Comparisons of the Effect of Different Metal Oxide Nanoparticles on the Root and Shoot Growth under Shaking and Non-Shaking Incubation, Different Plants, and Binary Mixture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials, Preparation, and Analysis
2.2. Effects of Incubation Conditions on Root and Shoot Growth
2.3. Effects of Binary NP Mixtures on Root Growth
3. Results
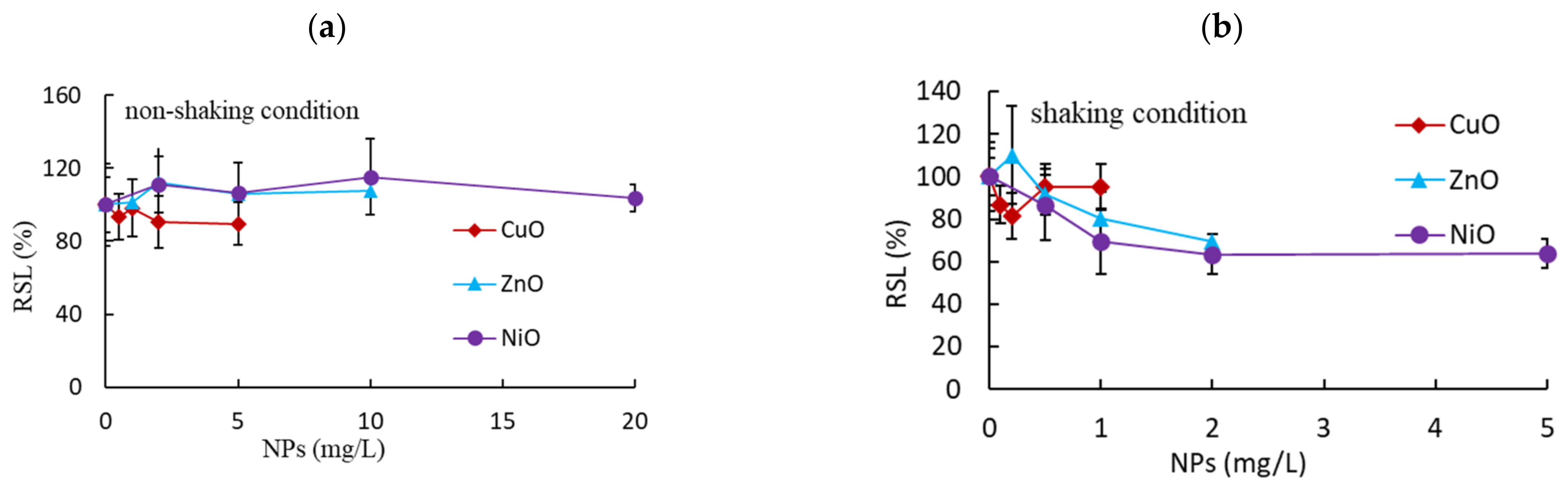
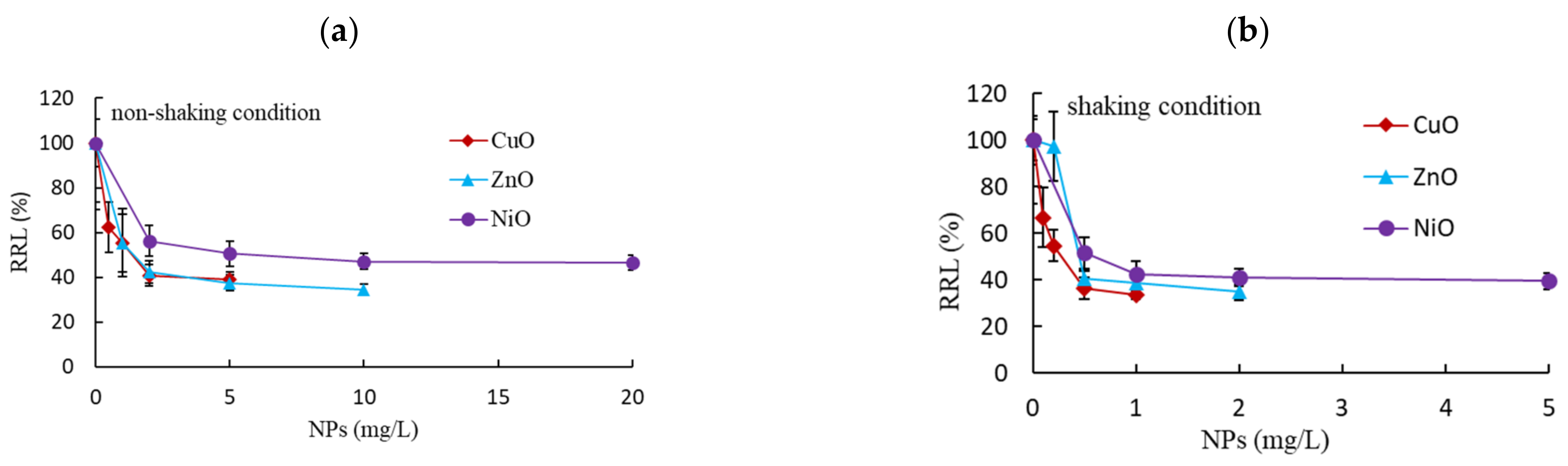
3.1. Effects of Non-Shaking and Shaking Incubation Conditions on Root and Shoot Growth
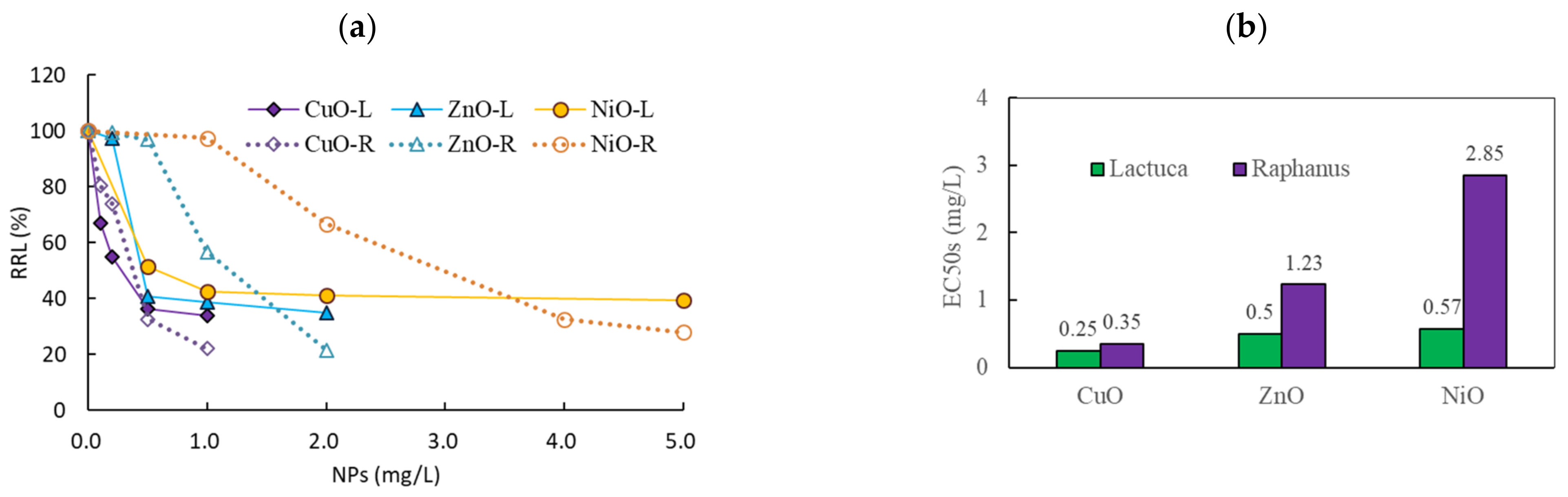
3.2. Comparison of the Effects of NPs on Two Plant Species
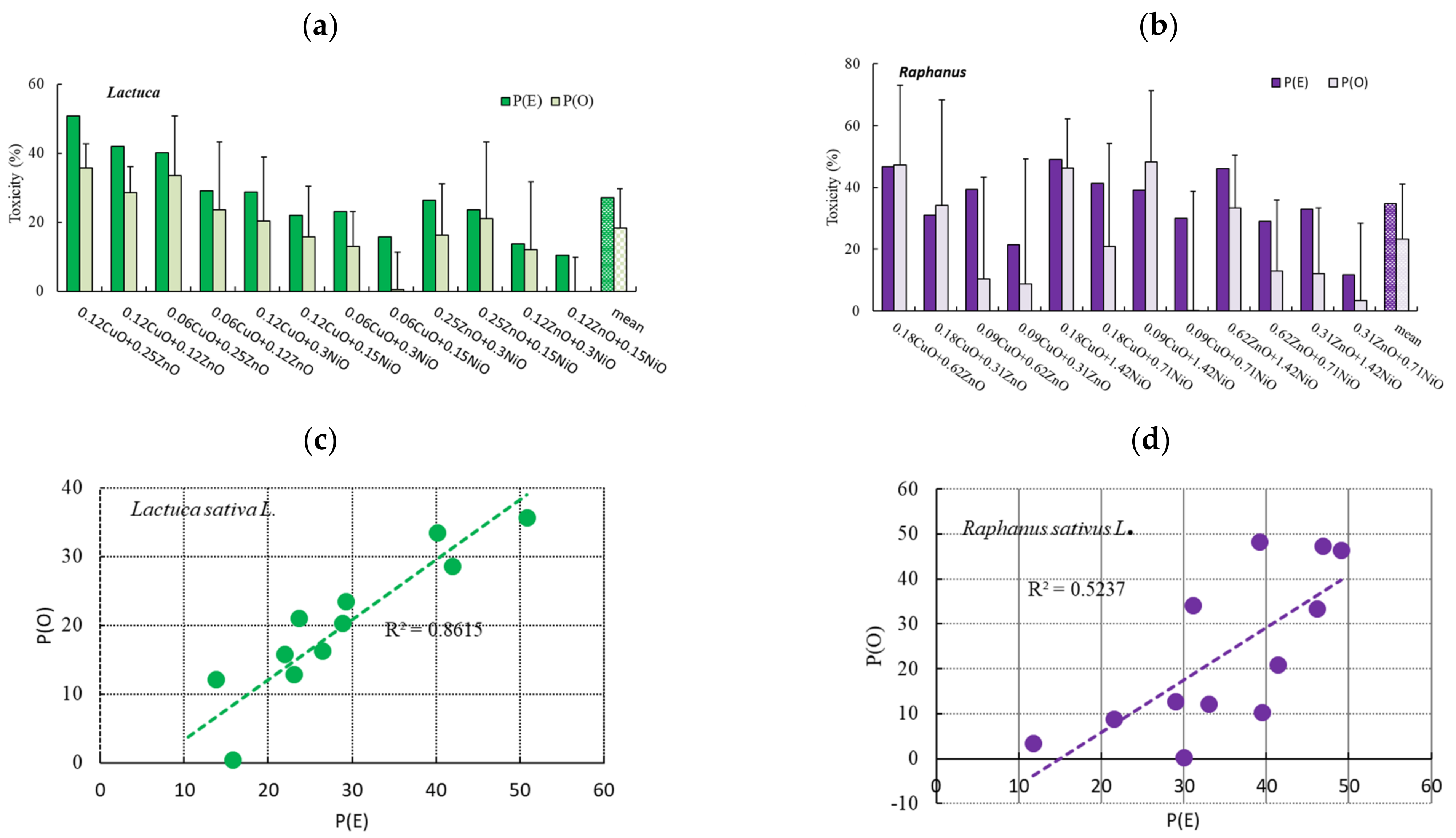
3.3. Effects of Binary NP Mixtures on the Root Growth of L. sativa and R. sativus
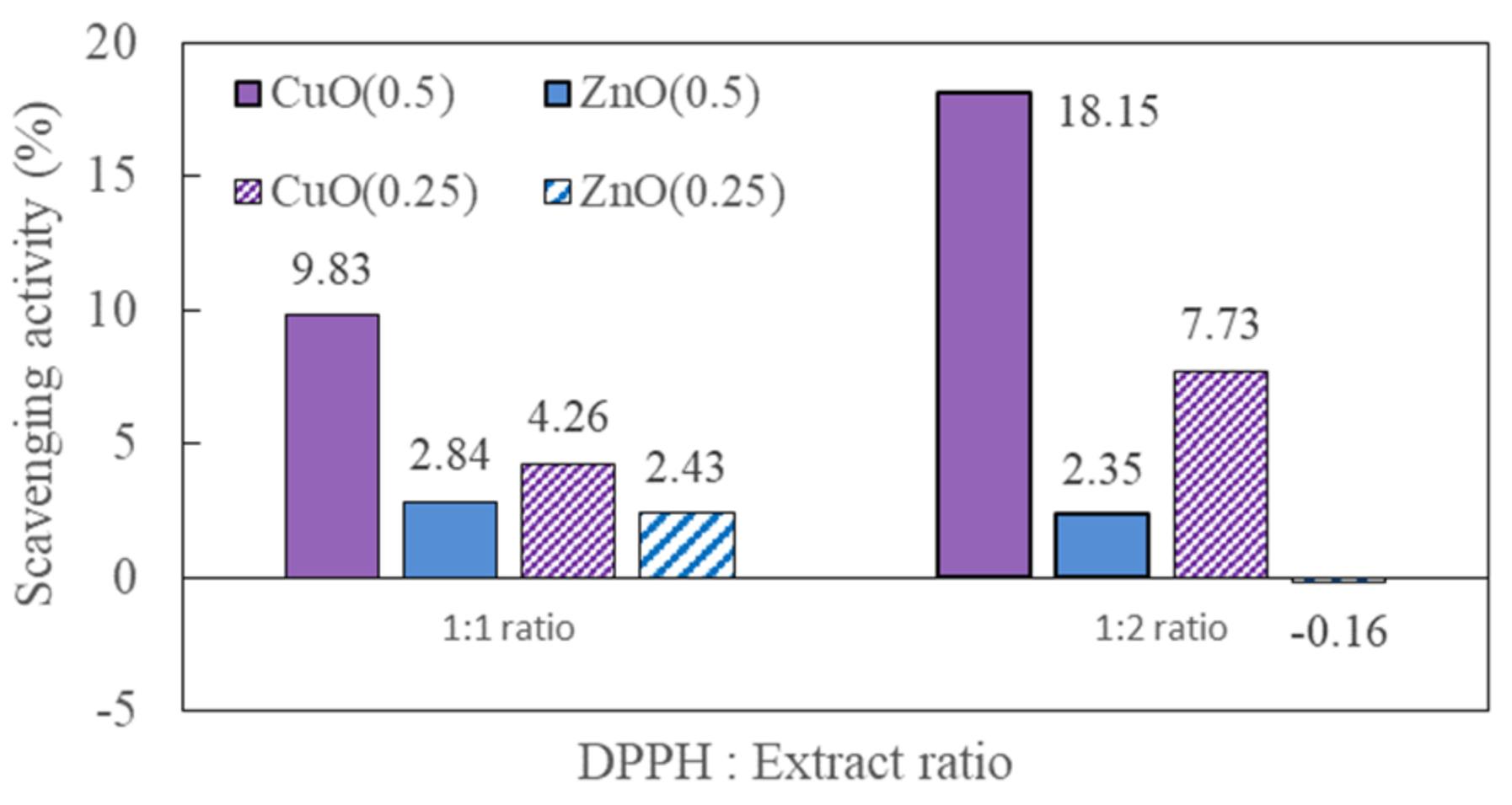
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.L.; Casman, E.A.; Lowry, G.V.; Lead, J.R.; Viparelli, E.; Baalousha, M. Modeling nanomaterial environmental fate in aquatic systems. Environ. Sci. Technol. 2015, 49, 2587–2593. [Google Scholar] [CrossRef]
- Selck, H.; Handy, R.D.; Fernandes, T.F.; Klaine, S.J.; Petersen, E.J. Nanomaterials in the aquatic environment: A European Union-Unite States perspective on the status ecotoxicology testing, research priorities, and challenges ahead. Environ. Toxicol. Chem. 2016, 35, 1055–1067. [Google Scholar] [CrossRef] [Green Version]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf. Environ. Protec. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Nemcek, L.; Šebesta, M.; Urík, M.; Bujdoš, M.; Dobrocka, E.; Vávra, I. Impact of bulk ZnO, ZnO nanoparticles and dissolved Zn on early growth stages of barley-a pot experiment. Plants 2020, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.H.; Yu, I.J. The concepts of nanotoxicology and risk assessment of the nanoparticles. J. Toxicol. Pub. Health 2005, 21, 87–98. [Google Scholar]
- Ates, M.; Demir, V.; Arslan, Z.; Camas, M.; Celik, F. Toxicity of engineered nickel oxide and cobalt oxide nanoparticles to Artemia salina in seawater. Water Air Soil Pollut. 2016, 227, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abudayyak, M.; Gurkaynak, T.A.; Ozhan, G. In vitro evaluation of cobalt oxide nanoparticle-induced toxicity. Toxicol. Indust. Health 2017, 33, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Baek, Y.W.; An, Y.J. Assay-dependent effect of silver nanoparticles to Escherichia coli and Bacillus subtilis. Appl. Microbiol. Biotechnol. 2011, 92, 1045–1052. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Grassian, V.H. Biological and environmental media control oxide nanoparticle surface composition: The roles of biological components (proteins and amino acids), inorganic oxyanions and humic acid. Environ. Sci. Nano 2015, 2, 429–439. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrisond, L.; Fogarty, A.; Cormican, M.; Dockeryf, P.; Prendergast, M.; Rowanc, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.R.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.M.; Wang, W.X. Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna. Nanotoxicology 2012, 6, 361–370. [Google Scholar] [CrossRef]
- Nam, S.H.; An, Y.J. Size-and shape-dependent toxicity of silver nanomaterials in green alga Chlorococcum infusionum. Ecotoxicol. Environ. Saf. 2019, 168, 388–393. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health A 2008, 43, 278–284. [Google Scholar] [CrossRef]
- Sousa, V.S.; Teixeira, M.R. Metal-based engineered nanoparticles in the drinking water treatment systems: A critical review. Sci. Total Environ. 2020, 707, 136077. [Google Scholar] [CrossRef]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Dash, S.K.; Tripathy, S.; Das, B.; Mandal, D.; Pramanik, P.; Roy, S. Toxicity of cobalt oxide nanoparticles to normal cells; An in vitro and in vivo study. Chem.-Biol. Interact. 2015, 226, 58–71. [Google Scholar] [CrossRef]
- Li, X.; Fang, J.; Cheng, H. Toxicity of silver nanoparticles to green algae M. aeruginosa and alleviation by organic matter. Environ. Monit. Assess. 2018, 190, 667. [Google Scholar]
- Padmanabhan, P.; Jangle, S.N. Evaluation of DPPH radical scavenging activity and reducing power of four selected medicinal plants and their combination. Int. J. Pharm. Sci. Drug Res. 2012, 4, 143–146. [Google Scholar]
- Abd-Alla, M.H.; Nafady, N.A.; Khalaf, D.M. Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: Implications for induction of autophagy process in root nodule. Agric. Ecosyst. Environ. 2016, 218, 163–177. [Google Scholar] [CrossRef]
- Bossi, E.; Zanella, D.; Gornati, R.; Nernardini, G. Cobalt oxide nanoparticles can enter inside the cells by crossing plasma membranes. Sci. Rep. 2016, 6, 22254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, Z.; Mustafa, G.; Sakata, K.; Komatsu, S. Insights into the proteomic response of soybean towards Al3, ZnO, and Ag nanoparticles stress. J. Hazard. Mater. 2016, 304, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; Upadhyay, R.G.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Alinovi, R.; Goldoni, M.; Pinelli, S.; Campanini, S.; Aliatis, M.I.; Bersani, D.; Lottici, P.P.; Iavicoli, S.; Petyx, M.; Mozzoni, P.; et al. Oxidative and pro-inflammatory effects of cobalt and titanium oxide nanoparticles on aortic and venous endothelial cells. Toxicol. In Vitro 2015, 29, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Pavlaki, M.D.; Pereira, R.; Loureiro, S.; Soares, A.M.V.M. Effects of binary mixtures on the life traits of Daphnia magna. Ecotoxicol. Environ. Saf. 2011, 74, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fernández-Cruz, M.L.; Connolly, M.; Conde, E.; Fernández, M.; Schuster, M.; Navas, J.M. The potentiation effect makes the difference: Non-toxic concentrations of ZnO nanoparticles enhance Cu nanoparticle toxicity in vitro. Sci. Total Environ. 2015, 505, 253–260. [Google Scholar] [CrossRef]
- Kungolos, A.; Emmanouil, C.; Tsiridis, V.; Tsiropoulos, N. Evaluation of toxic and interactive toxic effects of three agrochemicals and copper using a battery of microbiotests. Sci. Total Environ. 2009, 407, 4610–4615. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- US EPA. Ecological Effects Test Guidelines, OPPTS 850.4200. EPA 712-C-96-154.
- Banks, M.K.; Schultz, K.E. Comparison of plants for germination toxicity tests in petroleum-contaminated soils. Water Air Soil Pollut. 2015, 167, 211–219. [Google Scholar] [CrossRef]
- Ko, K.-S.; Han, J.; Kong, I.C. Assessment of arsenite, arsenate, and chromate phytotoxicity based on the activity of seed germination and growth (root & shoot) of various plant seeds. Human Eco. Risk Assess. Int. J. 2013, 19, 742–753. [Google Scholar]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2018, 71, 1308–1316. [Google Scholar] [CrossRef]
- Crane, M.; Handy, R.D.; Garrod, J.; Owen, R. Ecotoxicity test methods and environmental hazard assessment for engineered nanoparticles. Ecotoxicology 2018, 17, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.-S.; Kong, I.C. Influence of incubation conditions on the nanoparticles toxicity based on seed germination and bacterial bioluminescence. J. Nanosci. Nanotechnol. 2017, 19, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Papis, E.; Rossi, F.; Raspanti, M.; Dalle-Donne, I.; Colombo, G.; Milzani, A.; Bernardini, G.; Gornati, R. Engineered cobalt oxide nanoparticles readily enter cells. Toxicol. Lett. 2009, 189, 253–259. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. Nanoimpact 2017, 7, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Layet, C.; Auffan, M.; Santaella, C.; Chevassus-Rosset, C.; Montes, M.; Ortet, P.; Barakat, M.; Collin, B.; Legros, S.; Bravin, M.N.; et al. Evidence that soil properties and organic coating drive the phytoavailability of cerium oxide nanoparticles. Environ. Sci. Technol. 2017, 51, 9756–9764. [Google Scholar] [CrossRef]
- Kong, I.C.; Ko, K.-S.; Koh, D.C. Evaluation of the effects of particle sizes of silver nanoparticles on various biological systems. Int. J. Mol. Sci. 2020, 21, 8645. [Google Scholar] [CrossRef]
- Kong, I.C.; Ko, K.-S.; Koh, D.C. Comparative effects of particle sizes of cobalt nanoparticles to nine biological activities. Int. J. Mol. Sci. 2020, 21, 6767. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A. Toxicological consideration, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Vijver, M.G.; de Snoo, G.R. Toxicological mixture models are based on inadequate assumptions. Environ. Sci. Technol. 2010, 44, 4841–4842. [Google Scholar] [CrossRef]
- Ko, K.-S.; Koh, D.C.; Kong, I.C. Evaluation of the effects of nanoparticle mixtures on Brassica seed germination and bacterial bioluminescence activity based on the theory of probability. Nanomaterials 2017, 7, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, K.-S.; Koh, D.C.; Kong, I.C. Toxicity evaluation of individual and mixtures of nanoparticles based on algal chlorophyll content and cell count. Materials 2018, 11, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, S.I.; Holz, T.; Rodrigues, J.; Monteiro, T.; Costa, F.M.; Soares, A.M.V.M.; Loureiro, S. A mixture toxicity approach to predict the toxicity of Ag decorated ZnO nanomaterials. Sci. Total Environ. 2017, 579, 337–344. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef]
- Liu, Y.; Baas, J.; Peijnenburg, W.G.M.; Vijver, M.G. Evaluating the combined toxicity of Cu and ZnO nanoparticles: Utility of the concept of additivity and a nested experimental design. Environ. Sci. Technol. 2016, 50, 5328–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Yuan, L.; Richardson, C.J.; Ho, M.; Willis, C.W.; Colman, B.P.; Wiesner, M.R. Stress responses of aquatic plants to silver nanoparticles. Environ. Sci. Technol. 2018, 52, 2558–2565. [Google Scholar] [CrossRef]
- Gui, X.; Rui, M.; Song, Y.; Ma, Y.; Rui, Y.; Zhang, P.; He, X.; Li, Y.; Zhang, Z.; Liu, L. Phytotoxicity of CeO2 nanoparticles on radish plant (Raphanus sativus). Environ. Sci. Pollut. Res. 2017, 24, 13775–13781. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faizan, M.; Faraz, A.; Hayat, S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant Biochem. Biotechnol. 2020, 29, 553–567. [Google Scholar] [CrossRef]
- Jiang, F.; Shen, Y.; Ma, C.; Zhang, X.; Cao, W.; Rui, Y. Effects of TiO2 nanoparticles on wheat (Triticum aestivum L.) seedlings cultivated under super-elevated and normal CO2 conditions. PLoS ONE 2017, 12, e0178088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Incubation Condition | EC50 (mg/L) | |||
|---|---|---|---|---|
| CuO NPs | ZnO NPs | NiO NPs | ||
| Non-shaking | Shoot | >5 a (90%) b | >10 (107%) | >20 (104%) |
| Root | 1.28 (0.75–2.17) c | 1.31 (0.89–1.92) | 5.57 (3.41–8.73) | |
| Shaking | Shoot | >1 (28%) | >2 (26%) | >5 (21%) |
| Root | 0.25 (0.18–0.37) | 0.50 (0.36–0.70) | 0.57 (0.32–1.02) | |
| Incubation Condition | Relative Growth Activity (%) | ||
|---|---|---|---|
| TiO2 NPs | Al2O3 NPs | ||
| Non-shaking | Shoot | 106 ± 11.7 | 108 ± 22.0 |
| Root | 65 ± 16.4 | 56 ± 10.1 | |
| Shaking | Shoot | 84 ± 13.0 | 89 ± 12.3 |
| Root | 99 ± 18.8 | 86 ± 26.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, I.C.; Ko, K.-S.; Koh, D.-C. Comparisons of the Effect of Different Metal Oxide Nanoparticles on the Root and Shoot Growth under Shaking and Non-Shaking Incubation, Different Plants, and Binary Mixture Conditions. Nanomaterials 2021, 11, 1653. https://doi.org/10.3390/nano11071653
Kong IC, Ko K-S, Koh D-C. Comparisons of the Effect of Different Metal Oxide Nanoparticles on the Root and Shoot Growth under Shaking and Non-Shaking Incubation, Different Plants, and Binary Mixture Conditions. Nanomaterials. 2021; 11(7):1653. https://doi.org/10.3390/nano11071653
Chicago/Turabian StyleKong, In Chul, Kyung-Seok Ko, and Dong-Chan Koh. 2021. "Comparisons of the Effect of Different Metal Oxide Nanoparticles on the Root and Shoot Growth under Shaking and Non-Shaking Incubation, Different Plants, and Binary Mixture Conditions" Nanomaterials 11, no. 7: 1653. https://doi.org/10.3390/nano11071653
APA StyleKong, I. C., Ko, K.-S., & Koh, D.-C. (2021). Comparisons of the Effect of Different Metal Oxide Nanoparticles on the Root and Shoot Growth under Shaking and Non-Shaking Incubation, Different Plants, and Binary Mixture Conditions. Nanomaterials, 11(7), 1653. https://doi.org/10.3390/nano11071653