Comparative Evaluation on Impacts of Fibronectin, Heparin–Chitosan, and Albumin Coating of Bacterial Nanocellulose Small-Diameter Vascular Grafts on Endothelialization In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. BNC Graft Production
2.2. Coating Procedures
2.2.1. Heparin–Chitosan Coating
2.2.2. Albumin and Fibronectin Coating
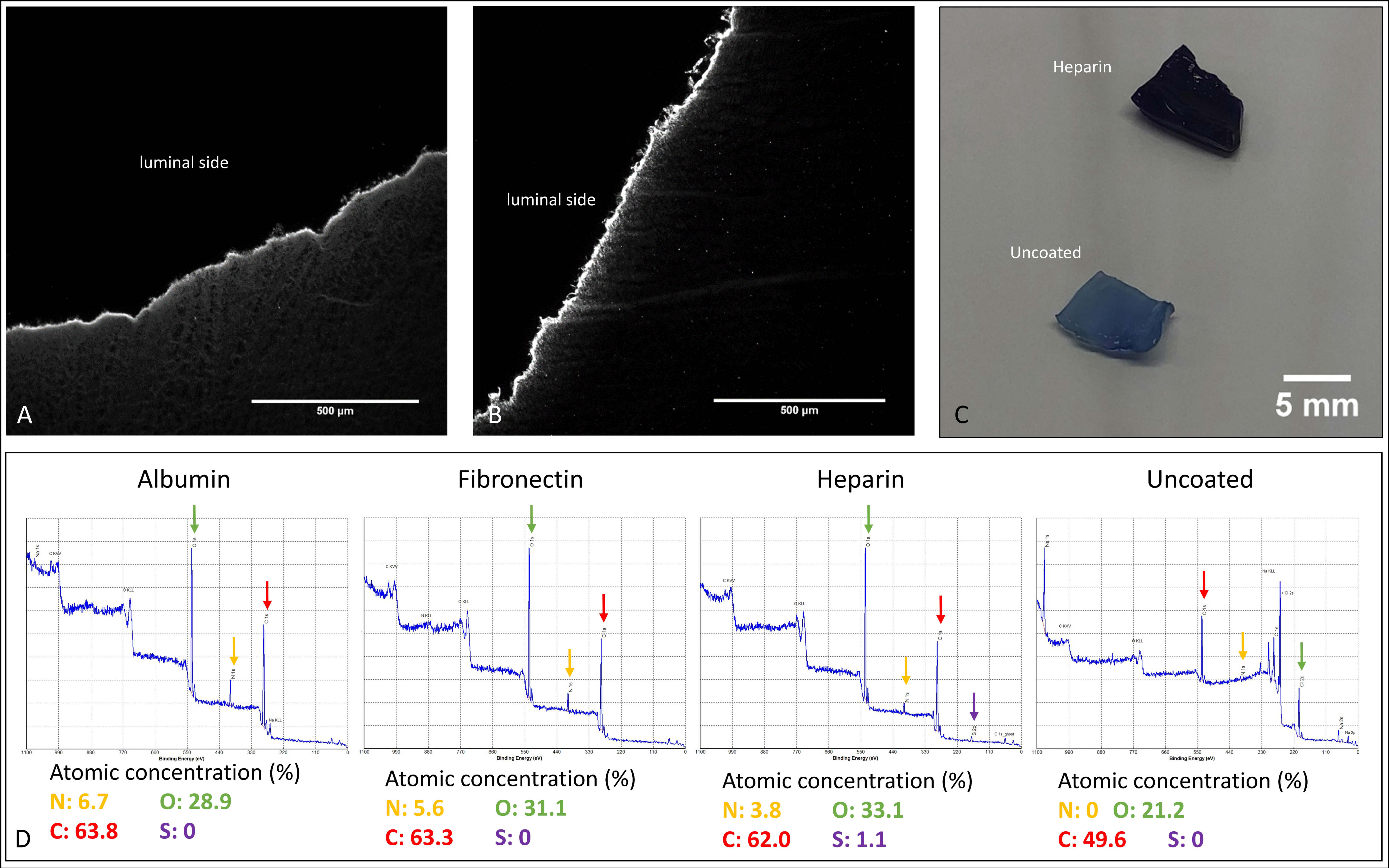
2.3. Coating Analyses for Quality Control
2.3.1. XPS Analyses
2.3.2. Heparin Detection
2.3.3. Albumin and Fibronectin Detection
2.4. Cell Isolation and Cell Culture
2.4.1. Human Saphenous Vein Endothelial Cells (VECs)
2.4.2. Human Endothelial Progenitor Cells (EPCs)
2.5. Cell Characterization
2.5.1. Phase Contrast Microscopy
2.5.2. AcLDL Assay
2.5.3. Flow Cytometry
2.6. Control Based Construct Culture
2.6.1. Static Culture Experiments
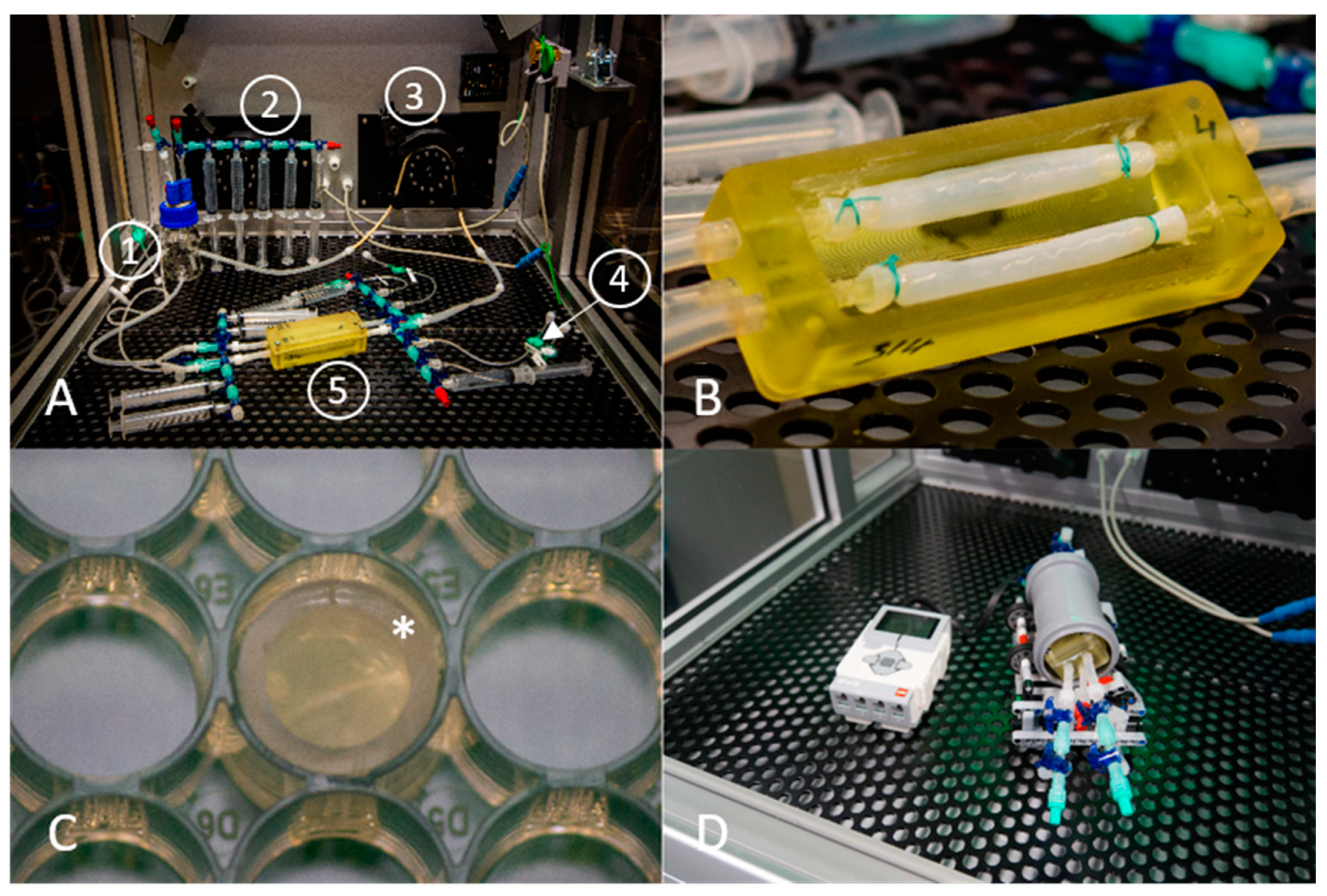
2.6.2. Perfusion Culture Experiments
Bioreactor Perfusion System
Seeding Technique
2.7. Metabolic Activity (WST-1 Assay) of Cells on BNC Constructs, Normalization
2.7.1. Static Cultures
2.7.2. Perfusion Cultures
2.8. AcLDL Assay on Seeded BNC Cell Constructs
2.9. Cryosectioning and Immunofluorescence of BNC Constructs
2.10. Data Analysis and Image Processing
3. Results
3.1. Comparative Coating Efficiencies of Fibronectin-, Heparin–Chitosan- and Albumin-Coated BNC Grafts
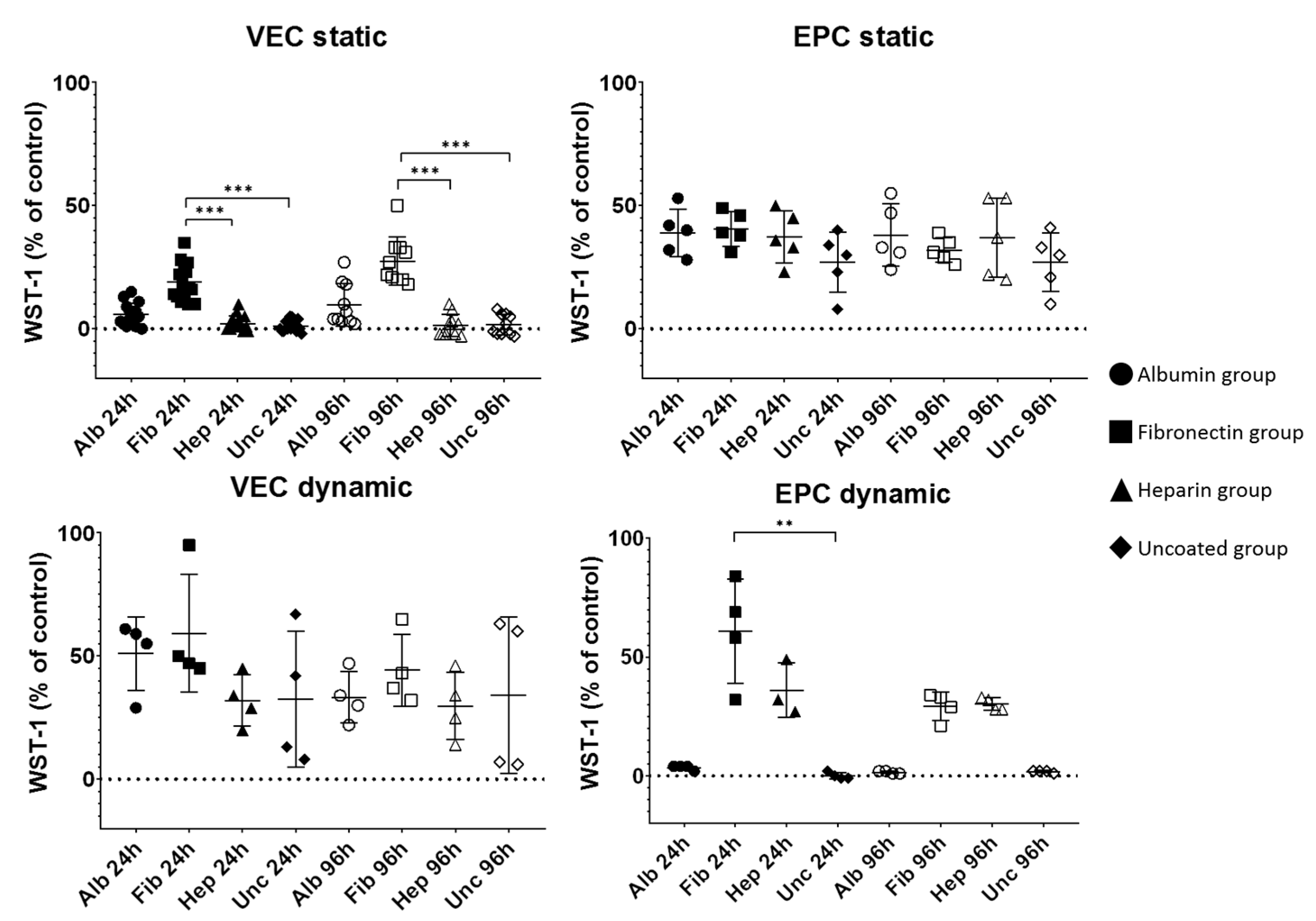
3.2. Progress of Metabolic Activity
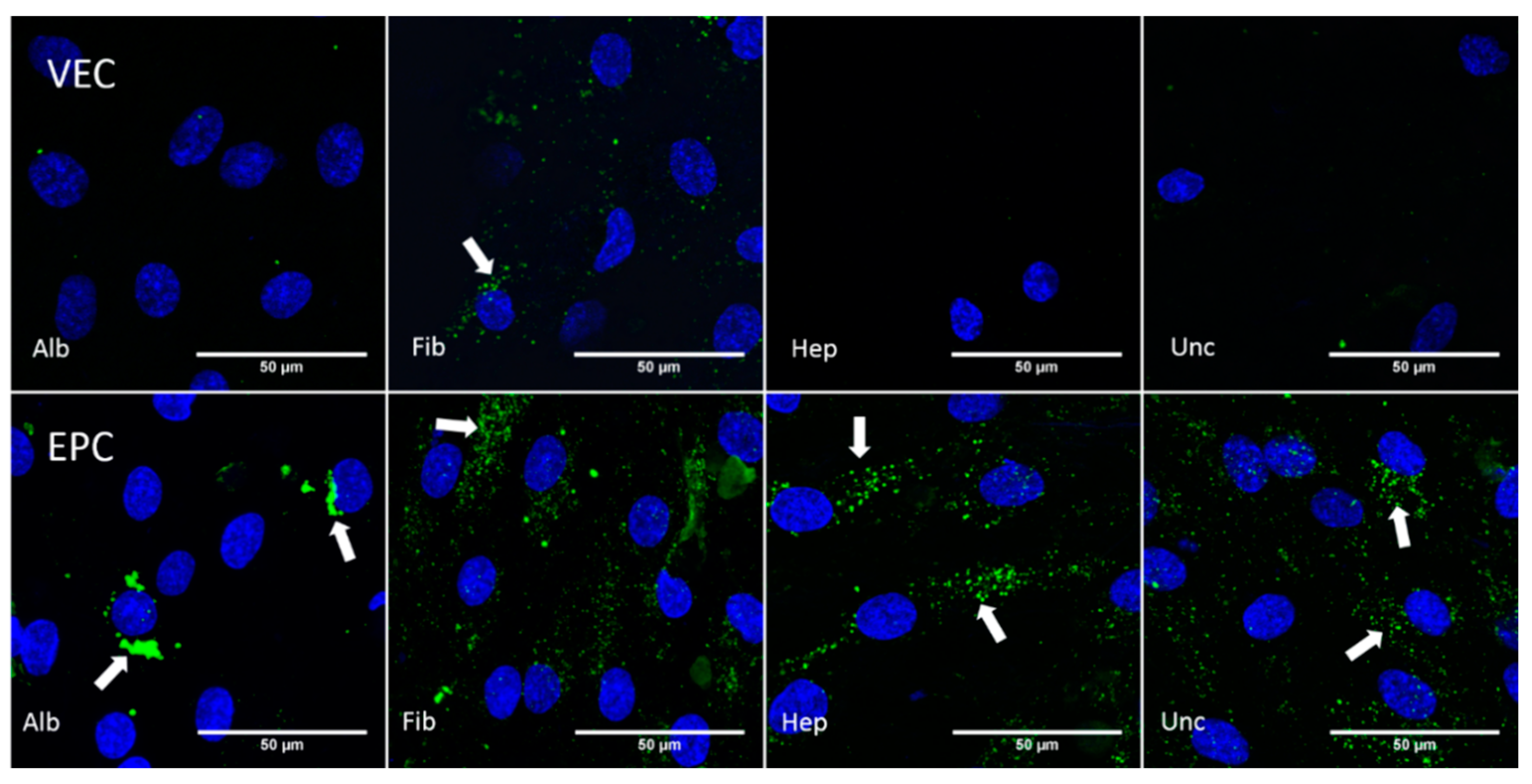
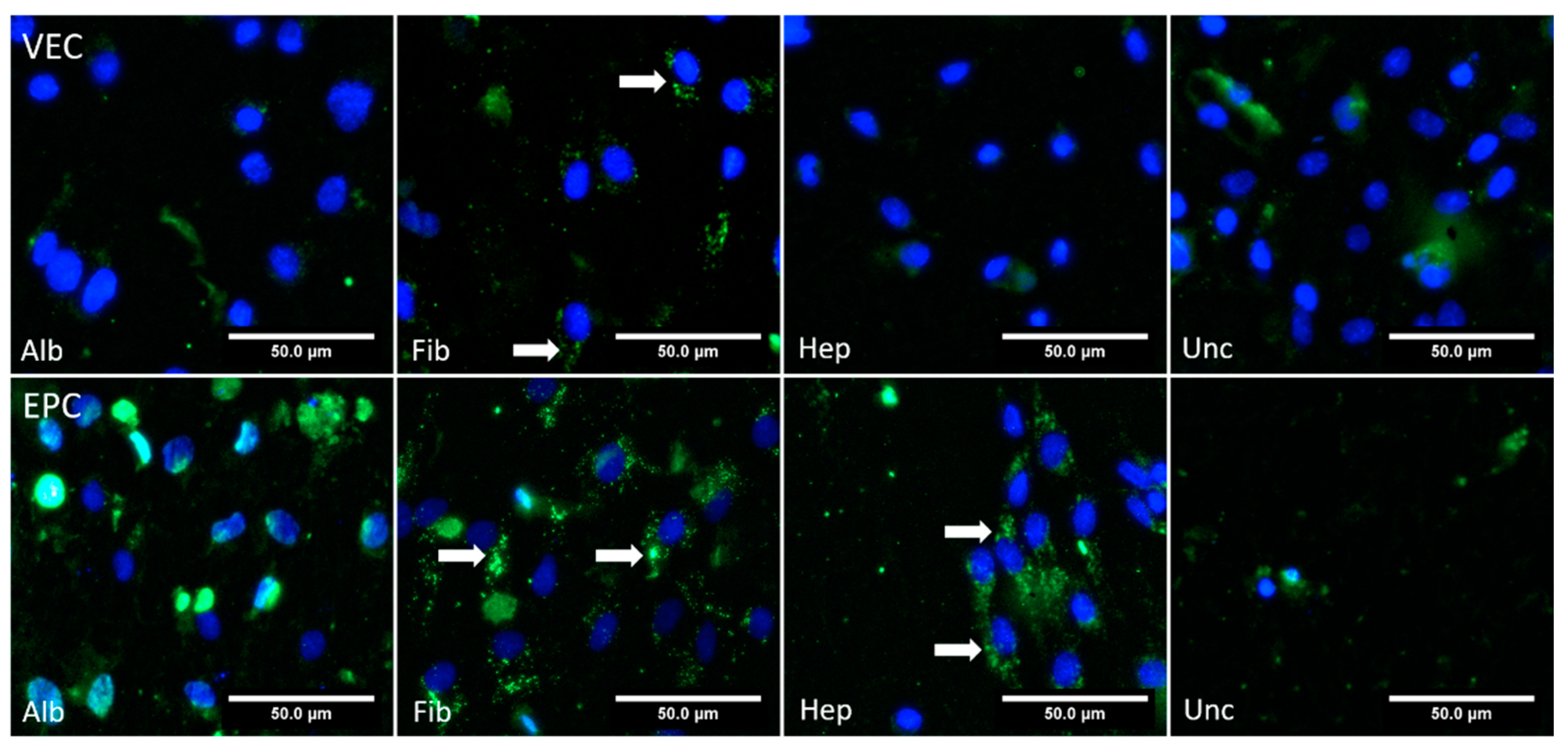
3.3. Histological Analyses
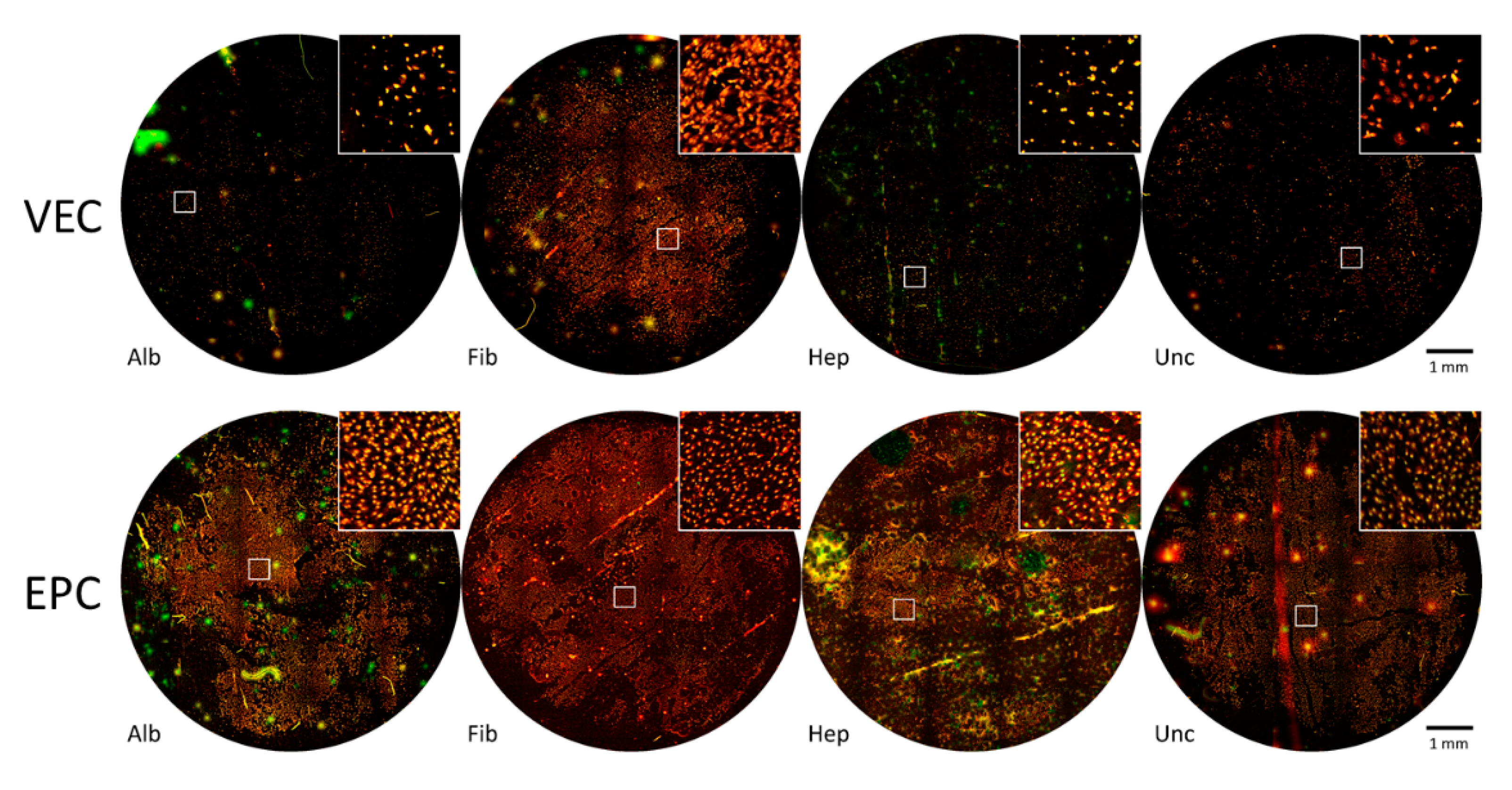
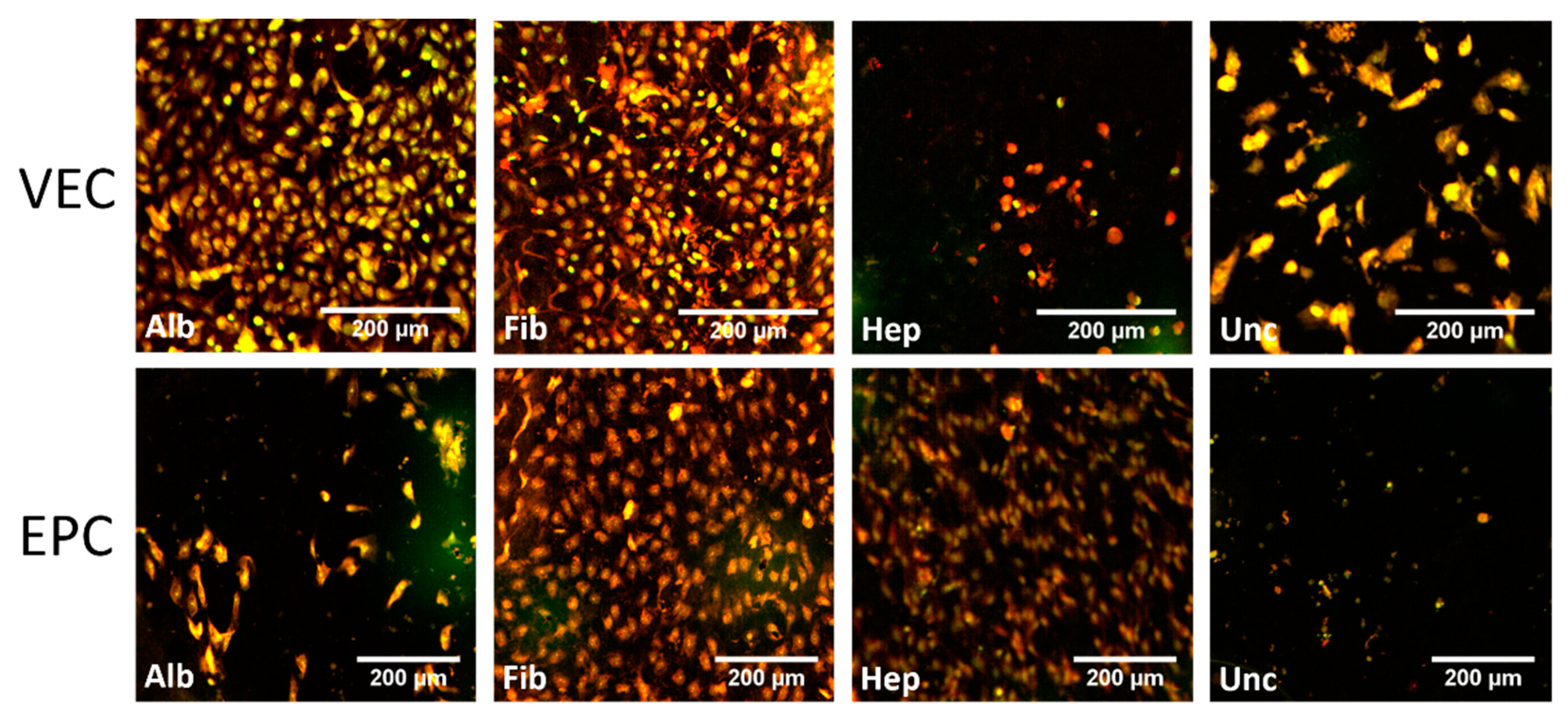
3.3.1. Graft Colonization under Static Conditions: Acridine Orange Staining
3.3.2. Graft Colonization under Dynamic Conditions: Acridine Orange Staining
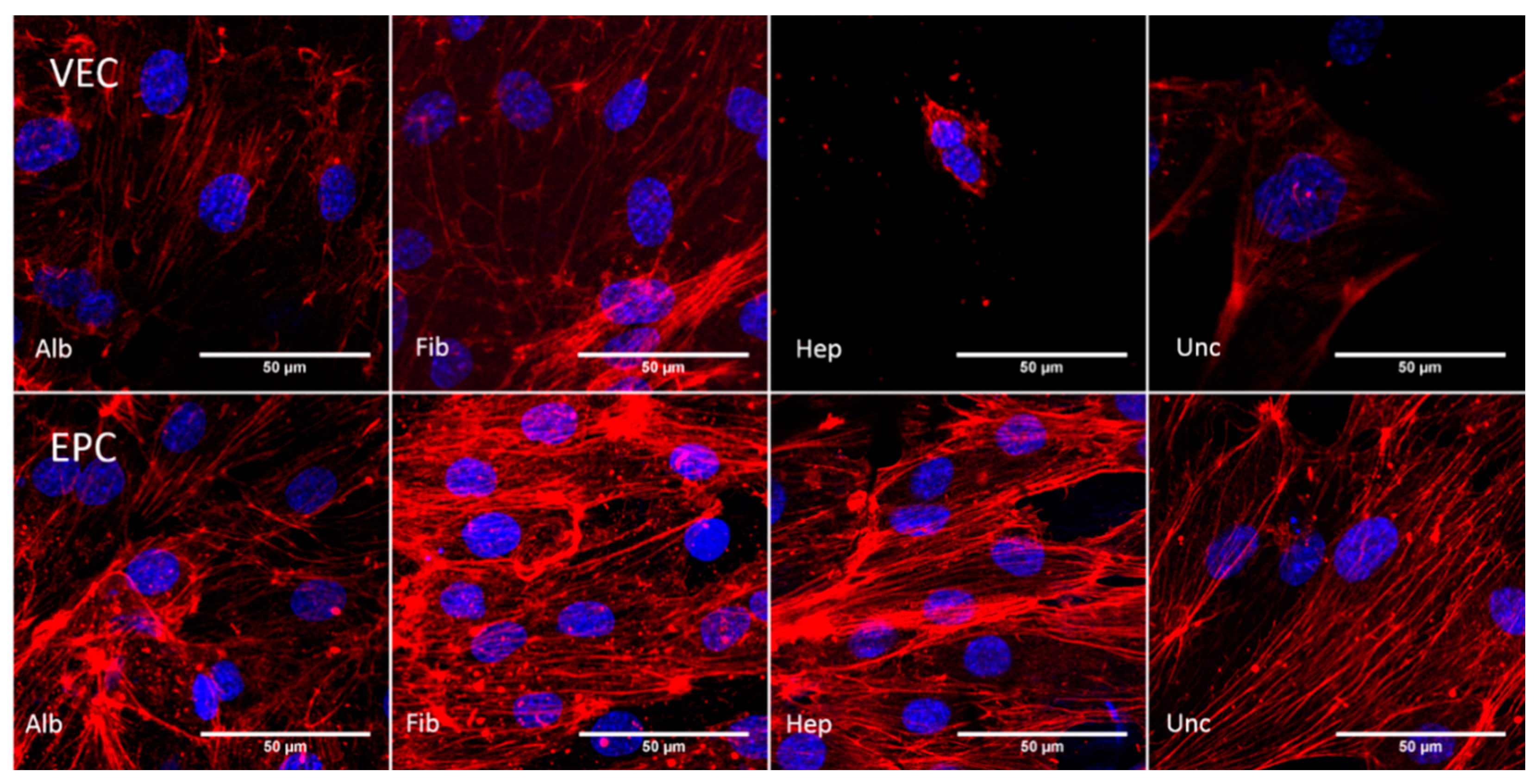
3.3.3. Cytoskeleton—Static Conditions
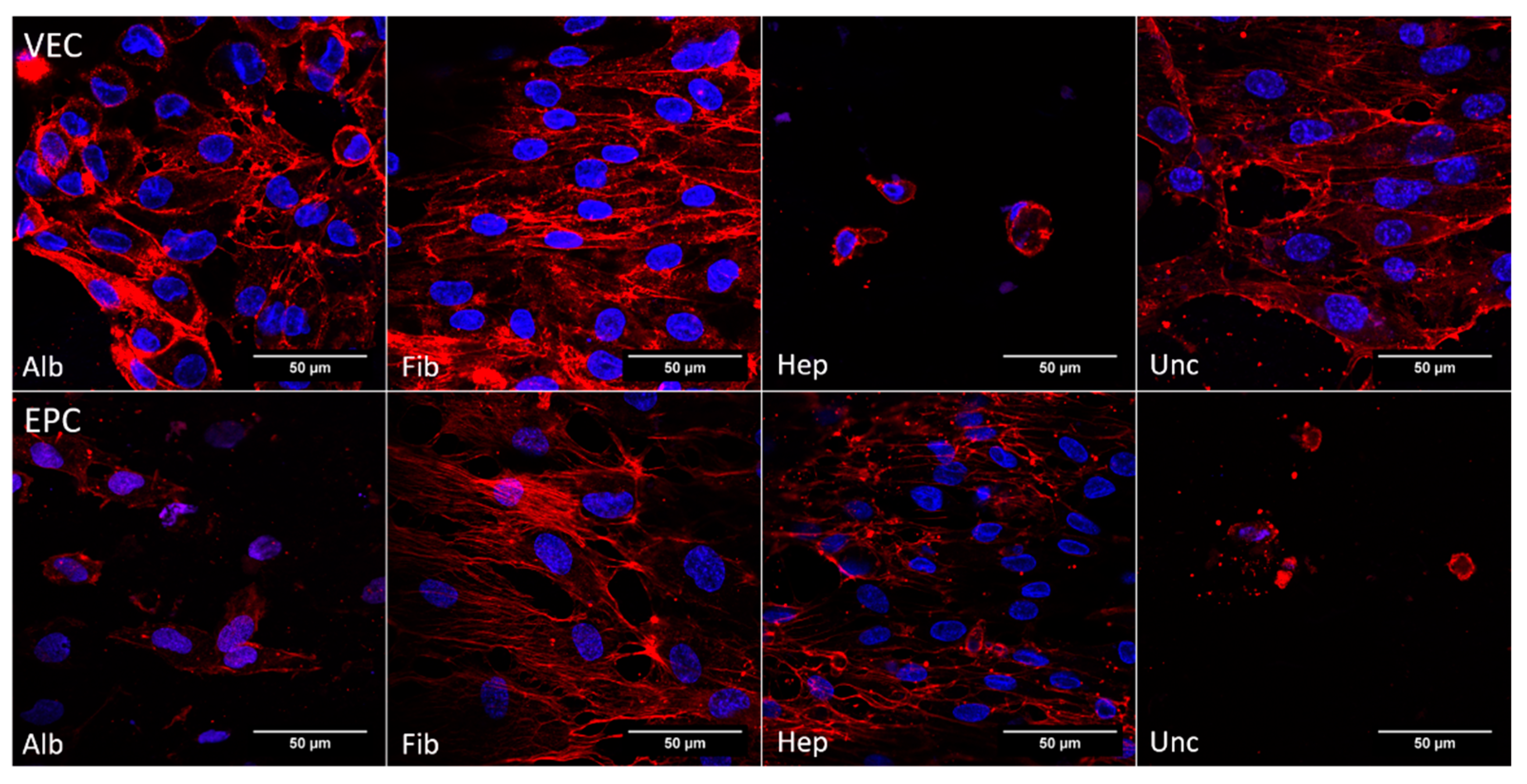
3.3.4. Cytoskeleton—Dynamic Conditions
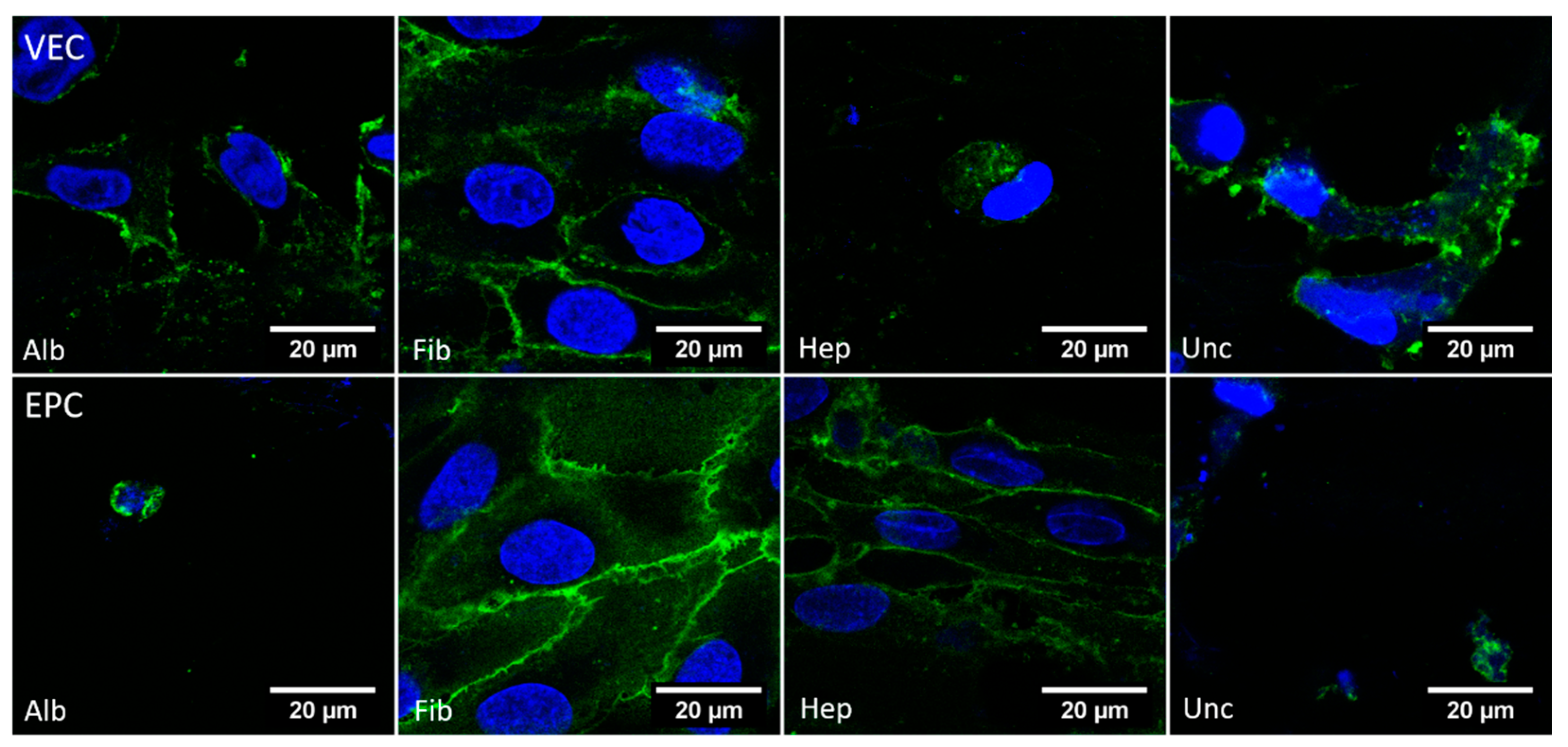
3.3.5. CD31—Dynamic Conditions
3.4. AcLDL Assay
3.4.1. Static Conditions
3.4.2. Dynamic Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diodato, M.; Chedrawy, E.G. Coronary artery bypass graft surgery: The past, present, and future of myocardial revascularisation. Surg. Res. Pract. 2014, 2014, 726158. [Google Scholar] [CrossRef]
- Beckmann, A.; Funkat, A.K.; Lewandowski, J.; Frie, M.; Ernst, M.; Hekmat, K.; Schiller, W.; Gummert, J.F.; Cremer, J.T. Cardiac Surgery in Germany during 2014: A Report on Behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2015, 63, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, B.; Reyes-Hernandez, C.G.; Quiroga-Garza, A.; Rodriguez-Rodriguez, V.E.; Esparza-Hernandez, C.N.; Elizondo-Omana, R.E.; Guzman-Lopez, S. Conduits Used in Coronary Artery Bypass Grafting: A Review of Morphological Studies. Ann. Thorac. Cardiovasc. Surg. 2017, 23, 55–65. [Google Scholar] [CrossRef] [PubMed]
- He, G.W. Arterial grafts for coronary artery bypass grafting: Biological characteristics, functional classification, and clinical choice. Ann. Thorac. Surg. 1999, 67, 277–284. [Google Scholar] [CrossRef]
- Gaudino, M.; Glieca, F.; Luciani, N.; Pragliola, C.; Tsiopoulos, V.; Bruno, P.; Farina, P.; Bonalumi, G.; Pavone, N.; Nesta, M.; et al. Systematic bilateral internal mammary artery grafting: Lessons learned from the CATHolic University EXtensive BIMA Grafting Study. Eur. J. Cardiothorac. Surg. 2018, 54, 702–707. [Google Scholar] [CrossRef]
- Wang, X.; Lin, P.; Yao, Q.; Chen, C. Development of small-diameter vascular grafts. World J. Surg. 2007, 31, 682–689. [Google Scholar] [CrossRef]
- Piccone, V. Alternative techniques in coronary artery reconstruction. In Modern Vascular Grafts; PN, S., Ed.; McGraw-Hill: New York, NY, USA, 1987; pp. 253–260. [Google Scholar]
- Gaudino, M.; Antoniades, C.; Benedetto, U.; Deb, S.; Di Franco, A.; Di Giammarco, G.; Fremes, S.; Glineur, D.; Grau, J.; He, G.W.; et al. Mechanisms, Consequences, and Prevention of Coronary Graft Failure. Circulation 2017, 136, 1749–1764. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev. 2015, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.T.; Wong, E.; Farhatnia, Y.; Tan, A.; Seifalian, A.M. Accelerating in situ endothelialisation of cardiovascular bypass grafts. Int. J. Mol. Sci. 2014, 16, 597–627. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Waltham, M.; Wadoodi, A.; Burnand, K.G.; Smith, A. The role of endothelial cells and their progenitors in intimal hyperplasia. Ther. Adv. Cardiovasc. Dis. 2010, 4, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention 2019, 14, 1435–1534. [Google Scholar] [CrossRef] [PubMed]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Healthc. Mater. 2018, 7, e1701461. [Google Scholar] [CrossRef] [PubMed]
- Scherner, M.; Reutter, S.; Klemm, D.; Sterner-Kock, A.; Guschlbauer, M.; Richter, T.; Langebartels, G.; Madershahian, N.; Wahlers, T.; Wippermann, J. In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: Proof of concept? J. Surg. Res. 2014, 189, 340–347. [Google Scholar] [CrossRef]
- Wippermann, J.; Schumann, D.; Klemm, D.; Kosmehl, H.; Salehi-Gelani, S.; Wahlers, T. Preliminary results of small arterial substitute performed with a new cylindrical biomaterial composed of bacterial cellulose. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Reinhardt, S.; Eghbalzadeh, K.; Wacker, M.; Guschlbauer, M.; Maul, A.; Sterner-Kock, A.; Wahlers, T.; Wippermann, J.; Scherner, M. Patency and in vivo compatibility of bacterial nanocellulose grafts as small-diameter vascular substitute. J. Vasc. Surg. 2018, 68, 177S–187S. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Kiesswetter, V.; Slottosch, I.; Awad, G.; Paunel-Gorgulu, A.; Varghese, S.; Klopfleisch, M.; Kupitz, D.; Klemm, D.; Nietzsche, S.; et al. In vitro hemo- and cytocompatibility of bacterial nanocelluose small diameter vascular grafts: Impact of fabrication and surface characteristics. PLoS ONE 2020, 15, e0235168. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, C.; Cheng, M.; Huang, J.; Liu, Q.; Yuan, G.; Lin, K.; Yu, H. Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact. Mater. 2021, 6, 1791–1809. [Google Scholar] [CrossRef]
- Avci-Adali, M.; Perle, N.; Ziemer, G.; Wendel, H.P. Current concepts and new developments for autologous in vivo endothelialisation of biomaterials for intravascular applications. Eur. Cell Mater. 2011, 21, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.S.; Viji, R.I.; Kiran, M.S.; Sudhakaran, P.R. Angiogenic Response of Endothelial Cells to Fibronectin; Springer: New York, NY, USA, 2012; pp. 131–151. [Google Scholar]
- Kuzmenko, V.; Samfors, S.; Hagg, D.; Gatenholm, P. Universal method for protein bioconjugation with nanocellulose scaffolds for increased cell adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4599–4607. [Google Scholar] [CrossRef]
- Li, X.; Tang, J.; Bao, L.; Chen, L.; Hong, F.F. Performance improvements of the BNC tubes from unique double-silicone-tube bioreactors by introducing chitosan and heparin for application as small-diameter artificial blood vessels. Carbohydr. Polym. 2017, 178, 394–405. [Google Scholar] [CrossRef]
- Bao, L.; Hong, F.F.; Li, G.; Hu, G.; Chen, L. Improved Performance of Bacterial Nanocellulose Conduits by the Introduction of Silk Fibroin Nanoparticles and Heparin for Small-Caliber Vascular Graft Applications. Biomacromolecules 2021, 22, 353–364. [Google Scholar] [CrossRef]
- Follmann, H.D.; Naves, A.F.; Martins, A.F.; Felix, O.; Decher, G.; Muniz, E.C.; Silva, R. Advanced fibroblast proliferation inhibition for biocompatible coating by electrostatic layer-by-layer assemblies of heparin and chitosan derivatives. J. Colloid Interface Sci. 2016, 474, 9–17. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Feng, Y.; Yang, Y.; Wei, Z.; Zhao, W.; Zhao, C. A chitosan modified asymmetric small-diameter vascular graft with anti-thrombotic and anti-bacterial functions for vascular tissue engineering. J. Mater. Chem. B 2020, 8, 568–577. [Google Scholar] [CrossRef]
- Aussel, A.; Thebaud, N.B.; Berard, X.; Brizzi, V.; Delmond, S.; Bareille, R.; Siadous, R.; James, C.; Ripoche, J.; Durand, M.; et al. Chitosan-based hydrogels for developing a small-diameter vascular graft: In vitro and in vivo evaluation. Biomed. Mater. 2017, 12, 065003. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Q.; Chen, J.; Luo, R.; Maitz, M.F.; Huang, N. Immobilization of serum albumin and peptide aptamer for EPC on polydopamine coated titanium surface for enhanced in-situ self-endothelialization. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.; Neumann, B.; Kurz, J.; Perle, N.; Avci-Adali, M.; Cattaneo, G.; Wendel, H.P. Preclinical evaluation of the thrombogenicity and endothelialization of bare metal and surface-coated neurovascular stents. AJNR Am. J. Neuroradiol. 2015, 36, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Marois, Y.; Chakfe, N.; Guidoin, R.; Duhamel, R.C.; Roy, R.; Marois, M.; King, M.W.; Douville, Y. An albumin-coated polyester arterial graft: In vivo assessment of biocompatibility and healing characteristics. Biomaterials 1996, 17, 3–14. [Google Scholar] [CrossRef]
- Tan, P.H.; Chan, C.; Xue, S.A.; Dong, R.; Ananthesayanan, B.; Manunta, M.; Kerouedan, C.; Cheshire, N.J.; Wolfe, J.H.; Haskard, D.O.; et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis 2004, 173, 171–183. [Google Scholar] [CrossRef]
- Haug, V.; Torio-Padron, N.; Stark, G.B.; Finkenzeller, G.; Strassburg, S. Comparison between endothelial progenitor cells and human umbilical vein endothelial cells on neovascularization in an adipogenesis mouse model. Microvasc. Res. 2015, 97, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.V.; Matthew, H.W. Vascular cell responses to polysaccharide materials: In vitro and in vivo evaluations. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef]
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of nanocellulose with a xyloglucan-RGD conjugate enhances adhesion and proliferation of endothelial cells: Implications for tissue engineering. Biomacromolecules 2007, 8, 3697–3704. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.; Ahrenstedt, L.; Bodin, A.; Brumer, H.; Gatenholm, P.; Krettek, A.; Risberg, B. Bacterial cellulose modified with xyloglucan bearing the adhesion peptide RGD promotes endothelial cell adhesion and metabolism—A promising modification for vascular grafts. J. Tissue. Eng. Regen. Med. 2011, 5, 454–463. [Google Scholar] [CrossRef]
- Jalali, S.; del Pozo, M.A.; Chen, K.; Miao, H.; Li, Y.; Schwartz, M.A.; Shyy, J.Y.; Chien, S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. USA 2001, 98, 1042–1046. [Google Scholar] [CrossRef]
- Klemm, D.; Marsch, S.; Schumann, D.; Udhardt, U. Method and Device for Producing Shaped Microbial Cellulose for Use as Biomaterial, Especially for Microsurgery. U.S. Patent US 2003/0013163 A1, 16 January 2003. [Google Scholar]
- Klemm, D.; Kopsch, V.; Koth, D.; Kramer, F.; Moritz, S.; Richter, T.; Ulrike, U.; Fried, W. Device, Used To Prepare Hollow Bodies Made Of Microbial Cellulose, Includes Template, A First Reservoir, Which Is Filled With A Mixture Comprising A Liquid Culture Medium And A Polymer-Forming Microorganism, A Wetting Device, And A Housing; Deutsches Patent- und Markenamt: Jena, Germany, 2013. [Google Scholar]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech nanocellulose: A review on progress in product design and today′s state of technical and medical applications. Carbohydr. Polym. 2021, 254, 117313. [Google Scholar] [CrossRef]
- Hercules, D.M. Electron Spectroscopy: Applications for Chemical Analysis. J. Chem. Educ. 2004, 81, 1751. [Google Scholar] [CrossRef]
- Hinrichs, W.L.J.; ten Hoopen, H.W.M.; Wissink, M.J.B.; Engbers, G.H.M.; Feijen, J. Design of a new type of coating for the controlled release of heparin. J. Control. Release 1997, 45, 163–176. [Google Scholar] [CrossRef][Green Version]
- Watkins, M.T.; Sharefkin, J.B.; Zajtchuk, R.; Maciag, T.M.; D′Amore, P.A.; Ryan, U.S.; Van Wart, H.; Rich, N.M. Adult human saphenous vein endothelial cells: Assessment of their reproductive capacity for use in endothelial seeding of vascular prostheses. J. Surg. Res. 1984, 36, 588–596. [Google Scholar] [CrossRef]
- Ormiston, M.L.; Toshner, M.R.; Kiskin, F.N.; Huang, C.J.; Groves, E.; Morrell, N.W.; Rana, A.A. Generation and Culture of Blood Outgrowth Endothelial Cells from Human Peripheral Blood. J. Vis. Exp. 2015, 106, e53384. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Kado, T.; Sakai, J.; Karino, T. The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC-SMC coculture. Ann. Biomed. Eng. 2004, 32, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Shnyra, A.; Lindberg, A.A. Scavenger receptor pathway for lipopolysaccharide binding to Kupffer and endothelial liver cells in vitro. Infect. Immun. 1995, 63, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Voyta, J.C.; Via, D.P.; Butterfield, C.E.; Zetter, B.R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J. Cell Biol. 1984, 99, 2034–2040. [Google Scholar] [CrossRef]
- Melchiorri, A.J.; Bracaglia, L.G.; Kimerer, L.K.; Hibino, N.; Fisher, J.P. In Vitro Endothelialization of Biodegradable Vascular Grafts Via Endothelial Progenitor Cell Seeding and Maturation in a Tubular Perfusion System Bioreactor. Tissue. Eng. Part. C Methods 2016, 22, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Schuerlein, S.; Schwarz, T.; Krziminski, S.; Gatzner, S.; Hoppensack, A.; Schwedhelm, I.; Schweinlin, M.; Walles, H.; Hansmann, J. A versatile modular bioreactor platform for Tissue Engineering. Biotechnol. J. 2017, 12, 1600326. [Google Scholar] [CrossRef] [PubMed]
- Hulsmann, J.; Aubin, H.; Kranz, A.; Godehardt, E.; Munakata, H.; Kamiya, H.; Barth, M.; Lichtenberg, A.; Akhyari, P. A novel customizable modular bioreactor system for whole-heart cultivation under controlled 3D biomechanical stimulation. J. Artif. Organs 2013, 16, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.A.; Deng, K.; Liu, T.; Chen, J.Y.; Huang, N. The endothelialization and hemocompatibility of the functional multilayer on titanium surface constructed with type IV collagen and heparin. Colloids Surf. B Biointerfaces 2013, 108, 295–304. [Google Scholar] [CrossRef]
- Avari, H.; Rogers, K.A.; Savory, E. Quantification of Morphological Modulation, F-Actin Remodeling and PECAM-1 (CD-31) Re-distribution in Endothelial Cells in Response to Fluid-Induced Shear Stress under Various Flow Conditions. J. Biomech. Eng. 2019, 141, 041004-1–041004-12. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.; Jonak, P.; Leask, R.L. Statin therapy influences endothelial cell morphology and F-actin cytoskeleton structure when exposed to static and laminar shear stress conditions. Life Sci. 2013, 92, 859–865. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Hytonen, J.P.; Leppanen, O.; Taavitsainen, J.; Korpisalo, P.; Laidinen, S.; Alitalo, K.; Wadstrom, J.; Rissanen, T.T.; Yla-Herttuala, S. Improved endothelialization of small-diameter ePTFE vascular grafts through growth factor therapy. Vasc. Biol. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Budd, J.S.; Allen, K.E.; Hartley, G.; Bell, P.R. The effect of preformed confluent endothelial cell monolayers on the patency and thrombogenicity of small calibre vascular grafts. Eur. J. Vasc. Surg. 1991, 5, 397–405. [Google Scholar] [CrossRef]
- James, N.L.; Schindhelm, K.; Slowiaczek, P.; Milthorpe, B.; Graham, A.R.; Munro, V.F.; Johnson, G.; Steele, J.G. In vivo patency of endothelial cell-lined expanded polytetrafluoroethylene prostheses in an ovine model. Artif. Organs 1992, 16, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Fan, Y.; Xiao, W.; Liu, R.; Pivetti, C.; Walimbe, T.; Guo, F.; Zhang, X.; Farmer, D.L.; Wang, F.; et al. Rapid endothelialization of small diameter vascular grafts by a bioactive integrin-binding ligand specifically targeting endothelial progenitor cells and endothelial cells. Acta. Biomater. 2020, 108, 178–193. [Google Scholar] [CrossRef]
- Samano, N.; Geijer, H.; Liden, M.; Fremes, S.; Bodin, L.; Souza, D. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J. Thorac. Cardiovasc. Surg. 2015, 150, 880–888. [Google Scholar] [CrossRef]
- Dashwood, M.R.; Savage, K.; Tsui, J.C.; Dooley, A.; Shaw, S.G.; Fernandez Alfonso, M.S.; Bodin, L.; Souza, D.S. Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension-induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J. Thorac. Cardiovasc. Surg. 2009, 138, 334–340. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, R.; Chen, H.; Lu, Y.; Zhou, J.; Chang, X.; Qiu, G.; Wu, Z.; Yang, G. Investigation on artificial blood vessels prepared from bacterial cellulose. Mater Sci. Eng. C Mater Biol. Appl. 2015, 46, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.K.; Costa, R.; Domingues, L.; Soares, R.; Gama, M. Improving bacterial cellulose for blood vessel replacement: Functionalization with a chimeric protein containing a cellulose-binding module and an adhesion peptide. Acta. Biomater. 2010, 6, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.H.; Lim, K.T.; Seonwoo, H.; Park, S.H.; Kim, Y.R.; Kim, Y.; Choung, Y.H.; Choung, P.H.; Chung, J.H. Charged nanomatrices as efficient platforms for modulating cell adhesion and shape. Tissue Eng. Part C Methods 2012, 18, 913–923. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Johns, M.A.; Galembeck, F.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Scott, J.L.; Sharma, R.I. Surface modified cellulose scaffolds for tissue engineering. Cellulose 2017, 24, 253–267. [Google Scholar] [CrossRef]
- Lindl, T.; Gstraunthaler, G. Zell- und Gewebekultur, 6th ed.; Spektrum Akademischer Verlag: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Watanabe, K.; Eto, Y.; Takano, S.; Nakamori, S.; Shibai, H.; Yamanaka, S. A new bacterial cellulose substrate for mammalian cell culture. A new bacterial cellulose substrate. Cytotechnology 1993, 13, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Kolmannskog, O.; Tenstad, O.; Bert, J.L. Effect of charge on interstitial distribution of albumin in rat dermis in vitro. J. Physiol. 2003, 550, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Baler, K.; Martin, O.A.; Carignano, M.A.; Ameer, G.A.; Vila, J.A.; Szleifer, I. Electrostatic unfolding and interactions of albumin driven by pH changes: A molecular dynamics study. J. Phys. Chem. B 2014, 118, 921–930. [Google Scholar] [CrossRef]
- Chokhawala, H.A.; Yu, H.; Chen, X. Enzymatic Approaches to O-Glycoside Introduction: Glycosyltransferases☆. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef] [PubMed]
- Sami El-banna, F.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.; Hanafy, N.A.N. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels. Appl. Sci. 2019, 9, 2193. [Google Scholar] [CrossRef]
- He, S.; Chen, B.; Li, W.; Yan, J.; Chen, L.; Wang, X.; Xiao, Y. Ventilator-associated pneumonia after cardiac surgery: A meta-analysis and systematic review. J. Thorac. Cardiovasc. Surg. 2014, 148, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Mie, M.; Mihara, H.; Nakamura, M.; Kobatake, E. Construction of multi-functional extracellular matrix proteins that promote tube formation of endothelial cells. Biomaterials 2008, 29, 2977–2986. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Andrade, F.K.; Silva, J.P.; Carvalho, M.; Castanheira, E.M.; Soares, R.; Gama, M. Studies on the hemocompatibility of bacterial cellulose. J. Biomed. Mater. Res. A 2011, 98, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Tersteeg, C.; Roest, M.; Mak-Nienhuis, E.M.; Ligtenberg, E.; Hoefer, I.E.; de Groot, P.G.; Pasterkamp, G. A fibronectin-fibrinogen-tropoelastin coating reduces smooth muscle cell growth but improves endothelial cell function. J. Cell Mol. Med. 2012, 16, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Bramfeldt, H.; Vermette, P. Enhanced smooth muscle cell adhesion and proliferation on protein-modified polycaprolactone-based copolymers. J. Biomed. Mater. Res. A 2009, 88, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Nishibe, T.; Miura, H.; Hazama, K.; Kato, H.; Kudo, F.; Murashita, T.; Okuda, Y. Improved healing of small-caliber, long-fibril expanded polytetrafluoroethylene vascular grafts by covalent bonding of fibronectin. Surg. Today 2004, 34, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, T.; Okuda, Y.; Kumada, T.; Tanabe, T.; Yasuda, K. Enhanced graft healing of high-porosity expanded polytetrafluoroethylene grafts by covalent bonding of fibronectin. Surg. Today 2000, 30, 426–431. [Google Scholar] [CrossRef]
- Seeger, J.M.; Klingman, N. Improved in vivo endothelialization of prosthetic grafts by surface modification with fibronectin. J. Vasc. Surg. 1988, 8, 476–482. [Google Scholar] [CrossRef]
- De Visscher, G.; Mesure, L.; Meuris, B.; Ivanova, A.; Flameng, W. Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1α. Acta Biomater. 2012, 8, 1330–1338. [Google Scholar] [CrossRef]
- Thomson, G.J.; Vohra, R.K.; Carr, M.H.; Walker, M.G. Adult human endothelial cell seeding using expanded polytetrafluoroethylene vascular grafts: A comparison of four substrates. Surgery 1991, 109, 20–27. [Google Scholar] [PubMed]
- Vohra, R.; Thomson, G.J.; Carr, H.M.; Sharma, H.; Walker, M.G. The response of rapidly formed adult human endothelial-cell monolayers to shear stress of flow: A comparison of fibronectin-coated Teflon and gelatin-impregnated Dacron grafts. Surgery 1992, 111, 210–220. [Google Scholar] [PubMed]
- Seeger, J.M.; Klingman, N. Improved endothelial cell seeding with cultured cells and fibronectin-coated grafts. J. Surg. Res. 1985, 38, 641–647. [Google Scholar] [CrossRef]
- Osorio, M.; Ortiz, I.; Ganan, P.; Naranjo, T.; Zuluaga, R.; van Kooten, T.G.; Castro, C. Novel surface modification of three-dimensional bacterial nanocellulose with cell-derived adhesion proteins for soft tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 697–705. [Google Scholar] [CrossRef]
- Daum, R.; Visser, D.; Wild, C.; Kutuzova, L.; Schneider, M.; Lorenz, G.; Weiss, M.; Hinderer, S.; Stock, U.A.; Seifert, M.; et al. Fibronectin Adsorption on Electrospun Synthetic Vascular Grafts Attracts Endothelial Progenitor Cells and Promotes Endothelialization in Dynamic In Vitro Culture. Cells 2020, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Wijelath, E.S.; Rahman, S.; Murray, J.; Patel, Y.; Savidge, G.; Sobel, M. Fibronectin promotes VEGF-induced CD34 cell differentiation into endothelial cells. J. Vasc. Surg. 2004, 39, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.S.; Berger, B.W.; Billings, P.C. The structure and function of platelet integrins. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 200–205. [Google Scholar] [CrossRef]
- Kieffer, N.; Melchior, C.; Guinet, J.M.; Michels, S.; Gouon, V.; Bron, N. Serine 752 in the cytoplasmic domain of the beta 3 integrin subunit is not required for alpha v beta 3 postreceptor signaling events. Cell Adhes. Commun. 1996, 4, 25–39. [Google Scholar] [CrossRef]
- Pytela, R.; Pierschbacher, M.D.; Ginsberg, M.H.; Plow, E.F.; Ruoslahti, E. Platelet membrane glycoprotein IIb/IIIa: Member of a family of Arg-Gly-Asp--specific adhesion receptors. Science 1986, 231, 1559–1562. [Google Scholar] [CrossRef] [PubMed]
- Beumer, S.; MJ, I.J.; de Groot, P.G.; Sixma, J.J. Platelet adhesion to fibronectin in flow: Dependence on surface concentration and shear rate, role of platelet membrane glycoproteins GP IIb/IIIa and VLA-5, and inhibition by heparin. Blood 1994, 84, 3724–3733. [Google Scholar] [CrossRef]
- Stellos, K.; Langer, H.; Daub, K.; Schoenberger, T.; Gauss, A.; Geisler, T.; Bigalke, B.; Mueller, I.; Schumm, M.; Schaefer, I.; et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation 2008, 117, 206–215. [Google Scholar] [CrossRef]
- Stronck, J.W.; van der Lei, B.; Wildevuur, C.R. Improved healing of small-caliber polytetrafluoroethylene vascular prostheses by increased hydrophilicity and by enlarged fibril length. An experimental study in rats. J. Thorac. Cardiovasc. Surg. 1992, 103, 146–152. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Ipema, J.; Brand, A.R.; GJ, D.E.B.; JP, D.E.V.; Unl, U.C. Antiplatelet and anticoagulation therapy after revascularization for lower extremity artery disease: A national survey and literature overview. J. Cardiovasc. Surg. 2021, 62, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Braghirolli, D.I.; Helfer, V.E.; Chagastelles, P.C.; Dalberto, T.P.; Gamba, D.; Pranke, P. Electrospun scaffolds functionalized with heparin and vascular endothelial growth factor increase the proliferation of endothelial progenitor cells. Biomed. Mater. 2017, 12, 025003. [Google Scholar] [CrossRef]
- Mazzucotelli, J.P.; Bertrand, P.; Benhaiem-Sigaux, N.; Leandri, J.; Loisance, D.Y. In vitro and in vivo evaluation of a small caliber vascular prosthesis fixed with a polyepoxy compound. Artif. Organs 1995, 19, 896–901. [Google Scholar] [CrossRef]
- Schaeffer, U.; Tanner, B.; Strohschneider, T.; Stadtmuller, A.; Hannekum, A. Damage to arterial and venous endothelial cells in bypass grafts induced by several solutions used in bypass surgery. Thorac. Cardiovasc. Surg. 1997, 45, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, S.; Murawsky, M.; Carpentier, A. Cytotoxicity of cardioplegic solutions: Evaluation by tissue culture. Circulation 1981, 64, II90–II95. [Google Scholar] [PubMed]
- Zoellner, H.; Hofler, M.; Beckmann, R.; Hufnagl, P.; Vanyek, E.; Bielek, E.; Wojta, J.; Fabry, A.; Lockie, S.; Binder, B.R. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J. Cell Sci. 1996, 109, 2571–2580. [Google Scholar] [CrossRef]
- Zhang, W.J.; Frei, B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc. Res. 2002, 55, 820–829. [Google Scholar] [CrossRef]
- Shen, M.; Martinson, L.; Wagner, M.S.; Castner, D.G.; Ratner, B.D.; Horbett, T.A. PEO-like plasma polymerized tetraglyme surface interactions with leukocytes and proteins: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2002, 13, 367–390. [Google Scholar] [CrossRef]
- Denizli, F.K.; Guven, O. Competitive adsorption of blood proteins on gamma-irradiated-polycarbonate films. J. Biomater. Sci. Polym. Ed. 2002, 13, 127–139. [Google Scholar] [CrossRef] [PubMed]
- McGee, G.S.; Shuman, T.A.; Atkinson, J.B.; Weaver, F.A.; Edwards, W.H. Experimental evaluation of a new albumin-impregnated knitted dacron prosthesis. Am. Surg. 1987, 53, 695–701. [Google Scholar]
- Fuchs, S.; Dohle, E.; Kolbe, M.; Kirkpatrick, C.J. Outgrowth endothelial cells: Sources, characteristics and potential applications in tissue engineering and regenerative medicine. Adv. Biochem. Eng. Biotechnol. 2010, 123, 201–217. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.H.; Kim, H.S.; Choi, J.H.; Kang, H.J.; Hwang, K.K.; Oh, B.H.; Lee, M.M.; Park, Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arter. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Chang, S.J.; Chueh, Y.N.; Huang, T.S.; Huang, P.H.; Cheng, S.M.; Tsai, T.N.; Chen, J.W.; Wang, H.W. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genomics 2013, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, K.; Elliot, W.; Tan, Y.; Monnet, E.; Tan, W. Performance of marrow stromal cell-seeded small-caliber multilayered vascular graft in a senescent sheep model. Biomed. Mater. 2018, 13, 055004. [Google Scholar] [CrossRef] [PubMed]
- Heise, M.; Schmidmaier, G.; Husmann, I.; Heidenhain, C.; Schmidt, J.; Neuhaus, P.; Settmacher, U. PEG-hirudin/iloprost coating of small diameter ePTFE grafts effectively prevents pseudointima and intimal hyperplasia development. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 418–424. [Google Scholar] [CrossRef]
- Clowes, A.W.; Karnowsky, M.J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature 1977, 265, 625–626. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacker, M.; Riedel, J.; Walles, H.; Scherner, M.; Awad, G.; Varghese, S.; Schürlein, S.; Garke, B.; Veluswamy, P.; Wippermann, J.; et al. Comparative Evaluation on Impacts of Fibronectin, Heparin–Chitosan, and Albumin Coating of Bacterial Nanocellulose Small-Diameter Vascular Grafts on Endothelialization In Vitro. Nanomaterials 2021, 11, 1952. https://doi.org/10.3390/nano11081952
Wacker M, Riedel J, Walles H, Scherner M, Awad G, Varghese S, Schürlein S, Garke B, Veluswamy P, Wippermann J, et al. Comparative Evaluation on Impacts of Fibronectin, Heparin–Chitosan, and Albumin Coating of Bacterial Nanocellulose Small-Diameter Vascular Grafts on Endothelialization In Vitro. Nanomaterials. 2021; 11(8):1952. https://doi.org/10.3390/nano11081952
Chicago/Turabian StyleWacker, Max, Jan Riedel, Heike Walles, Maximilian Scherner, George Awad, Sam Varghese, Sebastian Schürlein, Bernd Garke, Priya Veluswamy, Jens Wippermann, and et al. 2021. "Comparative Evaluation on Impacts of Fibronectin, Heparin–Chitosan, and Albumin Coating of Bacterial Nanocellulose Small-Diameter Vascular Grafts on Endothelialization In Vitro" Nanomaterials 11, no. 8: 1952. https://doi.org/10.3390/nano11081952
APA StyleWacker, M., Riedel, J., Walles, H., Scherner, M., Awad, G., Varghese, S., Schürlein, S., Garke, B., Veluswamy, P., Wippermann, J., & Hülsmann, J. (2021). Comparative Evaluation on Impacts of Fibronectin, Heparin–Chitosan, and Albumin Coating of Bacterial Nanocellulose Small-Diameter Vascular Grafts on Endothelialization In Vitro. Nanomaterials, 11(8), 1952. https://doi.org/10.3390/nano11081952






