Effects of Graphene Oxidation on Interaction Energy and Interfacial Thermal Conductivity of Polymer Nanocomposite: A Molecular Dynamics Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Interaction Energy
2.2. Kapitza Resistance (RK)
2.3. Phonon Density of State
3. Results and Discussions
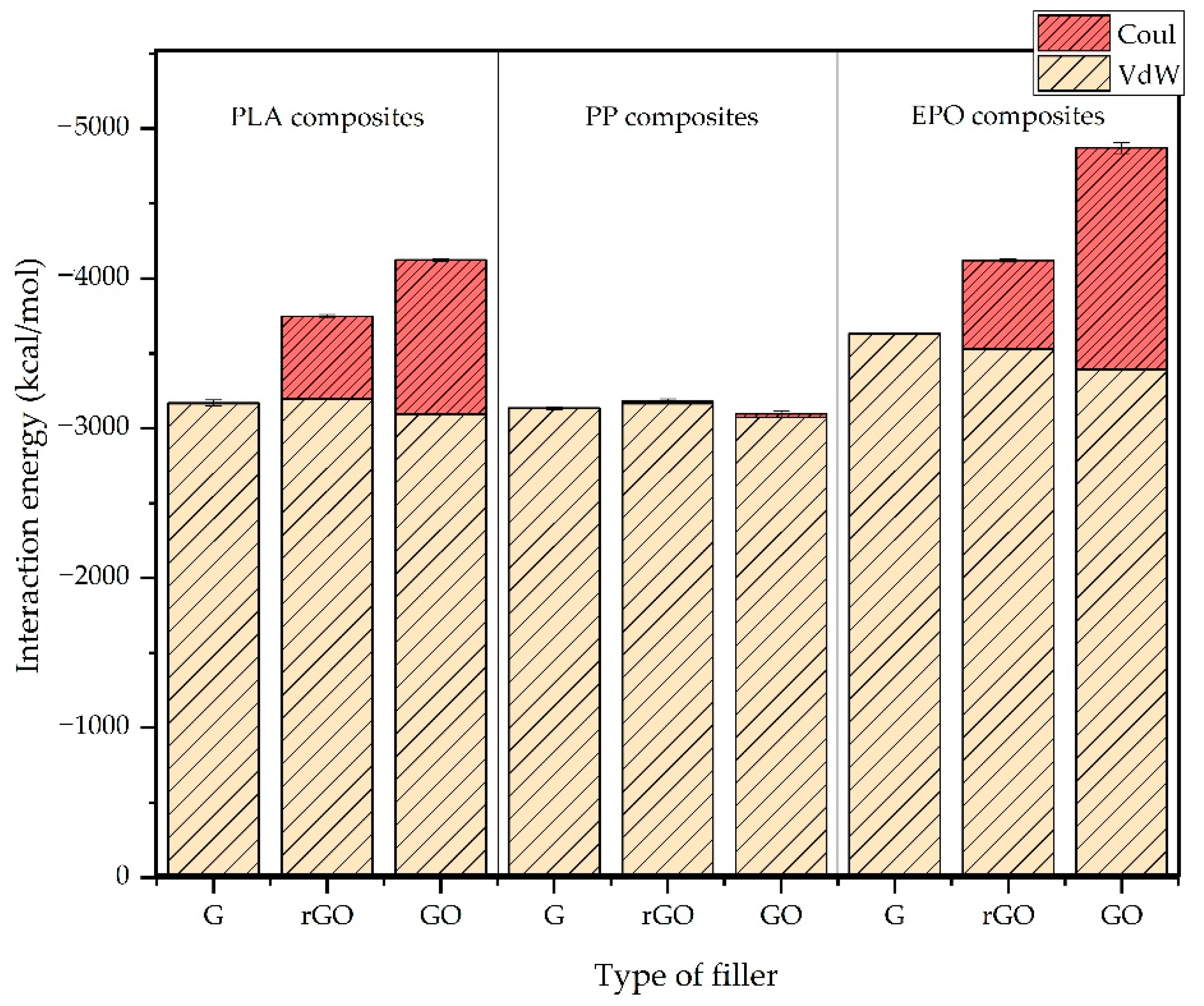
3.1. Interaction Energy
3.2. Kapitza Resistance (RK)
3.3. Phonon Density of States (DOS)
4. Conclusions
- The interaction energy of the three different polymer matrices with the graphene fillers depended on the polarity of the polymer, due to the different contribution of the Coulombic component. Thus, it was observed that the interaction energy increases in the order graphene < rGO < GO for polar polymers such as polylactic acid (PLA) and epoxy resin (EPO), whereas in the case of polypropylene, the functionalization did not affect sensibly its interaction with graphene fillers, due to the negligible Coulombic contribution. These results gave a relevant insight about the matrices–filler compatibility and how it is affected by the different functionalizations considered.
- The Kapitza resistance (RK) exhibited the same behavior/tendency in the three polymers, graphene > rGO ≈ GO. It was observed that the presence of functional groups in the graphene, in the form of oxidation (hydroxyl and oxirane groups) increases the thermal conductivity, which was associated with the appearance of high frequency phonon vibrations in the oxidized graphene fillers, GO and rGO.
Author Contributions
Funding
Conflicts of Interest
References
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part. B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photon. 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotech. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Chen, B. Reinforcement and interphase of polymer/graphene oxide nanocomposites. J. Mater. Chem. 2012, 22, 3637. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Li, J.J.; Weng, G.J. Theory of thermal conductivity of graphene-polymer nanocomposites with interfacial Kapitza resistance and graphene-graphene contact resistance. Carbon 2018, 137, 222–233. [Google Scholar] [CrossRef]
- Bigdeli, M.B.; Fasano, M. Thermal transmittance in graphene based networks for polymer matrix composites. Int. J. Therm. Sci. 2017, 117, 98–105. [Google Scholar] [CrossRef]
- Mohammad Nejad, S.; Bozorg Bigdeli, M.; Srivastava, R.; Fasano, M. Heat Transfer at the Interface of Graphene Nanoribbons with Different Relative Orientations and Gaps. Energies 2019, 12, 796. [Google Scholar] [CrossRef] [Green Version]
- Sáenz Ezquerro, C.; Laspalas, M.; Chiminelli, A.; Serrano, F.; Valero, C. Interface Characterization of Epoxy Resin Nanocomposites: A Molecular Dynamics Approach. Fibers 2018, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, L.; Cui, L.; Zhang, Y.; Du, X. Molecular dynamics simulation of cross-linked epoxy resin and its interaction energy with graphene under two typical force fields. Comput. Mater. Sci. 2018, 143, 240–247. [Google Scholar] [CrossRef]
- He, B.; Mortazavi, B.; Zhuang, X.; Rabczuk, T. Modeling Kapitza resistance of two-phase composite material. Compos. Struct. 2016, 152, 939–946. [Google Scholar] [CrossRef]
- Mortazavi, B.; Benzerara, O.; Meyer, H.; Bardon, J.; Ahzi, S. Combined molecular dynamics-finite element multiscale modeling of thermal conduction in graphene epoxy nanocomposites. Carbon 2013, 60, 356–365. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Molec. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Tersoff, J. Modeling solid-state chemistry: Interatomic potentials for multicomponent systems. Phys. Rev. B Condens. Matter 1989, 39, 5566–5568. [Google Scholar] [CrossRef]
- Yarovsky, I.; Evans, E. Computer simulation of structure and properties of crosslinked polymers: Application to epoxy resins. Polymer 2002, 43, 963–969. [Google Scholar] [CrossRef]
- Yu, K.; Li, Z.; Sun, J. Polymer Structures and Glass Transition: A Molecular Dynamics Simulation Study. Macromol. Theory Simul. 2001, 10, 624–633. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, Y.; Yang, W.; Hu, C.; Mu, Y.; Li, J. Study of the Mechanical and Thermal Properties of Poly(Lactic Acid) and Poly(Ethylene Glycol) Block Copolymer with Molecular Dynamics. Int. J. Polymer Anal. Charact. 2010, 15, 235–244. [Google Scholar] [CrossRef]
- Hadipeykani, M.; Aghadavoudi, F.; Toghraie, D. A molecular dynamics simulation of the glass transition temperature and volumetric thermal expansion coefficient of thermoset polymer based epoxy nanocomposite reinforced by CNT: A statistical study. Physica A 2020, 546, 123995. [Google Scholar] [CrossRef]
- Han, H.; Merabia, S.; Müller-Plathe, F. Thermal Transport at Solid-liquid Interfaces: High Pressure Facilitates Heat Flow through Non-local Liquid Structuring. J. Phys. Chem. Lett. 2017, 9, 1946–1951. [Google Scholar] [CrossRef]
- Merabia, S.; Lombard, J.; Alkurdi, A. Importance of viscoelastic and interface bonding effects in the thermal boundary conductance of solid–water interfaces. Int. J. Heat Mass Transf. 2016, 100, 287–294. [Google Scholar] [CrossRef]
- Chen, S.; Yang, M.; Liu, B.; Xu, M.; Zhang, T.; Zhuang, B.; Ding, D.; Huai, X.; Zhang, H. Enhanced Thermal Conductance at the Graphene–Water Interface Based on Functionalized Alkane Chains. RSC Adv. 2019, 9, 4563–4570. [Google Scholar] [CrossRef] [Green Version]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Allen, P.B.; Feldman, J.L.; Fabian, J.; Wooten, F. Diffusons, Locons and Propagons: Character of Atomie Yibrations in Amorphous Si. Philos. Mag. B 1999, 79, 1715–1731. [Google Scholar] [CrossRef]
- Moon, J.; Latour, B.; Minnich, A.J. Propagating Elastic Vibrations Dominate Thermal Conduction in Amorphous Silicon. Phys. Rev. B 2018, 97, 024201. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhan, H.F.; Xiang, Y.; Yang, C.; Wang, C.M.; Zhang, Y.Y. Effect of Covalent Functionalization on Thermal Transport across Graphene–Polymer Interfaces. J. Phys. Chem. C 2015, 119, 12731–12738. [Google Scholar] [CrossRef] [Green Version]
- Zabihi, Z.; Araghi, H. Effect of functional groups on thermal conductivity of graphene/paraffin nanocomposite. Phys. Lett. A 2016, 380, 3828–3831. [Google Scholar] [CrossRef]
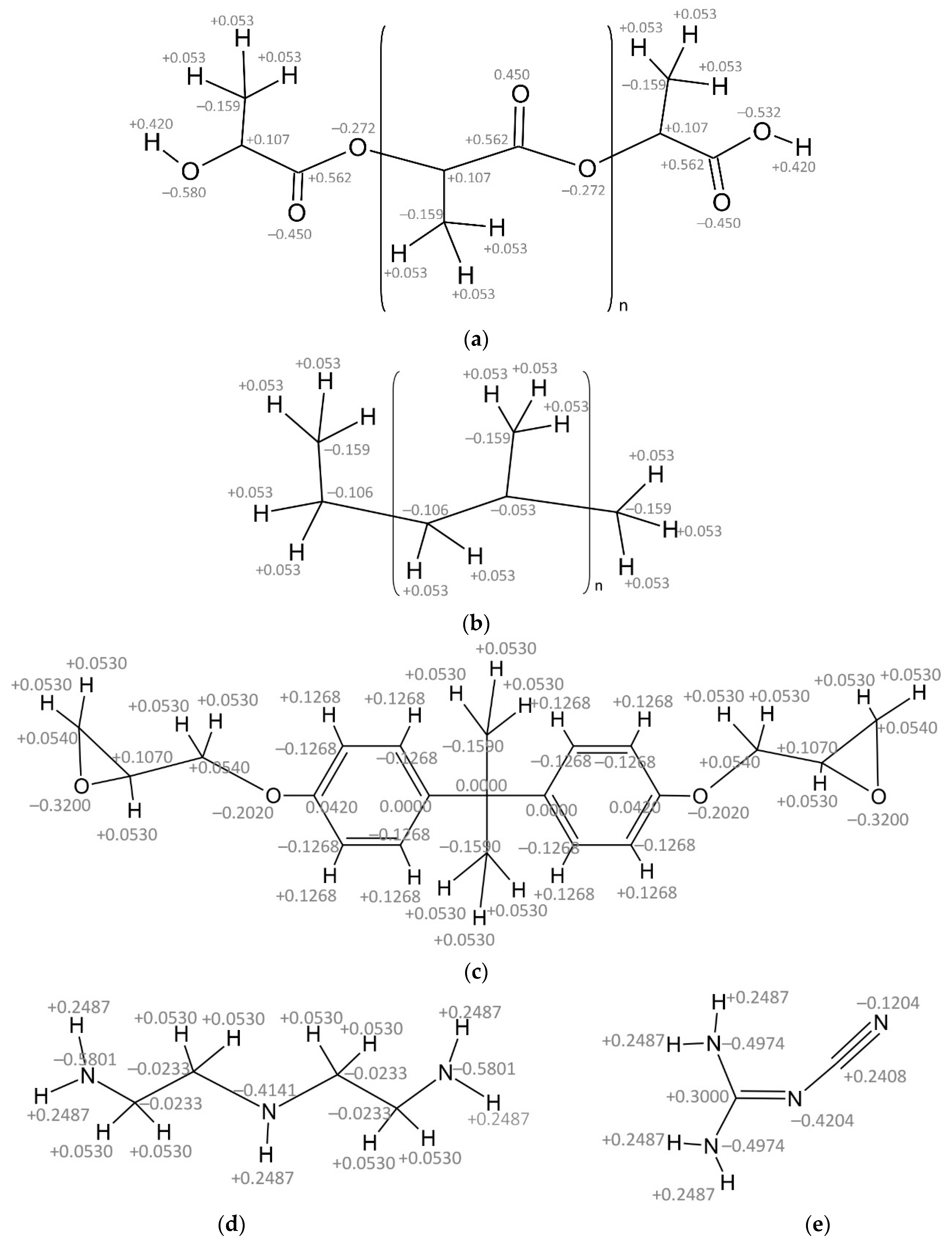

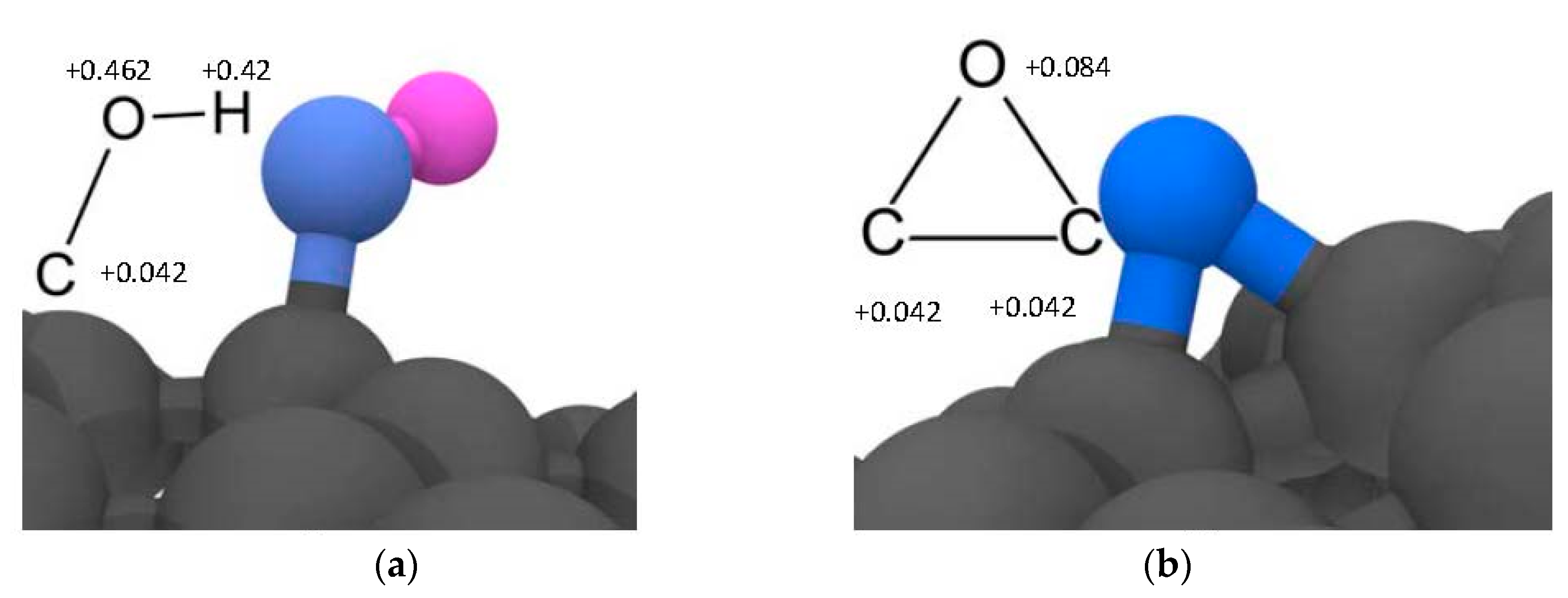
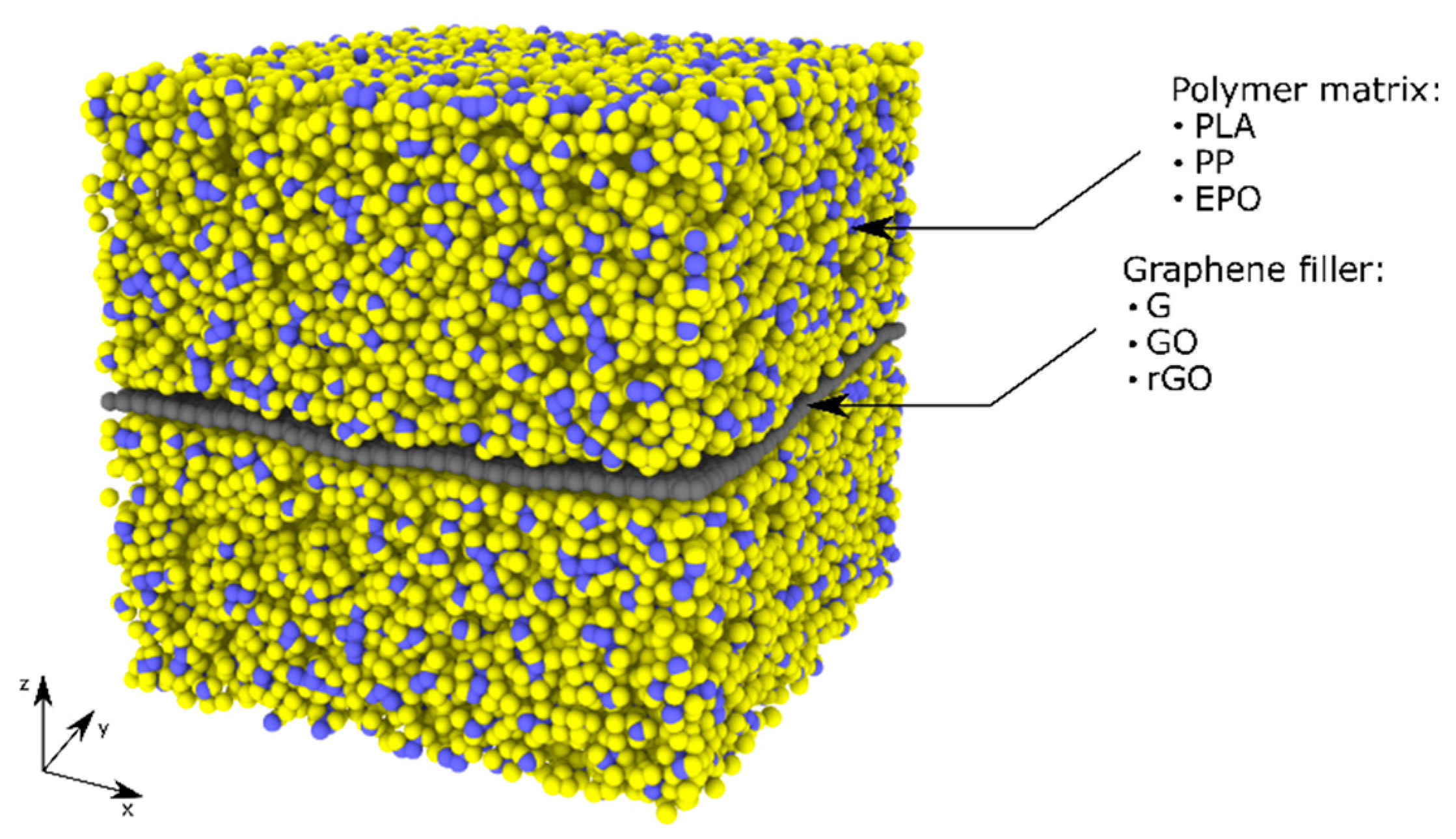
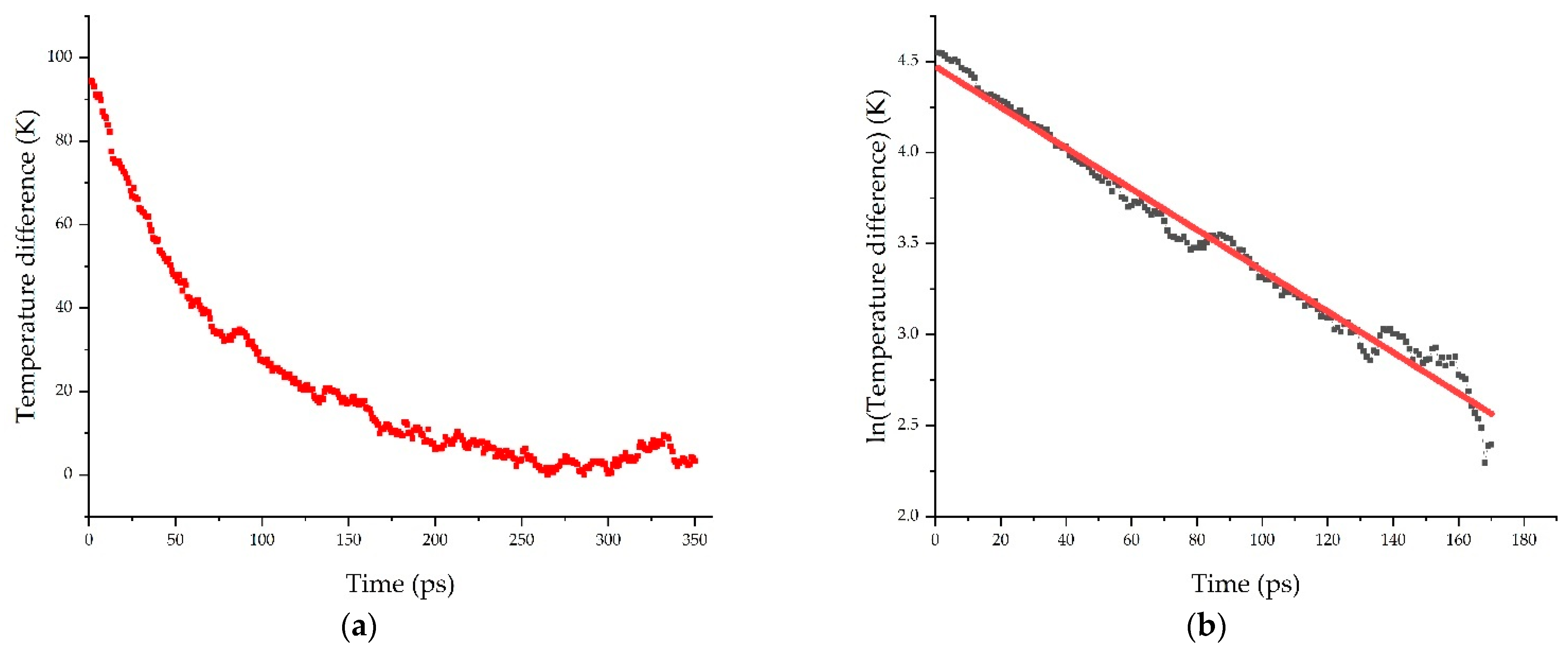


| ID Model | Polymer | Filler | N° Atoms | N° Molecules |
|---|---|---|---|---|
| PLA-G | PLA | G | 56,868 | 120 |
| PLA-GO | PLA | GO | 58,596 | 120 |
| PLA-rGO | PLA | rGO | 57,048 | 120 |
| PP-G | PP | G | 59,216 | 42 |
| PP-GO | PP | GO | 61,320 | 42 |
| PP-rGO | PP | rGO | 59,472 | 42 |
| EPO-G | EPO | G | 57,708 | 1000 + 292 + 164 (1) |
| EPO-GO | EPO | rGO | 59,763 | 1000 + 292 + 164 (1) |
| EPO-rGO | EPO | GO | 57,888 | 1000 + 292 + 164 (1) |
| ID Model | Polymer | Filler | EVdW | ECoul | Etotal | Standard Deviation |
|---|---|---|---|---|---|---|
| PLA-G | PLA | G | −3164 | −3 | −3167 | 14 |
| PLA-GO | PLA | rGO | −3197 | −548 | −3745 | 11 |
| PLA-rGO | PLA | GO | −3091 | −1022 | −4113 | 21 |
| PP-G | PP | G | −3132 | −0.1 | −3132 | 8 |
| PP-GO | PP | GO | −3073 | −24 | −3097 | 17 |
| PP-rGO | PP | rGO | −3151 | −14 | −3165 | 21 |
| EPO-G | EPO | G | −3635 | 1 | −3636 | 4 |
| EPO-GO | EPO | rGO | −3535 | −580 | −4115 | 12 |
| EPO-rGO | EPO | GO | −3395 | −1461 | −4856 | 34 |
| ID Model | Polymer | Filler | RK | Standard Deviation |
|---|---|---|---|---|
| PLA-G | PLA | G | 1.42 × 10−7 | 0.17 × 10−7 |
| PLA-GO | PLA | GO | 0.88 × 10−7 | 0.12 × 10−7 |
| PLA-rGO | PLA | rGO | 0.75 × 10−7 | 0.08 × 10−7 |
| PP-G | PP | G | 1.58 × 10−7 | 0.04 × 10−7 |
| PP-GO | PP | GO | 1.01 × 10−7 | 0.27 × 10−7 |
| PP-rGO | PP | rGO | 0.89 × 10−7 | 0.30 × 10−7 |
| EPO-G | EPO | G | 1.36 × 10−7 | 0.12 × 10−7 |
| EPO-GO | EPO | rGO | 0.75 × 10−7 | 0.03 × 10−7 |
| EPO-rGO | EPO | GO | 0.74 × 10−7 | 0.04 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellussi, F.M.; Sáenz Ezquerro, C.; Laspalas, M.; Chiminelli, A. Effects of Graphene Oxidation on Interaction Energy and Interfacial Thermal Conductivity of Polymer Nanocomposite: A Molecular Dynamics Approach. Nanomaterials 2021, 11, 1709. https://doi.org/10.3390/nano11071709
Bellussi FM, Sáenz Ezquerro C, Laspalas M, Chiminelli A. Effects of Graphene Oxidation on Interaction Energy and Interfacial Thermal Conductivity of Polymer Nanocomposite: A Molecular Dynamics Approach. Nanomaterials. 2021; 11(7):1709. https://doi.org/10.3390/nano11071709
Chicago/Turabian StyleBellussi, Francesco Maria, Carlos Sáenz Ezquerro, Manuel Laspalas, and Agustín Chiminelli. 2021. "Effects of Graphene Oxidation on Interaction Energy and Interfacial Thermal Conductivity of Polymer Nanocomposite: A Molecular Dynamics Approach" Nanomaterials 11, no. 7: 1709. https://doi.org/10.3390/nano11071709
APA StyleBellussi, F. M., Sáenz Ezquerro, C., Laspalas, M., & Chiminelli, A. (2021). Effects of Graphene Oxidation on Interaction Energy and Interfacial Thermal Conductivity of Polymer Nanocomposite: A Molecular Dynamics Approach. Nanomaterials, 11(7), 1709. https://doi.org/10.3390/nano11071709






