Cyanine-5-Driven Behaviours of Hyperbranched Polymers Designed for Therapeutic Delivery Are Cell-Type Specific and Correlated with Polar Lipid Distribution in Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Synthesis and Characterisation
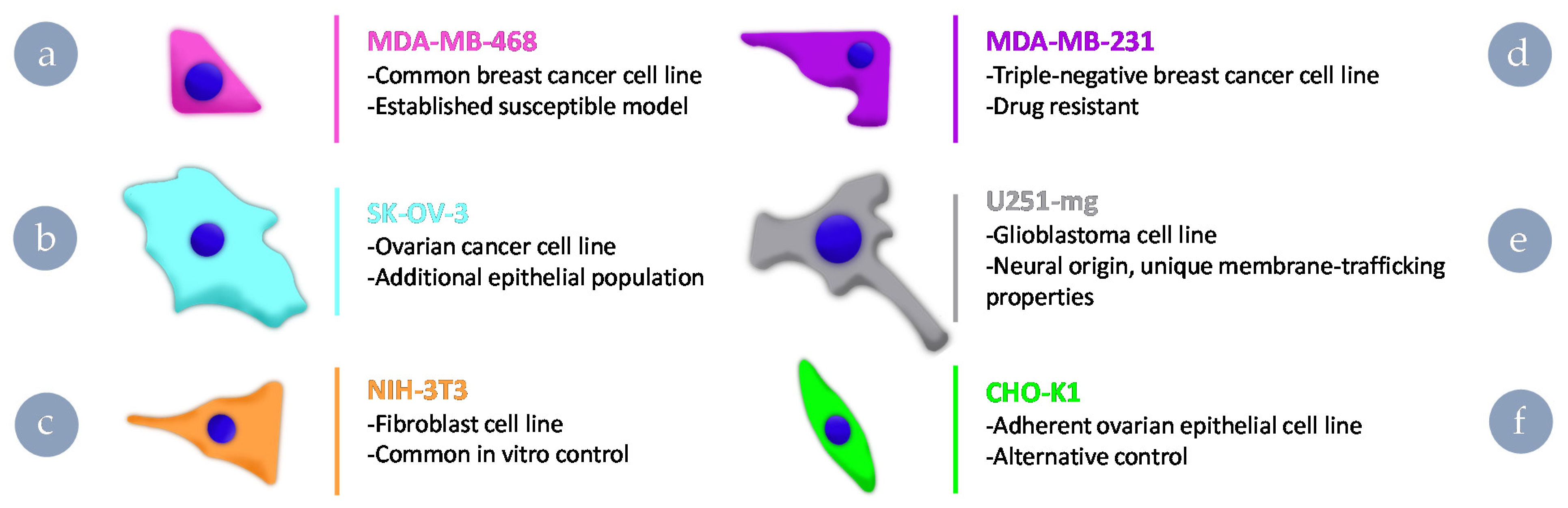
2.2. Cell Culture
2.3. Live Cell Confocal Microscopy
2.4. Internalisation Kinetics
2.5. Calcein Assay
2.6. Nile Red Staining
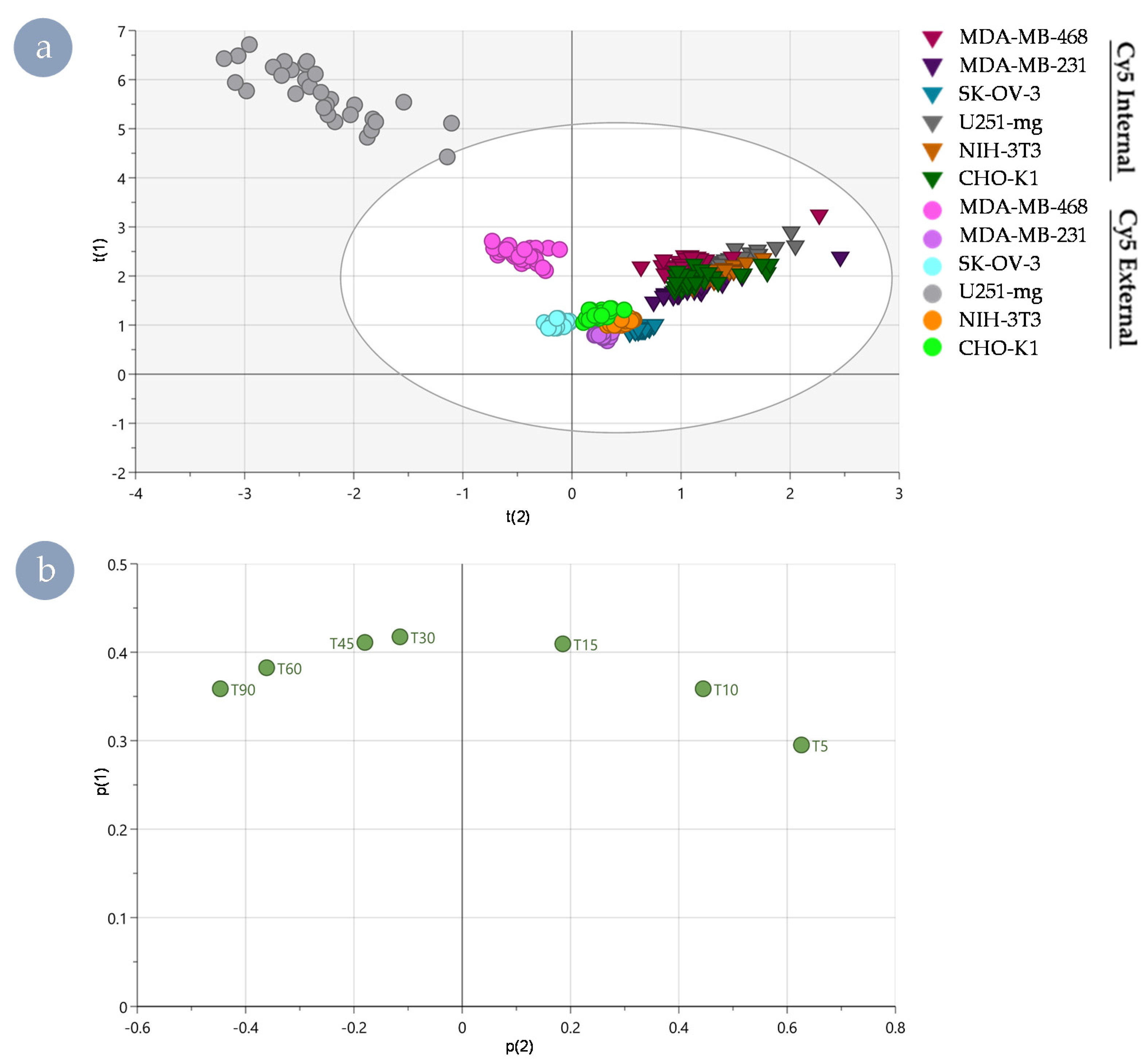
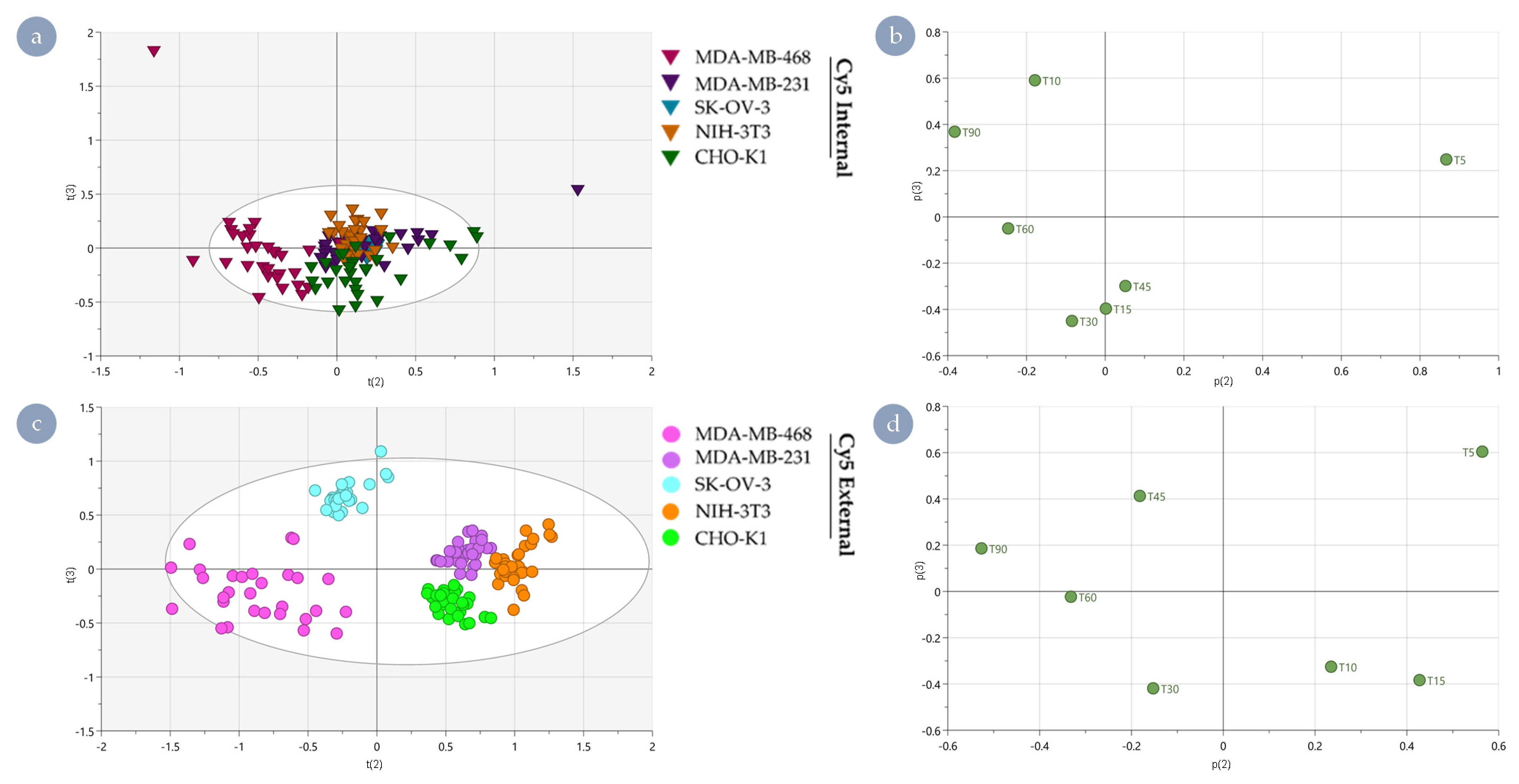
2.7. Principal Component Analysis of Rate Kinetic Data
2.8. Correlation Analysis
3. Results
3.1. Cy5-Labelled HBP Syntheses and Characterisation
3.2. Uptake Behaviours of Cy5-Labelled HBPs in Different Cell Lines
3.3. Distinguishing Underlying Factors through Principal Component Analysis
3.4. Endosomal Escape Behaviours of Cy5 HBPs across Cell Lines
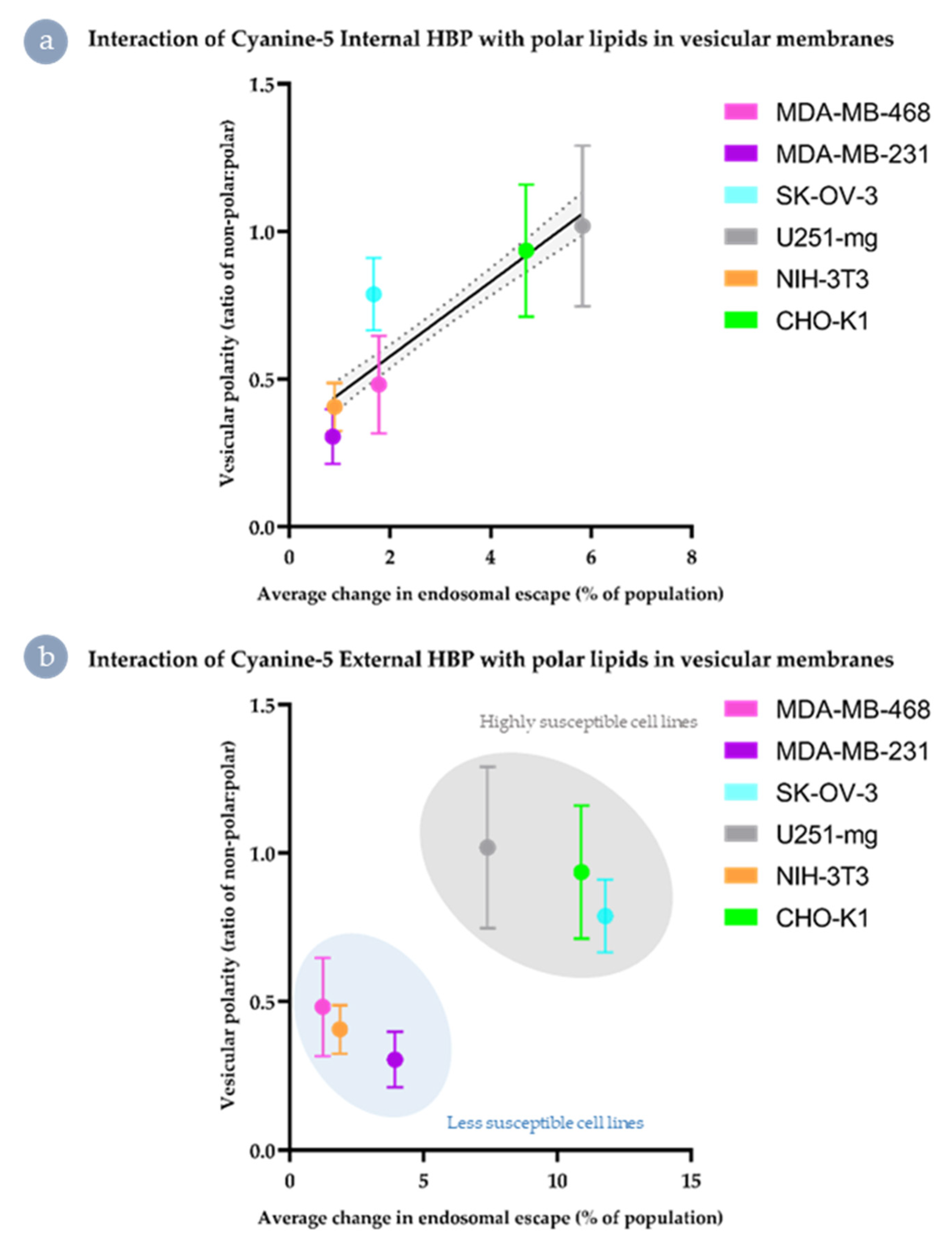
3.5. Both Internalisation Rates and Endosomal Escape Ability Are Correlated with Polar Lipid Distribution
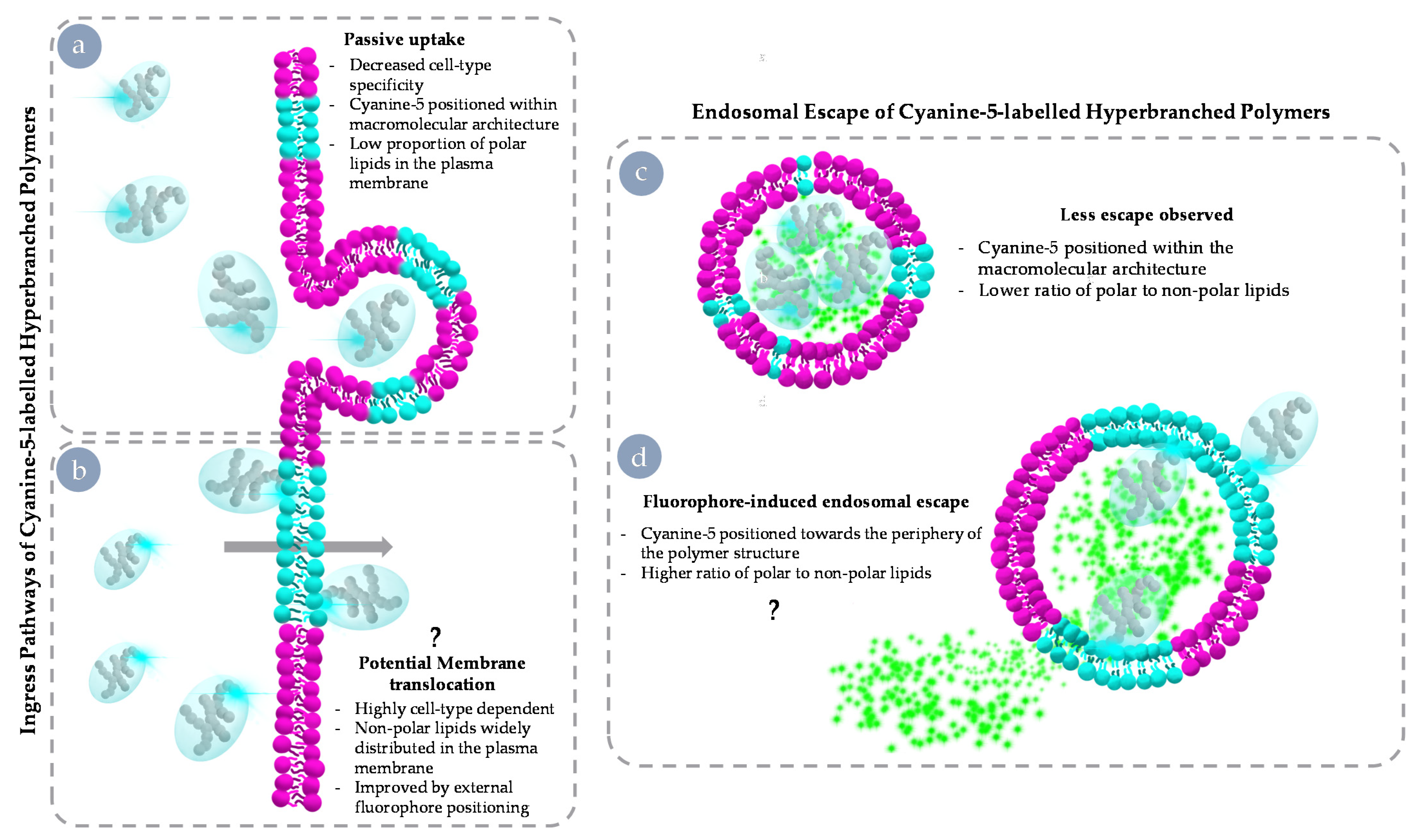
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Simpson, J.D.; Smith, S.A.; Thurecht, K.J.; Such, G.K. Engineered polymeric materials for biological applications: Overcoming challenges of the bio-nano interface. Polymers 2019, 11, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, V.; Liu, Z.; Hirvonen, J.; Santos, H.A. Bridging the Knowledge of Different Worlds to Understand the Big Picture of Cancer Nanomedicines. Adv. Healthc. Mater. 2018, 7, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1893–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014–16026. [Google Scholar] [CrossRef]
- Fletcher, N.L.; Kempe, K.; Thurecht, K.J. Next-Generation Polymeric Nanomedicines for Oncology: Perspectives and Future Directions. Macromol. Rapid Commun. 2020, 41, 2000319. [Google Scholar] [CrossRef]
- Ediriweera, G.R.; Simpson, J.D.; Fuchs, A.V.; Venkatachalam, T.K.; Van De Walle, M.; Howard, C.B.; Mahler, S.M.; Blinco, J.P.; Fletcher, N.L.; Houston, Z.H.; et al. Targeted and modular architectural polymers employing bioorthogonal chemistry for quantitative therapeutic delivery. Chem. Sci. 2020, 11, 3268–3280. [Google Scholar] [CrossRef] [Green Version]
- Cai, K.; Wang, A.Z.; Yin, L.; Cheng, J. Bio-nano interface: The impact of biological environment on nanomaterials and their delivery properties. J. Control. Release 2017, 263, 211–222. [Google Scholar] [CrossRef]
- Qiu, T.A.; Clement, P.L.; Haynes, C.L. Linking nanomaterial properties to biological outcomes: Analytical chemistry challenges in nanotoxicology for the next decade. Chem. Commun. 2018, 54, 12787–12803. [Google Scholar] [CrossRef]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Philippe, G.J.B.; Gaspar, D.; Sheng, C.; Huang, Y.H.; Benfield, A.H.; Condon, N.D.; Weidmann, J.; Lawrence, N.; Lower, A.; Castanho, M.A.; et al. Cell Membrane Composition Drives Selectivity and Toxicity of Designed Cyclic Helix–Loop–Helix Peptides with Cell Penetrating and Tumor Suppressor Properties. ACS Chem. Biol. 2019, 14, 2071–2087. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, C.; Zoschke, C.; Wolff, C.; Darvin, M.E.; Sochorová, M.; Kováčik, A.; Wanjiku, B.; Schumacher, F.; Tigges, J.; Kleuser, B.; et al. Fibroblast origin shapes tissue homeostasis, epidermal differentiation, and drug uptake. Sci. Rep. 2019, 9, 2913. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Kettler, K.; Veltman, K.; van de Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.T.; White, T.F.; Deal, A.M.; Herity, L.B.; Song, G.; Santos, C.M.; Zamboni, W.C. Profiling the relationship between tumor-associated macrophages and pharmacokinetics of liposomal agents in preclinical murine models. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genomics 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; de Jongh, P.A.J.M.; Briere, S.; Chen, M.; Nowell, C.J.; Johnston, A.P.R.; Davis, T.P.; Haddleton, D.M.; Kempe, K. Carboxylated Cy5-Labeled Comb Polymers Passively Diffuse the Cell Membrane and Target Mitochondria. ACS Appl. Mater. Interfaces 2019, 11, 31302–31310. [Google Scholar] [CrossRef]
- Simpson, J.D.; Ediriweera, G.R.; Howard, C.B.; Fletcher, N.L.; Bell, C.A.; Thurecht, K.J. Polymer design and component selection contribute to uptake, distribution & trafficking behaviours of polyethylene glycol hyperbranched polymers in live MDA-MB-468 breast cancer cells. Biomater. Sci. 2019, 7, 4661–4674. [Google Scholar] [CrossRef]
- Dougherty, C.A.; Vaidyanathan, S.; Orr, B.G.; Banaszak Holl, M.M. Fluorophore:Dendrimer ratio impacts cellular uptake and intracellular fluorescence lifetime. Bioconjug. Chem. 2015, 26, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Hughes, L.D.; Rawle, R.J.; Boxer, S.G. Choose your label wisely: Water-soluble fluorophores often interact with lipid bilayers. PLoS ONE 2014, 9, e87649. [Google Scholar] [CrossRef] [Green Version]
- Selby, L.I.; Cortez-Jugo, C.M.; Such, G.K.; Johnston, A.P.R. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017. [Google Scholar] [CrossRef]
- Wang, F.; Hu, K.; Cheng, Y. Structure-activity relationship of dendrimers engineered with twenty common amino acids in gene delivery. Acta Biomater. 2016, 29, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Dobay, M.P.; Schmidt, A.; Mendoza, E.; Bein, T.; Rädler, J.O. Cell type determines the light-induced endosomal escape kinetics of multifunctional mesoporous silica nanoparticles. Nano Lett. 2013, 13, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fletcher, N.L.; Liu, T.; Gemmell, A.C.; Houston, Z.H.; Blakey, I.; Thurecht, K.J. In vivo therapeutic evaluation of polymeric nanomedicines: Effect of different targeting peptides on therapeutic efficacy against breast cancer. Nanotheranostics 2018, 2, 360–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, N.L.; Houston, Z.H.; Simpson, J.D.; Veedu, R.N.; Thurecht, K.J. Designed multifunctional polymeric nanomedicines: Long-term biodistribution and tumour accumulation of aptamer-targeted nanomaterials. Chem. Commun. 2018, 54, 11538–11541. [Google Scholar] [CrossRef] [PubMed]
- Boase, N.R.B.; Blakey, I.; Rolfe, B.E.; Mardon, K.; Thurecht, K.J. Synthesis of a multimodal molecular imaging probe based on a hyperbranched polymer architecture. Polym. Chem. 2014, 5, 4450–4458. [Google Scholar] [CrossRef]
- Pearce, A.K.; Simpson, J.D.; Fletcher, N.L.; Houston, Z.H.; Fuchs, A.V.; Russell, P.J.; Whittaker, A.K.; Thurecht, K.J. Localised delivery of doxorubicin to prostate cancer cells through a PSMA-targeted hyperbranched polymer theranostic. Biomaterials 2017, 141, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Mignani, S.; Shi, X.; Rodrigues, J.; Roy, R.; Muñoz-Fernández, Á.; Ceña, V.; Majoral, J.-P. Dendrimers toward Translational Nanotherapeutics: Concise Key Step Analysis. Bioconjug. Chem. 2020, 31, 2060–2071. [Google Scholar] [CrossRef]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef]
- He, M.; Guo, S.; Li, Z. In situ characterizing membrane lipid phenotype of breast cancer cells using mass spectrometry profiling. Sci. Rep. 2015, 5, 11298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.S.M.; Mann, S.K.; Czuba, E.; Sahut, A.; Liu, H.; Suekama, T.C.; Bickerton, T.; Johnston, A.P.R.; Such, G.K. Self-assembling dual component nanoparticles with endosomal escape capability. Soft. Matter. 2015, 11, 2993–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
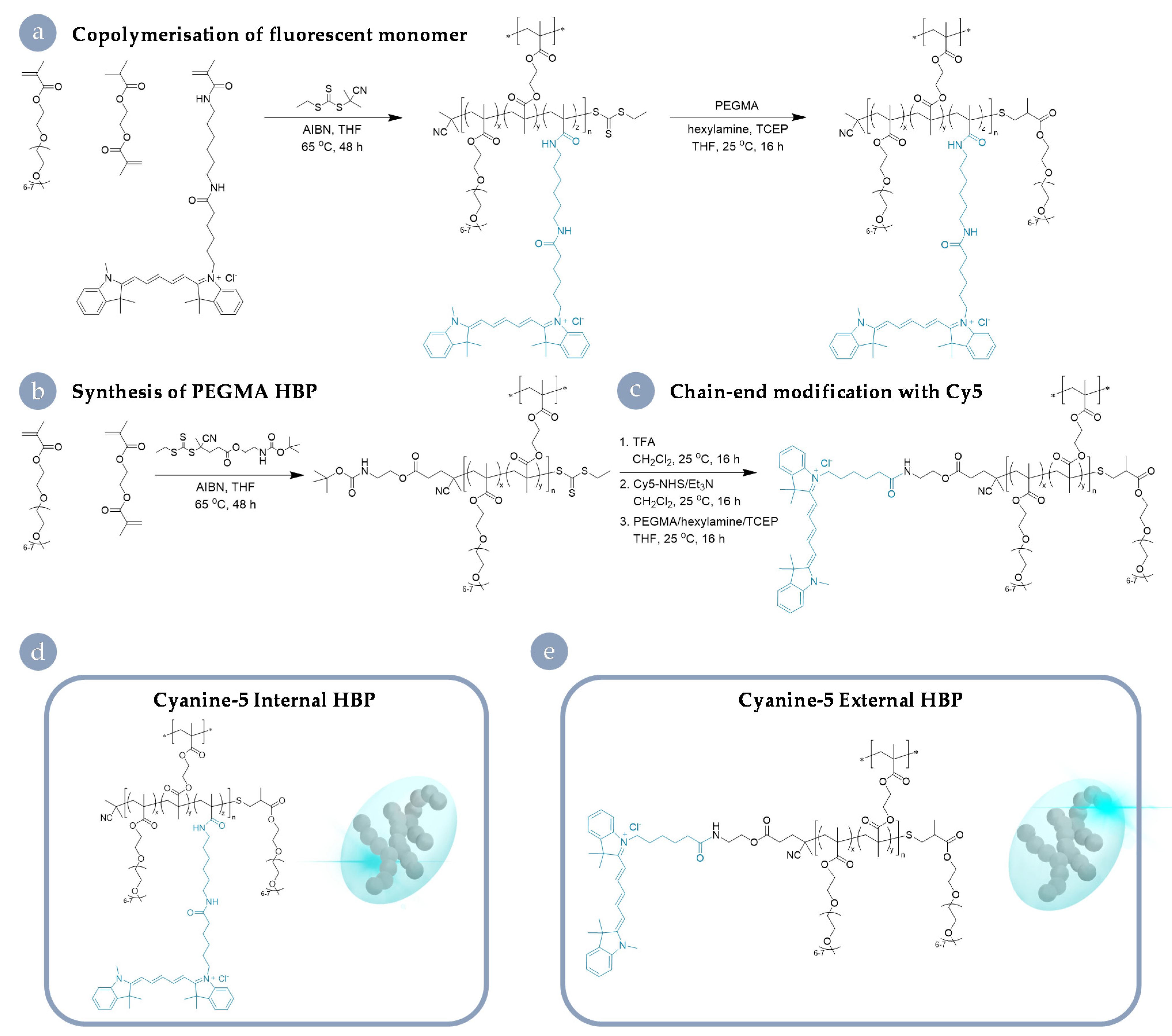



| Size (nm) | Labelling (a.u. per Particle) b | ζ Potential (mV) | Mn,NMR (kDa) | Mn,SEC (kDa) | ĐM | |
|---|---|---|---|---|---|---|
| Cyanine-5 internal HBP | 7.5 | 0.17 | −0.1 | 13.6 | 58.7 | 1.48 |
| Cyanine-5 external HBP a | 7.1 | 0.29 | −0.8 | 10.3 | 30.3 | 1.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simpson, J.D.; Currin-Ross, D.L.; Ediriweera, G.R.; Schirra, H.J.; Fletcher, N.L.; Bell, C.A.; Arno, M.C.; Thurecht, K.J. Cyanine-5-Driven Behaviours of Hyperbranched Polymers Designed for Therapeutic Delivery Are Cell-Type Specific and Correlated with Polar Lipid Distribution in Membranes. Nanomaterials 2021, 11, 1745. https://doi.org/10.3390/nano11071745
Simpson JD, Currin-Ross DL, Ediriweera GR, Schirra HJ, Fletcher NL, Bell CA, Arno MC, Thurecht KJ. Cyanine-5-Driven Behaviours of Hyperbranched Polymers Designed for Therapeutic Delivery Are Cell-Type Specific and Correlated with Polar Lipid Distribution in Membranes. Nanomaterials. 2021; 11(7):1745. https://doi.org/10.3390/nano11071745
Chicago/Turabian StyleSimpson, Joshua D., Denni L. Currin-Ross, Gayathri R. Ediriweera, Horst Joachim Schirra, Nicholas L. Fletcher, Craig A. Bell, Maria C. Arno, and Kristofer J. Thurecht. 2021. "Cyanine-5-Driven Behaviours of Hyperbranched Polymers Designed for Therapeutic Delivery Are Cell-Type Specific and Correlated with Polar Lipid Distribution in Membranes" Nanomaterials 11, no. 7: 1745. https://doi.org/10.3390/nano11071745
APA StyleSimpson, J. D., Currin-Ross, D. L., Ediriweera, G. R., Schirra, H. J., Fletcher, N. L., Bell, C. A., Arno, M. C., & Thurecht, K. J. (2021). Cyanine-5-Driven Behaviours of Hyperbranched Polymers Designed for Therapeutic Delivery Are Cell-Type Specific and Correlated with Polar Lipid Distribution in Membranes. Nanomaterials, 11(7), 1745. https://doi.org/10.3390/nano11071745









