Growth and Crystallization of SiO2/GeO2 Thin Films on Si(100) Substrates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
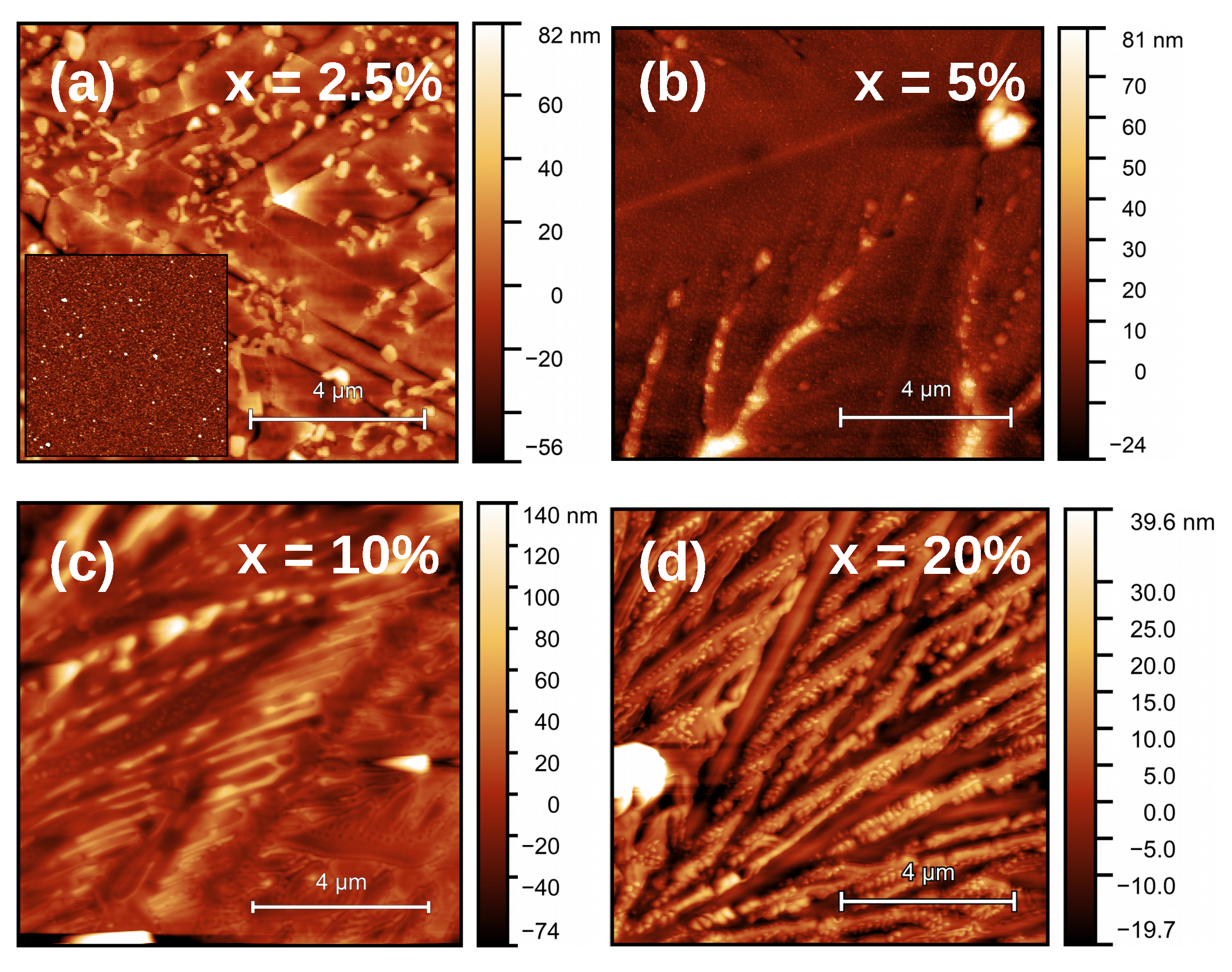
3.1. SiO/GeO Film Growth
3.2. Film Crystallization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, M. An overview of quartz MEMS devices. In Proceedings of the 2010 IEEE International Frequency Control Symposium, Newport Beach, CA, USA, 1–4 June 2010; pp. 162–167. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Nakamura, T. 5G Evolution and 6G. In Proceedings of the 2020 International Symposium on VLSI Design, Automation and Test (VLSI-DAT), Honolulu, HI, USA, 16–19 June 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Spezia, G. La pressione e’ chimicamente inattive nella solubilite e riecostituzione del quarzo. Atti della Reale Accademia delle scienze di Torino 1904, 40, 262. [Google Scholar]
- Iwasaki, F.; Iwasaki, H. Historical review of quartz crystal growth. J. Cryst. Growth 2002, 237–239, 820–827. [Google Scholar] [CrossRef]
- Johnson, G. History of the industrial production and technical development of single crystal cultured quartz. In Proceedings of the 2004 IEEE International Frequency Control Symposium and Exposition, Montreal, QC, Canada, 23–27 August 2004; pp. 35–45. [Google Scholar] [CrossRef]
- Stratton, F.; Chang, D.; Kirby, D.; Joyce, R.; Hsu, T.-Y.; Kubena, R.; Yong, Y.-K. A MEMS-based quartz resonator technology for GHz applications. In Proceedings of the 2004 IEEE International Frequency Control Symposium and Exposition, Montreal, QC, Canada, 23–27 August 2004; pp. 27–34. [Google Scholar] [CrossRef]
- Kubena, R.; Stratton, F.; Chang, D.; Joyce, R.; Hsu, T.; Lim, M.; M’Closkey, R. Next Generation Quartz Oscillators and Filters for VHF-UHF Systems. In Proceedings of the 2006 IEEE MTT-S International Microwave Symposium Digest, San Francisco, CA, USA, 11–16 June 2006; pp. 668–671. [Google Scholar] [CrossRef]
- Takahashi, N.; Hoshogi, M.; Nakumura, T.; Momose, Y.; Nonaka, S.; Yagi, H.; Sinriki, Y.; Tamanuki, K. Catalyst-enhanced vapor-phase epitaxy of quartz thin films under atmospheric pressure. J. Mater. Chem. 2002, 12, 719–722. [Google Scholar] [CrossRef]
- Takahashi, N.; Majima, J.; Nakumura, T.; Nonaka, S.; Yagi, H.; Shinriki, Y.; Tamanuki, K. Growth of a High Quality Quartz Film on Sapphire by Epitaxy Using Buffer Layers. Chem. Mater. 2003, 15, 2889–2891. [Google Scholar] [CrossRef]
- Takahashi, N.; Nakumura, T.; Nonaka, S.; Yagi, H.; Sinriki, Y.; Tamanuki, K. Rapid Growth of Thick Quartz Films by Catalyst-Enhanced Vapor-Phase Epitaxy under Atmospheric Pressure. Electrochem. Solid State Lett. 2003, 6, C77–C78. [Google Scholar] [CrossRef]
- Takahashi, N.; Nakamura, T. Quartz Oscillator Films Prepared under Atmospheric Pressure. Electrochem. Solid State Lett. 2003, 6, H25–H26. [Google Scholar] [CrossRef]
- Carretero-Genevrier, A.; Gich, M.; Picas, L.; Gazquez, J.; Drisko, G.L.; Boissiere, C.; Grosso, D.; Rodriguez-Carvajal, J.; Sanchez, C. Soft-chemistry-based routes to epitaxial alpha-quartz thin films with tunable textures. Science 2013, 340, 827–831. [Google Scholar] [CrossRef]
- Drisko, G.L.; Carretero-Genevrier, A.; Gich, M.; Gàzquez, J.; Ferrah, D.; Grosso, D.; Boissière, C.; Rodriguez-Carvajal, J.; Sanchez, C. Water-induced phase separation forming macrostructured epitaxial quartz films on silicon. Adv. Funct. Mater. 2014, 24, 5494–5502. [Google Scholar] [CrossRef]
- Carretero-Genevrier, A.; Gich, M.; Picas, L.; Sanchez, C.; Rodriguez-Carvajal, J. Chiral habit selection on nanostructured epitaxial quartz films. Faraday Discuss 2015, 179, 227–233. [Google Scholar] [CrossRef]
- Zhang, Q.; Sánchez-Fuentes, D.; Gómez, A.; Desgarceaux, R.; Charlot, B.; Gázquez, J.; Carretero-Genevrier, A.; Gich, M. Tailoring the crystal growth of quartz on silicon for patterning epitaxial piezoelectric films. Nanoscale Adv. 2019, 1, 3741–3752. [Google Scholar] [CrossRef] [Green Version]
- Jolly, C.; Gomez, A.; Sánchez-Fuentes, D.; Cakiroglu, D.; Rathar, R.; Maurin, N.; Garcia-Bermejo, R.; Charlot, B.; Gich, M.; Bahriz, M.; et al. Soft-Chemistry-Assisted On-Chip Integration of Nanostructured α-Quartz Microelectromechanical System. Adv. Mater. Technol. 2021, 6, 2000831. [Google Scholar] [CrossRef]
- Zhou, S.; Antoja-Lleonart, J.; Nukala, P.; Ocelik, V.; Lutjes, N.R.; Noheda, B. Crystallization of GeO2 thin films into alpha-quartz: From spherulites to single crystals. arXiv 2020, arXiv:2007.03916. [Google Scholar]
- Swamy, V.; Saxena, S.K. A thermodynamic assessment of silica phase diagram. J. Geophys. Res. 1994, 99, 11787–11794. [Google Scholar] [CrossRef]
- Heaney, P. Structure and chemistry of the low-pressure silica polymorphs. In Silica: Physical Behavior, Geochemistry, and Materials Applications; Heaney, P.J., Prewitt, C.T., Gibbs, G.V., Eds.; Number 29; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 1–40. [Google Scholar] [CrossRef]
- Balitskii, D.B.; Sil’vestrova, O.Y.; Balitskii, V.S.; Pisarevskii, Y.V.; Pushcharovskii, D.Y.; Philippot, E. Elastic, piezoelectric, and dielectric properties of alpha-GeO2 single crystals. Crystallogr. Rep. 2000, 45, 145–147. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The atomic arrangement in glass. J. Am. Chem. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]
- Le Bourhis, E. Glass: Mechanics and Technology, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2014; p. 416. [Google Scholar]
- Nagasima, N.; Enari, H. Local Crystallization of Thermal Oxide Film of Silicon. Jpn. J. Appl. Phys. 1971, 10, 441–447. [Google Scholar] [CrossRef]
- Drisko, G.L.; Carretero-Genevrier, A.; Perrot, A.; Gich, M.; Gàzquez, J.; Rodriguez-Carvajal, J.; Favre, L.; Grosso, D.; Boissière, C.; Sanchez, C. Crystallization of hollow mesoporous silica nanoparticles. Chem. Commun. 2015, 51, 4164–4167. [Google Scholar] [CrossRef]
- Roccaforte, F.; Bolse, W.; Lieb, K.P. Solid phase epitaxial regrowth of ion beam-amorphized alpha-quartz. Appl. Phys. Lett. 1998, 73, 1349–1351. [Google Scholar] [CrossRef]
- Roccaforte, F.; Dhar, S.; Harbsmeier, F.; Lieb, K.P. Epitaxial crystallization of amorphous SiO2 films deposited on single-crystalline alpha-quartz. Appl. Phys. Lett. 1999, 75, 2903–2905. [Google Scholar] [CrossRef]
- Gasiorek, S.; Dhar, S.; Lieb, K.P.; Schaaf, P. Laser-induced epitaxial recrystallization after alkali-ion implantion into a-quartz. Appl. Surf. Sci. 2005, 247, 396–400. [Google Scholar] [CrossRef]
- Zhao, L.; Li, N.; Langner, A.; Steinhart, M.; Tan, T.Y.; Pippel, E.; Hofmeister, H.; Tu, K.N.; Gösele, U. Crystallization of amorphous SiO2 microtubes catalyzed by lithium. Adv. Funct. Mater. 2007, 17, 1952–1957. [Google Scholar] [CrossRef]
- Antoja-Lleonart, J.; Zhou, S.; de Hond, K.; Huang, S.; Koster, G.; Rijnders, G.; Noheda, B. Atomic layer deposition of SiO2-GeO2 multilayers. Appl. Phys. Lett. 2020, 117, 041601. [Google Scholar] [CrossRef]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX—Free and Open Source Software Toolbox. Solid State Phenom. 2010, 160, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.; Aziz, M. Stoichiometry issues in pulsed-laser deposition of alloys grown from multicomponent targets. Appl. Phys. A Mater. Sci. Process. 1999, 69, S23–S27. [Google Scholar] [CrossRef]
- Wei, P.H. The Structure of a-Quartz. Z. Kristallogr.-Crystal. Mater. 1935, 92, 355–362. [Google Scholar] [CrossRef]
- Nieuwenkamp, W. Die Kristallstruktur des Tief-Cristobalits SiO2. Z. Kristallogr.-Crystal. Mater. 1935, 92, 82–88. [Google Scholar] [CrossRef]
- Nishi, F. Strontium Metasilicate, SrSiO3. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 534–536. [Google Scholar] [CrossRef]
- Nishi, F. Strontium Tetragermanate, SrGe4O9. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1996, 52, 2393–2395. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; Punin, Y.O.; Gunn, E.; Kahr, B. Spherulites. Chem. Rev. 2012, 112, 1805–1838. [Google Scholar] [CrossRef]
- Frondel, C. Secondary Dauphiné twinning in quartz. Am. Mineral. J. Earth Planet. Mater. 1945, 30, 447–460. [Google Scholar]
- Gránásy, L.; Pusztai, T.; Tegze, G.; Warren, J.A.; Douglas, J.F. Growth and form of spherulites. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 72, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlström, D. Micro X-ray diffraction for use in histochemistry. J. Histochem. Cytochem. 1954, 2, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, R.L. Micro X-ray diffraction (XRD): A versatile technique for characterization of Earth and planetary materials. Can. J. Earth Sci. 2007, 44, 1333–1346. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoja-Lleonart, J.; Ocelík, V.; Zhou, S.; de Hond, K.; Koster, G.; Rijnders, G.; Noheda, B. Growth and Crystallization of SiO2/GeO2 Thin Films on Si(100) Substrates. Nanomaterials 2021, 11, 1654. https://doi.org/10.3390/nano11071654
Antoja-Lleonart J, Ocelík V, Zhou S, de Hond K, Koster G, Rijnders G, Noheda B. Growth and Crystallization of SiO2/GeO2 Thin Films on Si(100) Substrates. Nanomaterials. 2021; 11(7):1654. https://doi.org/10.3390/nano11071654
Chicago/Turabian StyleAntoja-Lleonart, Jordi, Václav Ocelík, Silang Zhou, Kit de Hond, Gertjan Koster, Guus Rijnders, and Beatriz Noheda. 2021. "Growth and Crystallization of SiO2/GeO2 Thin Films on Si(100) Substrates" Nanomaterials 11, no. 7: 1654. https://doi.org/10.3390/nano11071654
APA StyleAntoja-Lleonart, J., Ocelík, V., Zhou, S., de Hond, K., Koster, G., Rijnders, G., & Noheda, B. (2021). Growth and Crystallization of SiO2/GeO2 Thin Films on Si(100) Substrates. Nanomaterials, 11(7), 1654. https://doi.org/10.3390/nano11071654





