Microdialysis on Ex Vivo Porcine Ear Skin Can Validly Study Dermal Penetration including the Fraction of Transfollicular Penetration—Demonstrated on Caffeine Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Formulations: Caffeine Nanocrystals and Saturated Caffeine Gel
2.1.2. Skin Model: Porcine Ear Skin
2.2. Methods
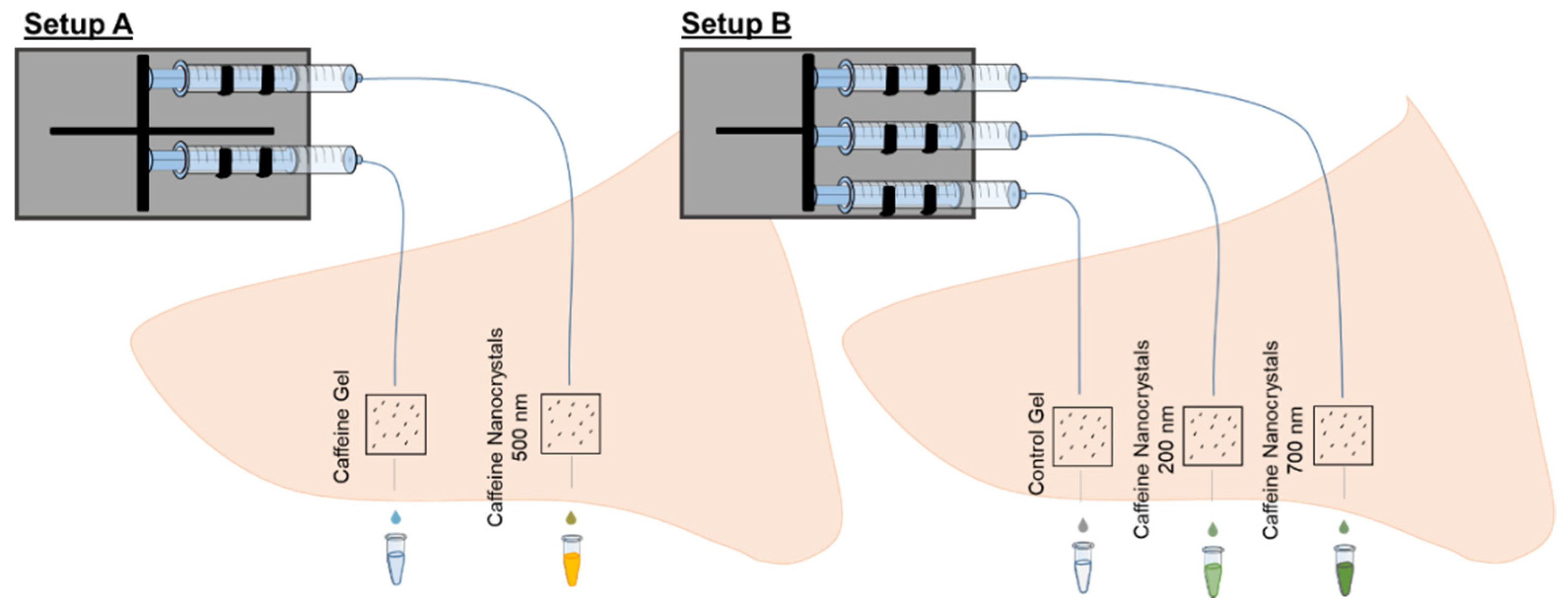
2.2.1. Experimental Setups
2.2.2. Skin Preparation and Application of Formulations
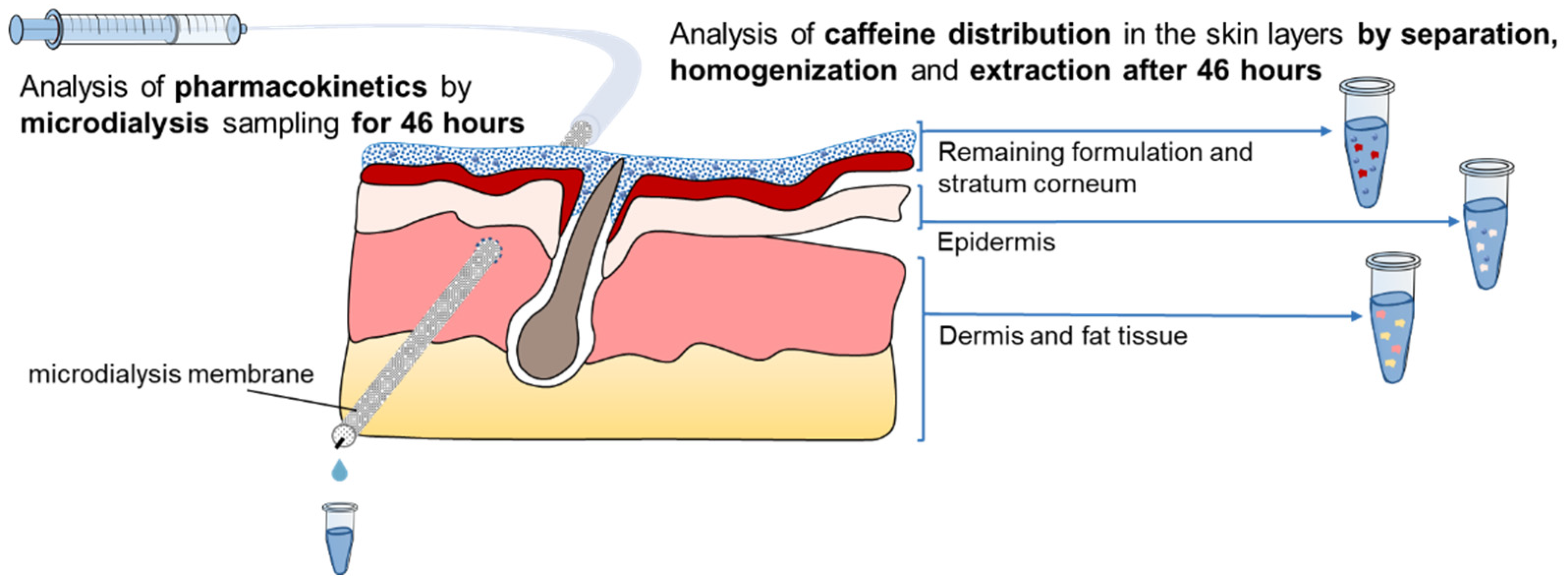
2.2.3. Dermal Microdialysis
2.2.4. Skin Separation, Homogenization and Extraction after 46 h
2.2.5. Determination of Caffeine by HPLC
2.2.6. Statistical Analysis
3. Results
3.1. Setup A—Comparison of Caffeine Gel and Caffeine Nanocrystal Gel Formulation
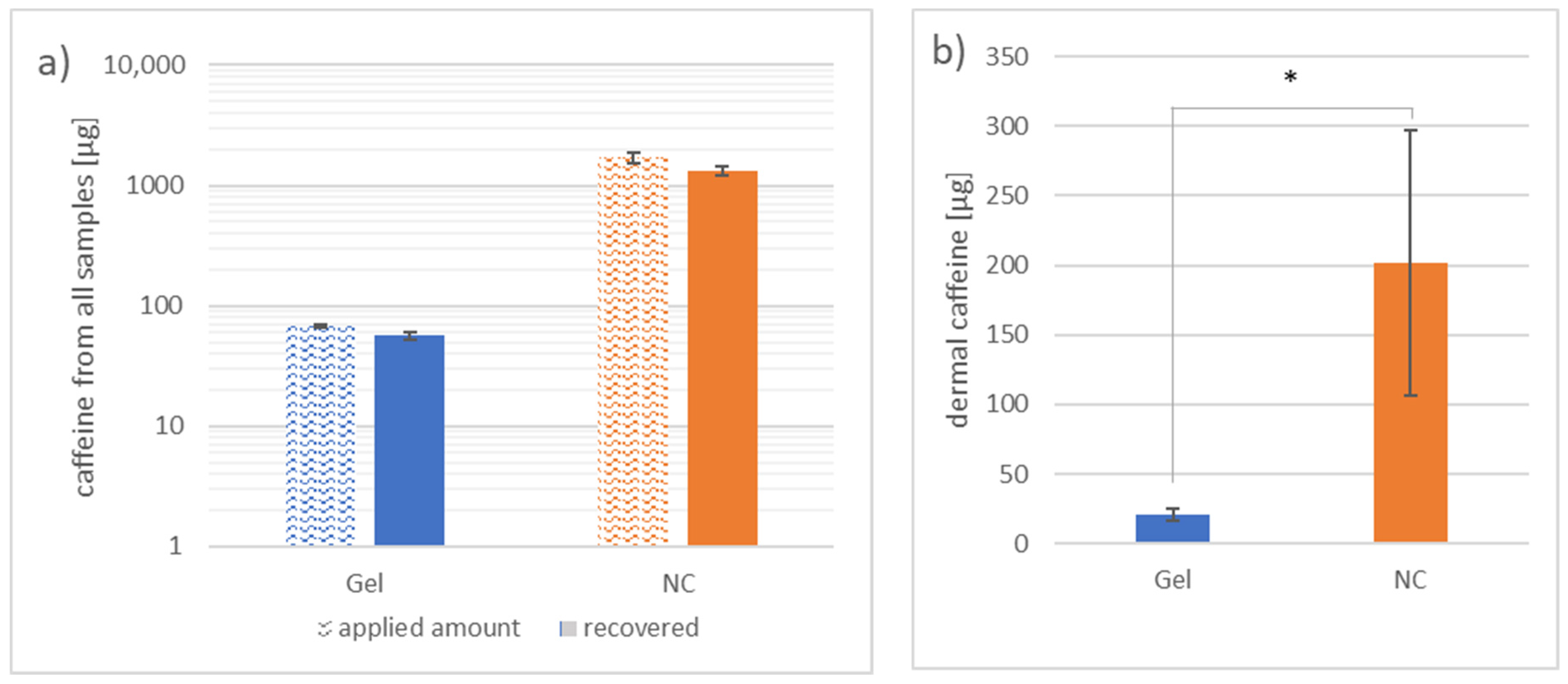
3.1.1. Investigation of Caffeine Distribution in the Skin Layers by Separation, Homogenization and Extraction after 46 h
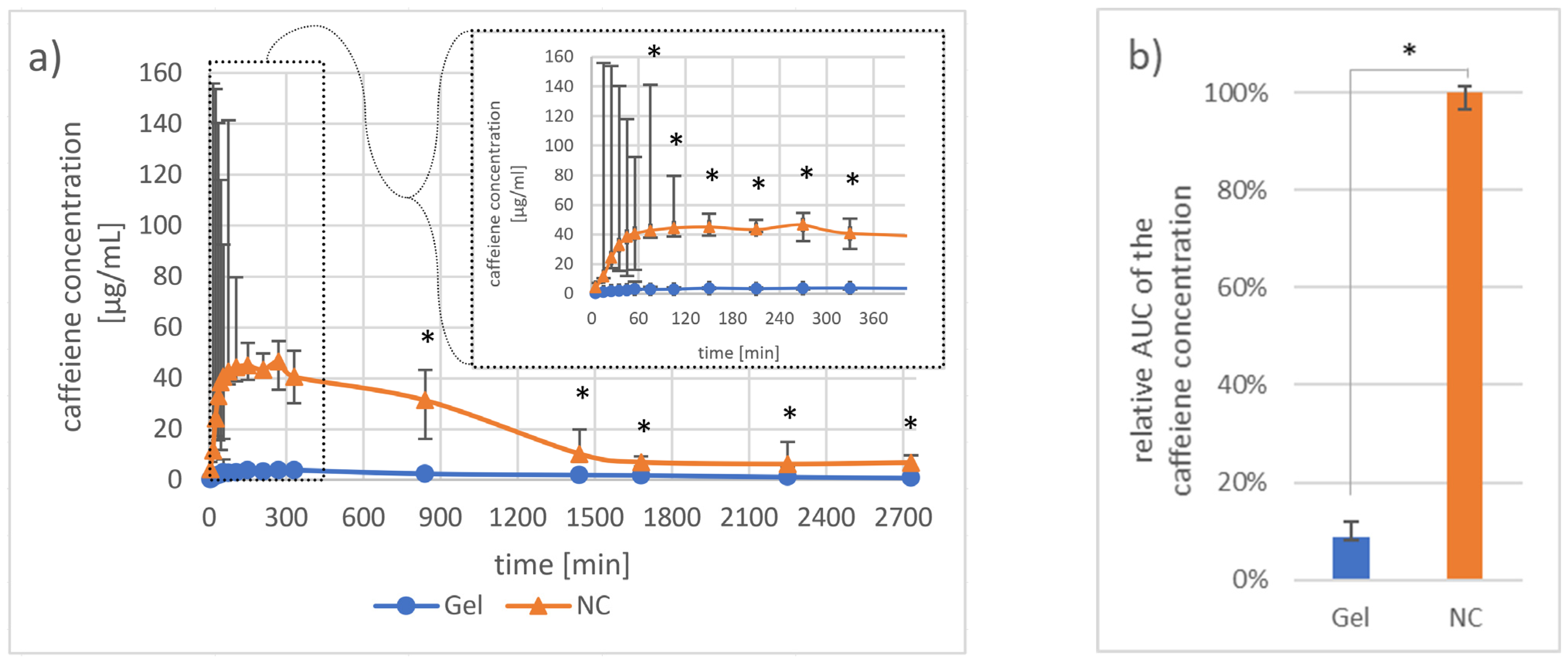
3.1.2. Investigation of Pharmacokinetics by Dermal Microdialysis for 46 h
3.2. Setup B—Comparison of Two Sizes of Caffeine Nanocrystals
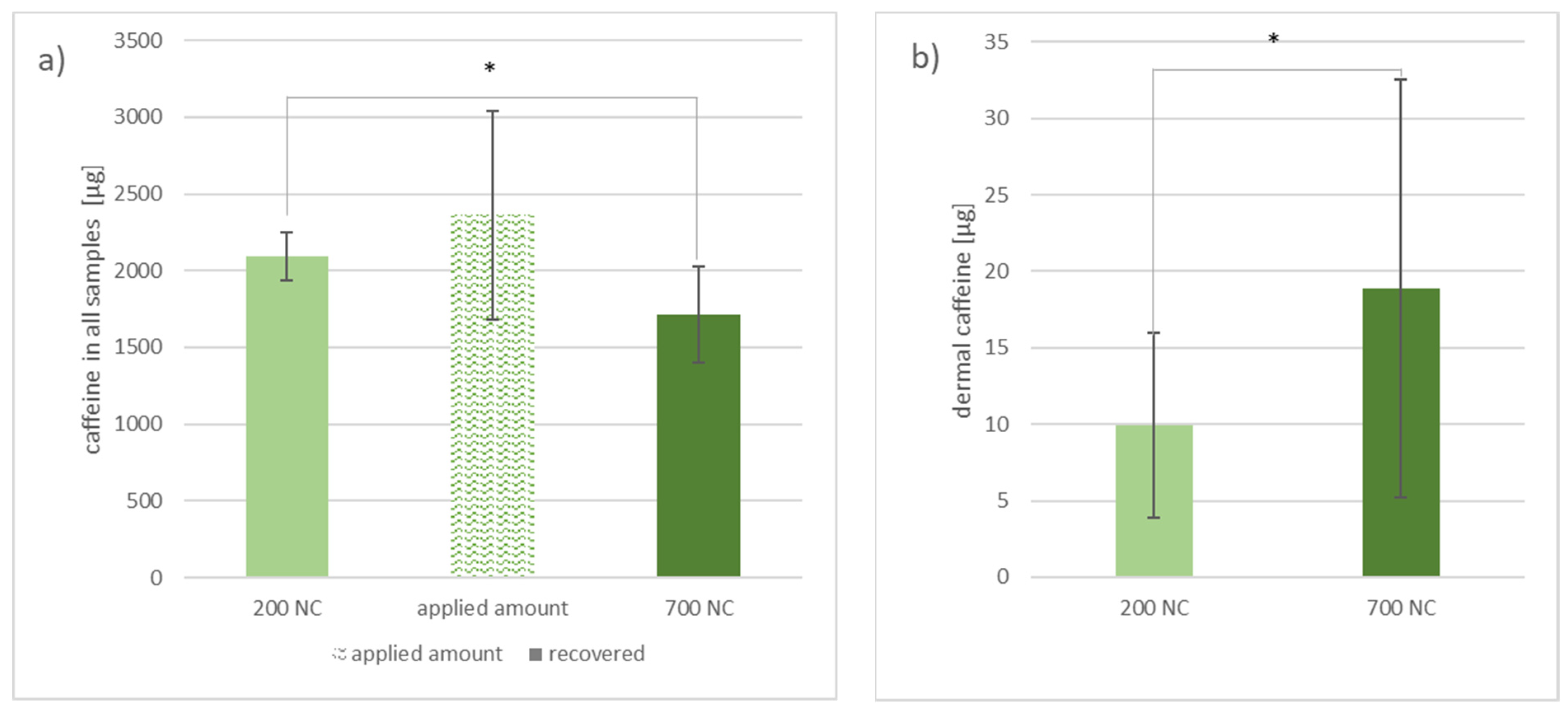
3.2.1. Investigation of Caffeine Distribution in the Skin Layers by Separation, Homogenization and Extraction after 46 h
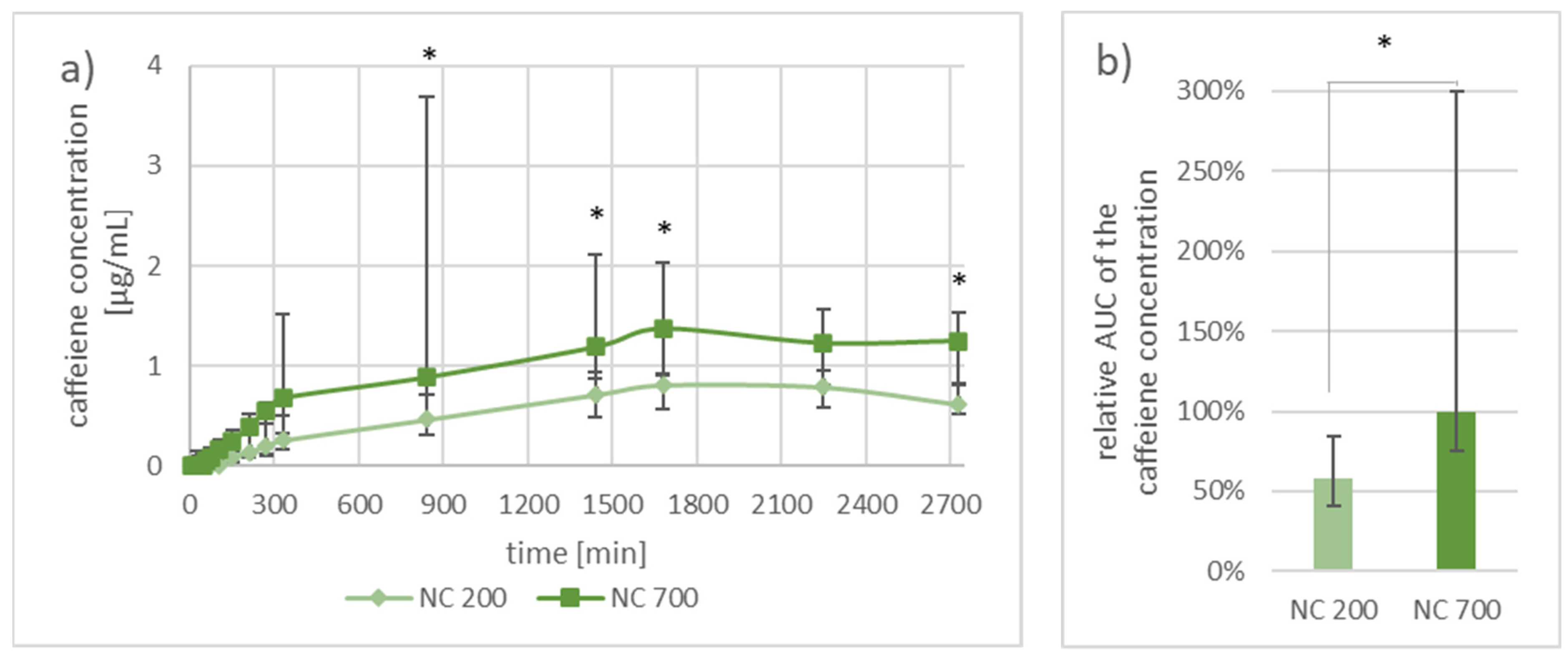
3.2.2. Investigation of Pharmacokinetics by Dermal Microdialysis for 46 h
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franz, T.J. Percutaneous absorption on the relevance of in vitro data. J. Invest. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Mitragotri, S. Engineering approaches to transdermal drug delivery: A tribute to contributions of prof. Robert Langer. Skin Pharmacol. Physiol. 2013, 26, 263–276. [Google Scholar] [CrossRef]
- Kattou, P.; Lian, G.; Glavin, S.; Sorrell, I.; Chen, T. Development of a two-dimensional model for predicting transdermal permeation with the follicular pathway: Demonstration with a caffeine study. Pharm. Res. 2017, 34, 2036–2048. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Lubda, M.; Skov, P.S.; Vogt, A.; Keck, C.M.; Lademann, J.; Beckers, I.; von Hagen, J.; Patzelt, A. Investigation of transfollicular caffeine penetration using microdialysis on ex vivo porcine ear skin. Eur. J. Pharm. Biopharm. 2020, 157, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Lademann, J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin. Drug Deliv. 2020, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Radtke, M.; Patzelt, A.; Knorr, F.; Lademann, J.; Netz, R.R. Ratchet effect for nanoparticle transport in hair follicles. Eur. J. Pharm. Biopharm. 2017, 116, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.-M.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- Pelikh, O.; Keck, C.M. Hair follicle targeting and dermal drug delivery with curcumin drug nanocrystals—Essential influence of excipients. Nanomaterials 2020, 10, 2323. [Google Scholar] [CrossRef]
- Patzelt, A.; Mak, W.C.; Jung, S.; Knorr, F.; Meinke, M.C.; Richter, H.; Rühl, E.; Cheung, K.Y.; Tran, N.B.N.N.; Lademann, J. Do nanoparticles have a future in dermal drug delivery? J. Control. Release 2017, 246, 174–182. [Google Scholar] [CrossRef]
- Dong, P.; Sahle, F.; Lohan, S.; Saeidpour, S.; Albrecht, S.; Teutloff, C.; Bodmeier, R.; Unbehauen, M.; Wolff, C.; Haag, R.; et al. pH-sensitive Eudragit® L 100 nanoparticles promote cutaneous penetration and drug release on the skin. J. Control. Release 2018, 295. [Google Scholar] [CrossRef]
- Breuckmann, P.; Meinke, M.C.; Jaenicke, T.; Krutmann, J.; Rasulev, U.; Keck, C.M.; Müller, R.H.; Klein, A.L.; Lademann, J.; Patzelt, A. Influence of nanocrystal size on the in vivo absorption kinetics of caffeine after topical application. Eur. J. Pharm. Biopharm. 2021. (under review). [Google Scholar] [CrossRef]
- Kreilgaard, M. Assessment of cutaneous drug delivery using microdialysis. Adv. Drug Deliv. Rev. 2002, 54, S99–S121. [Google Scholar] [CrossRef]
- Bito, L.; Davson, H.; Levin, E.; Murray, M.; Snider, N. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J. Neurochem. 1966, 13, 1057–1067. [Google Scholar] [CrossRef]
- Anderson, C.; Andersson, T.; Molander, M. Ethanol absorption across human skin measured by in vivo microdialysis technique. Acta Derm. Venereol. 1991, 71, 389–393. [Google Scholar] [PubMed]
- Müller, M.; Schmid, R.; Wagner, O.; Osten, B.v.; Shayganfar, H.; Eichler, H.G. In vivo characterization of transdermal drug transport by microdialysis. J. Control. Release 1995, 37, 49–57. [Google Scholar] [CrossRef]
- Cross, S.E.; Anderson, C.; Roberts, M.S. Topical penetration of commercial salicylate esters and salts using human isolated skin and clinical microdialysis studies. Br. J. Clin. Pharmacol. 1998, 46, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutsiouki, P.; Thompson, J.P.; Clough, G.F. Effects of local blood flow on the percutaneous absorption of the organophosphorus compound malathion: A microdialysis study in man. Arch. Toxicol. 2001, 75, 321–328. [Google Scholar] [CrossRef]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin-assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Richter, H.; Buettemeyer, R.; Huber, H.J.R.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Differential stripping demonstrates a significant reduction of the hair follicle reservoir in vitro compared to in vivo. Eur. J. Pharm. Biopharm. 2008, 70, 234–238. [Google Scholar] [CrossRef]
- Mangelsdorf, S.; Vergou, T.; Sterry, W.; Lademann, J.; Patzelt, A. Comparative study of hair follicle morphology in eight mammalian species and humans. Ski. Res. Technol. 2014, 20, 147–154. [Google Scholar] [CrossRef]
- Zambrano, A.; Klein, A.; Patzelt, A. Analysis of the morphometric parameters of pig ear hair follicles. Ski. Res. Technol. 2021. [Google Scholar] [CrossRef]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharmacol. 2008, 65, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Trauer, S.; Patzelt, A.; Otberg, N.; Knorr, F.; Rozycki, C.; Balizs, G.; Büttemeyer, R.; Linscheid, M.; Liebsch, M.; Lademann, J. Permeation of topically applied caffeine through human skin–a comparison of in vivo and in vitro data. Br. J. Clin. Pharmacol. 2009, 68, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Skin Absorption: In Vivo Method; OCED: Paris, France, 2004. [Google Scholar]
- Zhai, X.; Lademann, J.; Keck, C.M.; Müller, R.H. Nanocrystals of medium soluble actives—Novel concept for improved dermal delivery and production strategy. Int. J. Pharm. 2014, 470, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lademann, J.; Keck, C.M.; Müller, R.H. Dermal nanocrystals from medium soluble actives—Physical stability and stability affecting parameters. Eur. J. Pharm. Biopharm. 2014, 88, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Weigmann, H.-J.; Baumann, M.; Reiche, A.-I.; Sterry, W.; Lademann, J. Lateral spreading of topically applied UV filter substances investigated by tape stripping. Ski. Pharmacol. Physiol. 2004, 17, 17–22. [Google Scholar] [CrossRef]
- Busch, L.; Keziban, Y.; Dähne, L.; Keck, C.; Meinke, M.; Lademann, J.; Patzelt, A. The impact of skin massage frequency on the intrafollicular transport of silica nanoparticles: Validation of the ratchet effect on an ex vivo porcine skin model. Eur. J. Pharm. Biopharm. 2020, 158, 266–272. [Google Scholar] [CrossRef]
- Baumberger, J.P. Methods for the separation of epidermis from dermis and some physiologic. J. Biomed. Opt. 1941, 2, 413. [Google Scholar]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Follicular penetration of caffeine from topically applied nanoemulsion formulations containing penetration enhancers: In vitro human skin studies. Ski. Pharmacol. Physiol. 2018, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.; Niedorf, F.; Wohlert, M.; Kietzmann, M. The in vitro use of the hair follicle closure technique to study the follicular and percutaneous permeation of topically applied drugs. Altern. Lab. Anim. 2012, 40, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Lademann, J. Drug delivery to hair follicles. Expert Opin. Drug Deliv. 2013, 10, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Lane, M.E. Topical and transdermal delivery of caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef] [PubMed]
| Formulations | Composition | z-Average [nm] | PDI | d(v)0.1 [nm] |
|---|---|---|---|---|
| d(v)0.5 [nm] | ||||
| d(v)0.9 [nm] | ||||
| caffeine gel | caffeine 2.5% (w/w) gel phase * add 100.0% (w/w) | n.a. (formulation contained dissolved caffeine) | ||
| caffeine nanocrystals (setup A) | caffeine 20.0% (w/w) gel phase * add 100.0% (w/w) | 535 | 0.66 | 351 |
| 462 | ||||
| 609 | ||||
| caffeine nanocrystals small size (setup B) | caffeine 20.0% (w/w) gel phase * add 100.0% (w/w) | 178 | 0.59 | 176 |
| 225 | ||||
| 288 | ||||
| caffeine nanocrystals medium size (setup B) | caffeine 20.0% (w/w) gel phase * add 100.0% (w/w) | 689 | 0.74 | 405 |
| 531 | ||||
| 691 | ||||
| Sample | Collection Time | Duration of Collecting |
|---|---|---|
| 1 | 10 min | 10 min |
| 2 | 20 min | 10 min |
| 3 | 30 min | 10 min |
| 4 | 40 min | 10 min |
| 5 | 50 min | 10 min |
| 6 | 1 h | 10 min |
| 7 | 1.5 h | 30 min |
| 8 | 2 h | 30 min |
| 9 | 3 h | 1 h |
| 10 | 4 h | 1 h |
| 11 | 5 h | 1 h |
| 12 | 6 h | 1 h |
| 13 | 22 h | 16 h |
| 14 | 26 h | 4 h |
| 15 | 30 h | 4 h |
| 16 | 45 h | 15 h |
| 17 | 46 h | 1 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.L.; Lubda, M.; Specht, D.; Pyo, S.-M.; Busch, L.; Lademann, J.; Meinke, M.C.; Beckers, I.; von Hagen, J.; Keck, C.M.; et al. Microdialysis on Ex Vivo Porcine Ear Skin Can Validly Study Dermal Penetration including the Fraction of Transfollicular Penetration—Demonstrated on Caffeine Nanocrystals. Nanomaterials 2021, 11, 2387. https://doi.org/10.3390/nano11092387
Klein AL, Lubda M, Specht D, Pyo S-M, Busch L, Lademann J, Meinke MC, Beckers I, von Hagen J, Keck CM, et al. Microdialysis on Ex Vivo Porcine Ear Skin Can Validly Study Dermal Penetration including the Fraction of Transfollicular Penetration—Demonstrated on Caffeine Nanocrystals. Nanomaterials. 2021; 11(9):2387. https://doi.org/10.3390/nano11092387
Chicago/Turabian StyleKlein, Anna Lena, Markus Lubda, David Specht, Sung-Min Pyo, Loris Busch, Jürgen Lademann, Martina C. Meinke, Ingeborg Beckers, Jörg von Hagen, Cornelia M. Keck, and et al. 2021. "Microdialysis on Ex Vivo Porcine Ear Skin Can Validly Study Dermal Penetration including the Fraction of Transfollicular Penetration—Demonstrated on Caffeine Nanocrystals" Nanomaterials 11, no. 9: 2387. https://doi.org/10.3390/nano11092387
APA StyleKlein, A. L., Lubda, M., Specht, D., Pyo, S.-M., Busch, L., Lademann, J., Meinke, M. C., Beckers, I., von Hagen, J., Keck, C. M., & Patzelt, A. (2021). Microdialysis on Ex Vivo Porcine Ear Skin Can Validly Study Dermal Penetration including the Fraction of Transfollicular Penetration—Demonstrated on Caffeine Nanocrystals. Nanomaterials, 11(9), 2387. https://doi.org/10.3390/nano11092387









