genes, inflammatory cascades, and changes in microRNAs: A toxicogenomic study. Environ. Mol. Mutagen. 2011, 52, 425–439. [Google Scholar] [CrossRef]
- Husain, M.; Wu, D.; Saber, A.T.; Decan, N.; Jacobsen, N.R.; Williams, A.; Yauk, C.L.; Wallin, H.; Vogel, U.; Halappanavar, S. Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice. Nanotoxicology 2015, 9, 1013–1022. [Google Scholar] [CrossRef]
- Hadrup, N.; Zhernovkov, V.; Jacobsen, N.R.; Voss, C.; Vogel, U. Acute phase response as a biological mechanism-of-action of (nano) particle—induced cardiovascular disease. Small 2020, 16, 1907476. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M. Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J. Appl. Toxicol. 2020, 40, 16–36. [Google Scholar] [CrossRef]
- Kansara, K.; Patel, P.; Shah, D.; Shukla, R.K.; Singh, S.; Kumar, A.; Dhawan, A. TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ. Mol. Mutagen. 2015, 56, 204–217. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2015, 22, 5519–5530. [Google Scholar] [CrossRef]
- Bengalli, R.; Ortelli, S.; Blosi, M.; Costa, A.; Mantecca, P.; Fiandra, L. In vitro toxicity of TiO2:SiO2 nanocomposites with different photocatalytic properties. Nanomaterials 2019, 9, 1041. [Google Scholar] [CrossRef]
- Loon, J.J.W.A.v. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Ricci, G.; Cucina, A.; Proietti, S.; Dinicola, S.; Catizone, A. Microgravity induces transient EMT in human keratinocytes by early down-regulation of E-cadherin and cell-adhesion remodeling. Appl. Sci. 2021, 11, 110. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.C.; Hu, Z.B.; Xu, H.Y.; Sun, J.; Gao, Y.; Li, X.T.; Yang, C.B.; Xie, C.; Li, C.F. Simulated microgravity promotes angiogenesis through RhoA-dependent rearrangement of the actin cytoskeleton. Cell. Physiol. Biochem. 2017, 41, 227–238. [Google Scholar] [CrossRef]
- Meloni, M.A.; Galleri, G.; Pippia, P.; Cogoli-Greuter, M. Cytoskeleton changes and impaired motility of monocytes at modelled low gravity. Protoplasma 2006, 229, 243–249. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Shi, S.; Li, Q.; Cao, Q.; Diao, Y.; Zhang, Y.; Yue, L.; Wei, L. EMT transcription factors are involved in the altered cell adhesion under simulated microgravity effect or overloading by regulation of E-cadherin. Int. J. Mol. Sci. 2020, 21, 1349. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin signaling in cancer: Mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, K.; Wang, N.; Qiu, X.M.; Wang, Y.B.; He, P. Involvement of phosphatidylinositol 3-Kinase/Akt signaling pathway in β1 integrin-mediated internalization of Staphylococcus aureus by alveolar epithelial cells. J. Microbiol. 2013, 51, 644–650. [Google Scholar] [CrossRef]
- Zhao, G.; Gong, L.; Su, D.; Jin, Y.; Guo, C.; Yue, M.; Yao, S.; Qin, Z.; Ye, Y.; Tang, Y.; et al. Cullin5 deficiency promotes small-cell lung cancer metastasis by stabilizing integrin β1. J. Clin. Investig. 2019, 129, 972–987. [Google Scholar] [CrossRef]
- Buken, C.; Sahana, J.; Corydon, T.J.; Melnik, D.; Grimm, D. Morphological and molecular changes in juvenile normal human fibroblasts exposed to simulated microgravity. Sci. Rep. 2019, 9, 11882. [Google Scholar] [CrossRef]
- Cary, L.A.; Chang, J.F.; Guan, J.L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 1996, 109, 1787–1794. [Google Scholar] [CrossRef]
- Aleshcheva, G.; Bauer, J.; Hemmersbach, R.; Slumstrup, L.; Wehland, M.; Infanger, M.; Grimm, D. Scaffold-free tissue formation under real and simulated microgravity conditions. Basic Clin. Pharmacol. Toxicol. 2016, 119, 26–33. [Google Scholar] [CrossRef]
- Chung, J.H.; Ahn, C.B.; Son, K.H.; Yi, E.; Son, H.S.; Kim, H.-S.; Lee, S.H. Simulated microgravity effects on nonsmall cell lung cancer cell proliferation and migration. Aviat. Space Environ. Med. 2017, 88, 82–89. [Google Scholar] [CrossRef]
- Ranieri, D.; Proietti, S.; Dinicola, S.; Masiello, M.G.; Rosato, B.; Ricci, G.; Cucina, A.; Catizone, A.; Bizzarri, M.; Torrisi, M.R. Simulated microgravity triggers epithelial mesenchymal transition in human keratinocytes. Sci. Rep. 2017, 7, 538. [Google Scholar] [CrossRef]
- Ranieri, D.; Cucina, A.; Bizzarri, M.; Alimandi, M.; Torrisi, M.R. Microgravity influences circadian clock oscillation in human keratinocytes. FEBS Open Bio. 2015, 5, 717–723. [Google Scholar] [CrossRef]
- Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Maniratanachote, R. Titanium dioxide nanoparticles-mediated in vitro cytotoxicity does not induce Hsp70 and Grp78 expression in human bronchial epithelial A549 cells. Biol. Trace Elem. Res. 2012, 149, 123–132. [Google Scholar] [CrossRef][Green Version]
- Mao, Z.; Yao, M.; Xu, B.; Ji, X.; Jiang, H.; Han, X.; Tang, Q.; Zhou, Z.; Chen, R.; Li, X.; et al. Cytoskeletons of two reproductive germ cell lines response differently to titanium dioxide nanoparticles mediating vary reproductive toxicity. J. Biomed. Nanotechnol. 2017, 13, 409–416. [Google Scholar] [CrossRef]
- Hekmat, A.; Rabizadeh, M.; Safavi, M.; Hajebrahimi, Z. The comparison of the apoptosis effects of titanium dioxide nanoparticles into MDA-MB-231 cell line in microgravity and gravity conditions. Nanomedicine 2019, 6, 120–127. [Google Scholar]
- Chang, D.; Xu, H.; Guo, Y.; Jiang, X.; Liu, Y.; Li, K.; Pan, C.; Yuan, M.; Wang, J.; Li, T. Simulated microgravity alters the metastatic potential of a human lung adenocarcinoma cell line. In Vitro Cell. Dev. Biol.-Anim. 2013, 49, 170–177. [Google Scholar] [CrossRef]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Krüger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K. Simulated microgravity influences VEGF, MAPK, and PAM signaling in prostate cancer cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef]
- Mann, V.; Grimm, D.; Corydon, T.; Krüger, M.; Wehland, M.; Riwaldt, S.; Sahana, J.; Kopp, S.; Bauer, J.; Reseland, J. Changes in human foetal osteoblasts exposed to the random positioning machine and bone construct tissue engineering. Int. J. Mol. Sci. 2019, 20, 1357. [Google Scholar] [CrossRef]
- Stutchbury, B.; Atherton, P.; Tsang, R.; Wang, D.-Y.; Ballestrem, C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 2017, 130, 1612–1624. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Grimm, D. Short-term microgravity influences cell adhesion in human breast cancer cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef]
- Chan, K.T.; Cortesio, C.L.; Huttenlocher, A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J. Cell Biol. 2009, 185, 357–370. [Google Scholar] [CrossRef]
- Gilmore, A.P.; Romer, L.H. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell 1996, 7, 1207–1224. [Google Scholar] [CrossRef]
- Junlin, G. Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell Biol. 1997, 29, 1085–1096. [Google Scholar]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef]
- Nie, R. Expression and Significance of Integrin β1, FAK and pY397FAK in Non-Small Cell Lung Carcinoma; Wuhan University: Wuhan, China, 2005. [Google Scholar]
- Imaizumi, M.; Nishimura, M.; Takeuchi, S.; Murase, M.; Hamaguchi, M. Role of tyrosine specific phosphorylation of cellular proteins, especially EGF receptor and p125FAK in human lung cancer cells. Lung Cancer 1997, 17, 69–84. [Google Scholar] [CrossRef]
- Al-Ghabkari, A.; Qasrawi, D.O.; Alshehri, M.; Narendran, A. Focal adhesion kinase (FAK) phosphorylation is a key regulator of embryonal rhabdomyosarcoma (ERMS) cell viability and migration. J. Cancer Res. Clin. Oncol. 2019, 145, 1461–1469. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, J.; Zhang, S.; You, Y.; Zhu, X.; Xiang, H.; Yan, L.; Zhao, F.; Li, Y. Effects of Titanium Dioxide Nanoparticles on Cell Growth and Migration of A549 Cells under Simulated Microgravity. Nanomaterials 2022, 12, 1879. https://doi.org/10.3390/nano12111879
Wang M, Li J, Zhang S, You Y, Zhu X, Xiang H, Yan L, Zhao F, Li Y. Effects of Titanium Dioxide Nanoparticles on Cell Growth and Migration of A549 Cells under Simulated Microgravity. Nanomaterials. 2022; 12(11):1879. https://doi.org/10.3390/nano12111879
Chicago/Turabian StyleWang, Mei, Jinxia Li, Shunyu Zhang, Yue You, Xianyu Zhu, Huandong Xiang, Liang Yan, Feng Zhao, and Yunhui Li. 2022. "Effects of Titanium Dioxide Nanoparticles on Cell Growth and Migration of A549 Cells under Simulated Microgravity" Nanomaterials 12, no. 11: 1879. https://doi.org/10.3390/nano12111879
APA StyleWang, M., Li, J., Zhang, S., You, Y., Zhu, X., Xiang, H., Yan, L., Zhao, F., & Li, Y. (2022). Effects of Titanium Dioxide Nanoparticles on Cell Growth and Migration of A549 Cells under Simulated Microgravity. Nanomaterials, 12(11), 1879. https://doi.org/10.3390/nano12111879