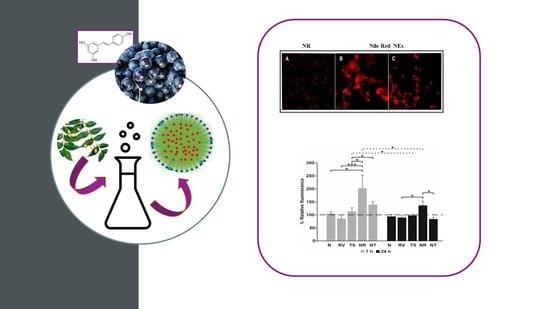
Resveratrol-Loaded Nanoemulsions: In Vitro Activity on Human T24 Bladder Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pseudoternary Phase Diagram Construction and Nanoemulsion Preparation
2.3. Preparation of Selected Nanoemulsions
2.4. Dynamic Light Scattering Measurements
2.5. Stability Studies
2.6. Fluorometric Measurements
2.7. Release Studies in RPMI 1640 Medium
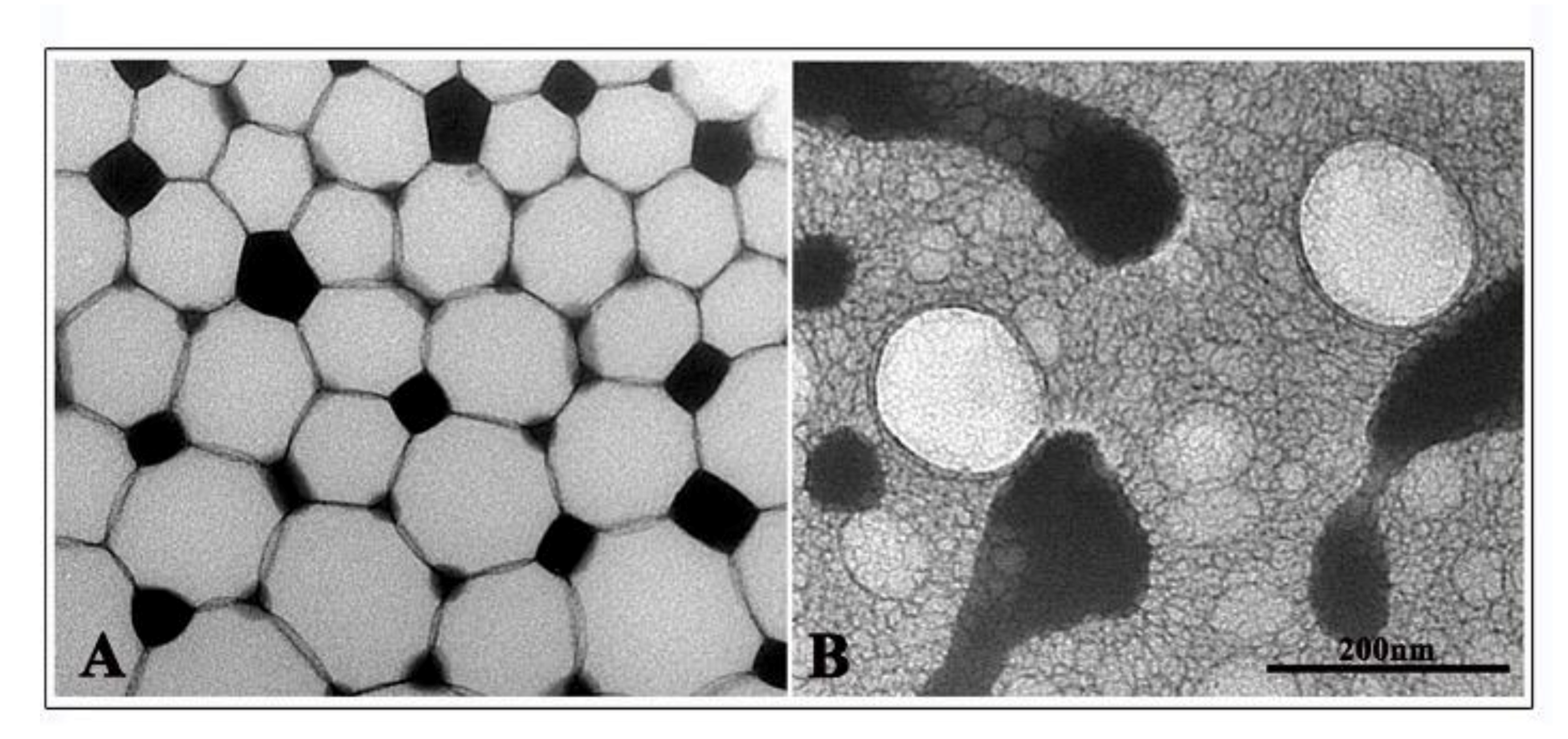
2.8. NE Transmission Electron Microscopy
2.9. Preparation of Polyphenols Solutions
2.10. Cell Culture
2.11. NE Fluorescence Assay
2.12. Effect of Free or NE Loaded RV on T24 Cells by MTT Assay
2.13. Effect of RV/TS or NR/NS on T24 Cells by Trypan Blue Assay
2.14. Reactive Oxygen Species (ROS) Production
2.15. Statistical Analysis
3. Results and Discussion
3.1. NE Design and Characterization
3.2. Characterization Studies: NE Physical–Chemical Features
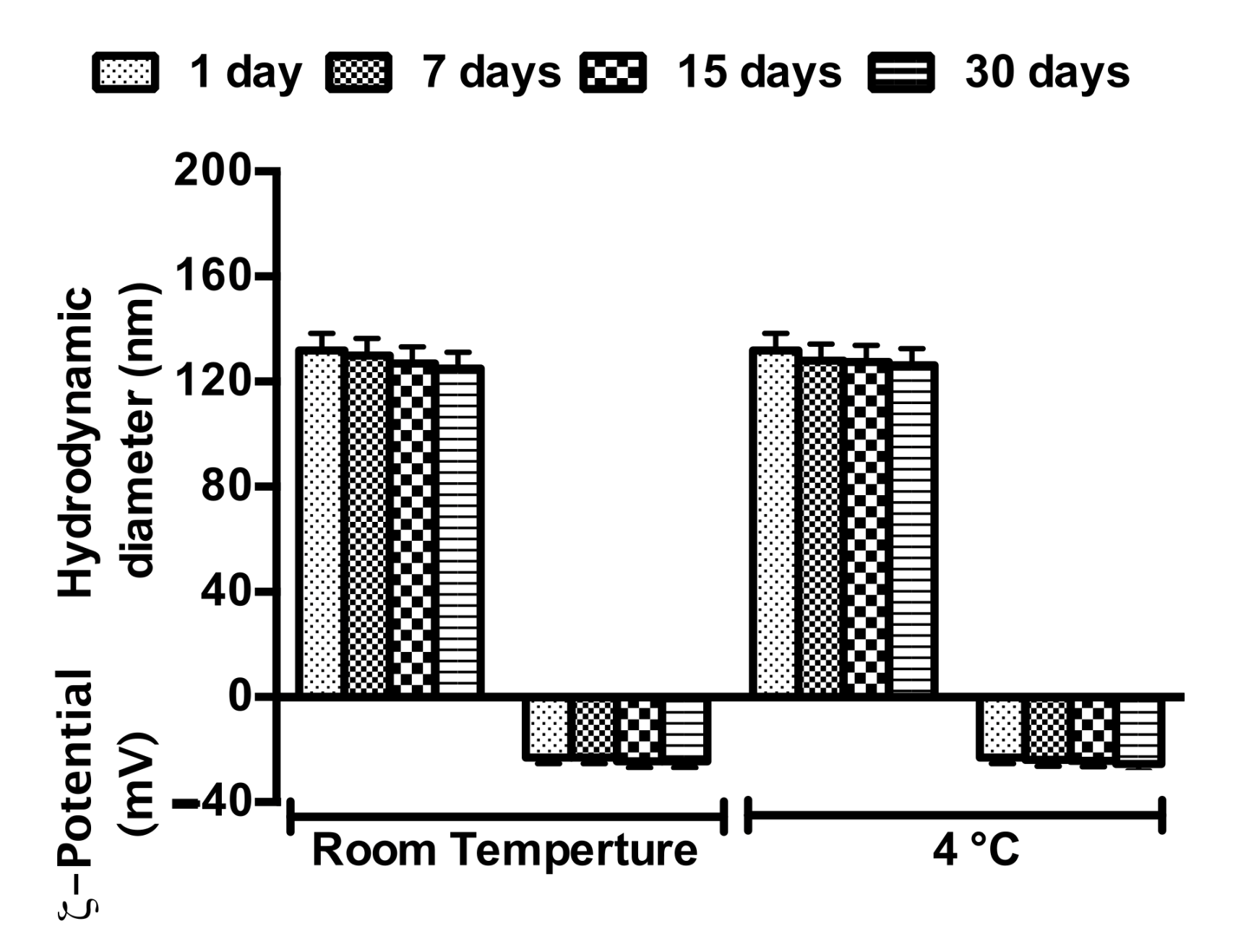
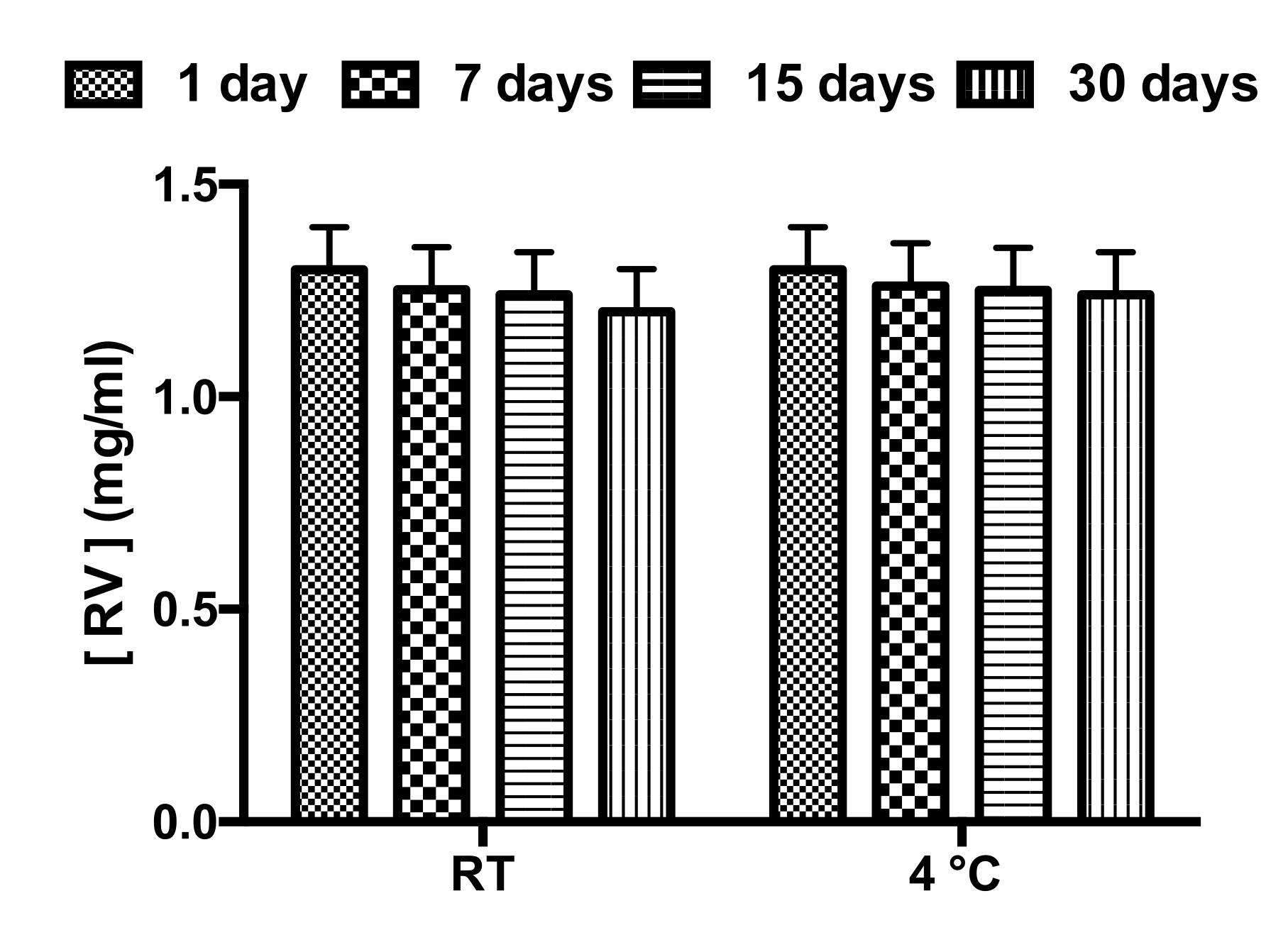
3.3. NE Stability
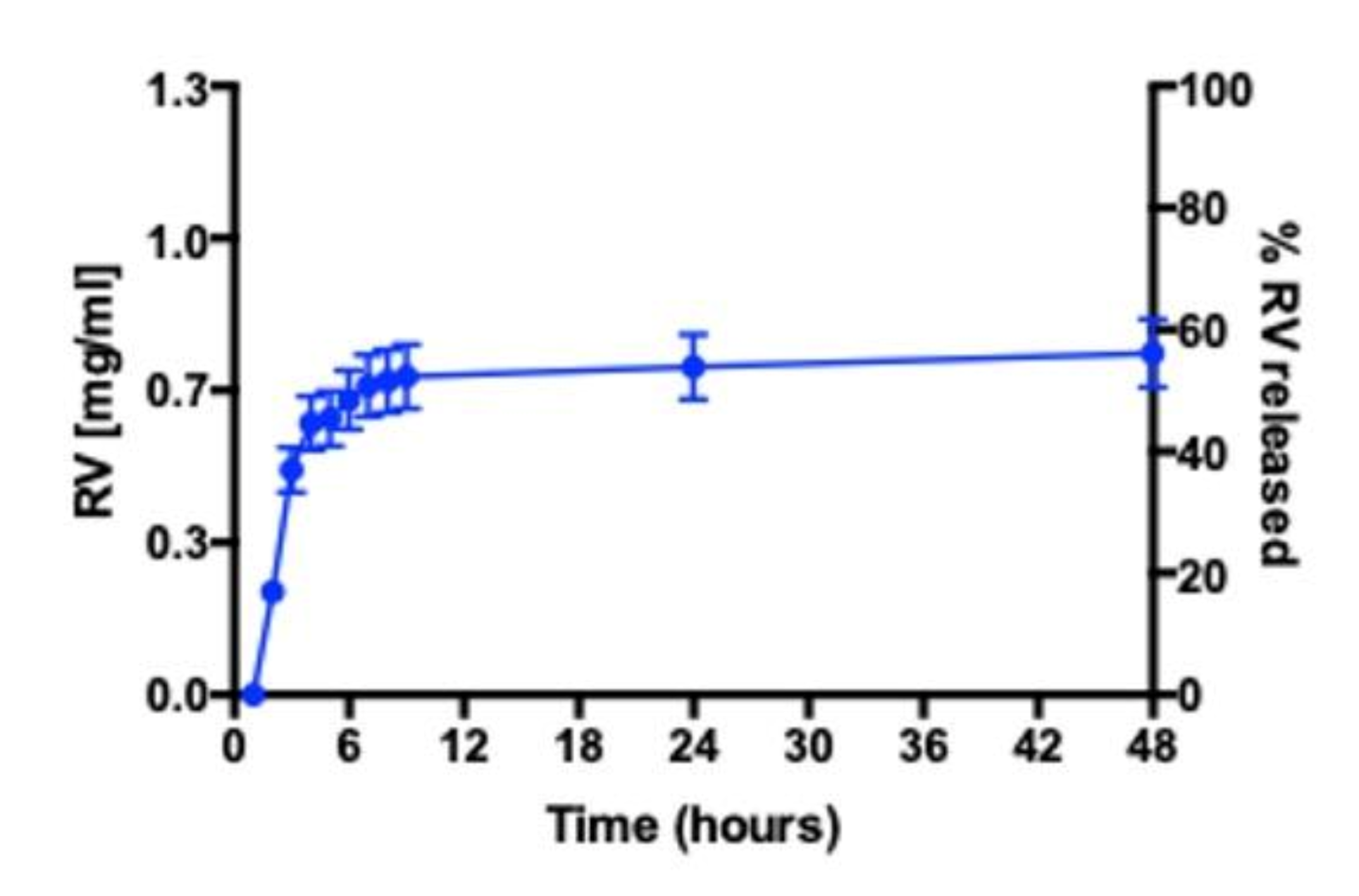
3.4. RV Release Studies in RPMI 1640 Medium
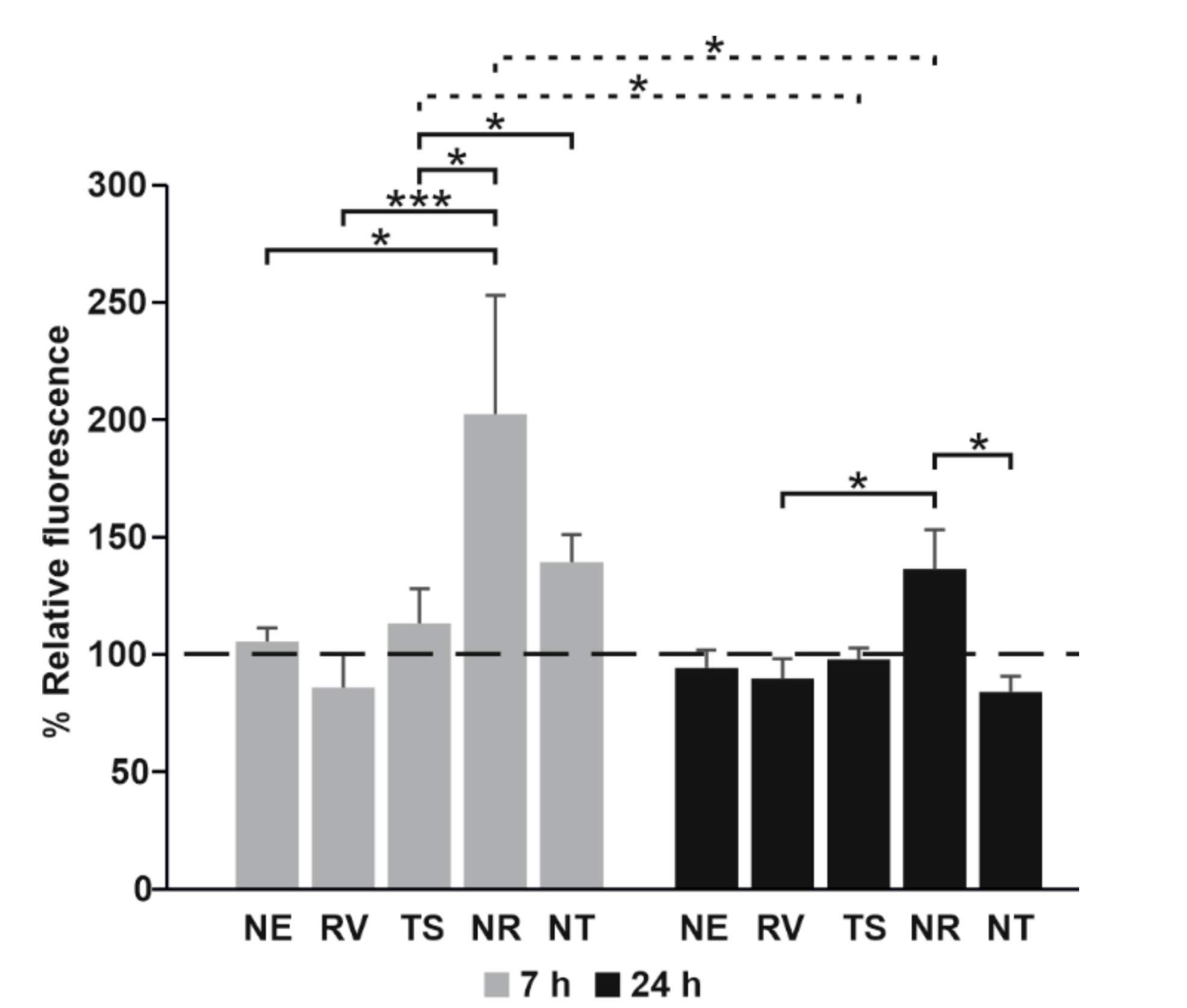
3.5. Cell Internalization of NE Loaded with Nile Red by Fluorescence Assay
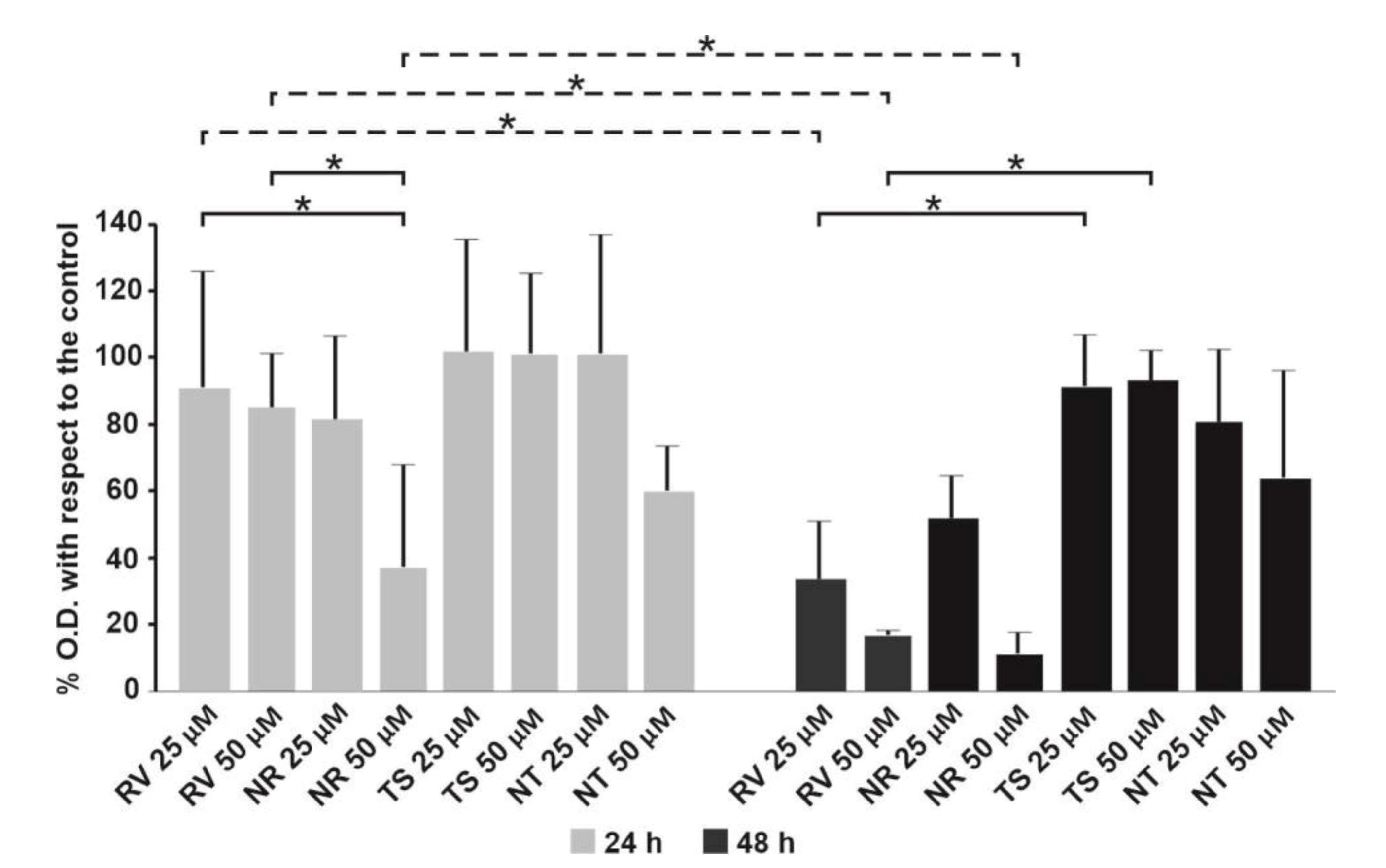
3.6. Effect of Free RV or Loaded in NEs towards T24 Bladder Cell Monolayers
3.7. Evaluation of the Oxidative Stress of T24 Cells Treated with Free RV or Loaded in NEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, C.-F.; Yang, J.-Y.; Wang, F.; Wang, X.-X. Resveratrol: Botanical Origin, Pharmacological Activity and Applications. Chin. J. Nat. Med. 2013, 11, 1–15. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol Addiction: To Die or Not to Die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsì, F. Evaluation of the Stability and Antioxidant Activity of Nanoencapsulated Resveratrol during in Vitro Digestion. J. Agric. Food Chem. 2011, 59, 12352–12360. [Google Scholar] [CrossRef]
- Aziz, M.H.; Nihal, M.; Fu, V.X.; Jarrard, D.F.; Ahmad, N. Resveratrol-Caused Apoptosis of Human Prostate Carcinoma LNCaP Cells Is Mediated via Modulation of Phosphatidylinositol 3′-Kinase/Akt Pathway and Bcl-2 Family Proteins. Mol. Cancer Ther. 2006, 5, 1335–1341. [Google Scholar] [CrossRef]
- Clément, M.-V.; Hirpara, J.L.; Chawdhury, S.-H.; Pervaiz, S. Chemopreventive Agent Resveratrol, a Natural Product Derived From Grapes, Triggers CD95 Signaling-Dependent Apoptosis in Human Tumor Cells. Blood 1998, 92, 996–1002. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Shih, A.; Cao, H.J.; Davis, F.B.; Davis, P.J.; Lin, H.-Y. Resveratrol-Induced Cyclooxygenase-2 Facilitates P53-Dependent Apoptosis in Human Breast Cancer Cells. Mol. Cancer Ther. 2006, 5, 2034–2042. [Google Scholar] [CrossRef]
- Almeida, T.C.; da Silva, G.N. Resveratrol Effects in Bladder Cancer: A Mini Review. Genet. Mol. Biol. 2021, 44. [Google Scholar] [CrossRef]
- Bai, Y.; Mao, Q.-Q.; Qin, J.; Zheng, X.-Y.; Wang, Y.-B.; Yang, K.; Shen, H.-F.; Xie, L.-P. Resveratrol Induces Apoptosis and Cell Cycle Arrest of Human T24 Bladder Cancer Cells in Vitro and Inhibits Tumor Growth in Vivo. Cancer Sci. 2010, 101, 488–493. [Google Scholar] [CrossRef]
- Rajan, M.; Raj, V. Formation and Characterization of Chitosan-Polylacticacid-Polyethylene Glycol-Gelatin Nanoparticles: A Novel Biosystem for Controlled Drug Delivery. Carbohydr. Polym. 2013, 98, 951–958. [Google Scholar] [CrossRef]
- Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Parekh, H.S.; Popat, A. Enhancing Delivery and Cytotoxicity of Resveratrol through a Dual Nanoencapsulation Approach. J. Colloid Interface Sci. 2016, 462, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Qu, Z.; Pujara, N.; Sheng, Y.; Jambhrunkar, S.; McGuckin, M.; Popat, A. Colloidal Mesoporous Silica Nanoparticles Enhance the Biological Activity of Resveratrol. Colloids Surf. B Biointerfaces 2016, 144, 1–7. [Google Scholar] [CrossRef]
- Donsì, F.; Senatore, B.; Huang, Q.; Ferrari, G. Development of Novel Pea Protein-Based Nanoemulsions for Delivery of Nutraceuticals. J. Agric. Food Chem. 2010, 58, 10653–10660. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Wang, Y.; Huang, Q. Freeze–Thaw Stability of Lecithin and Modified Starch-Based Nanoemulsions. Food Hydrocoll. 2011, 25, 1327–1336. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of Essential Oils to Enhance Their Antimicrobial Activity in Foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Pouton, C.W.; Cuine, J.F.; Charman, W.N. Enhancing Intestinal Drug Solubilisation Using Lipid-Based Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef]
- Giuliani, A.; Balducci, A.G.; Zironi, E.; Colombo, G.; Bortolotti, F.; Lorenzini, L.; Galligioni, V.; Pagliuca, G.; Scagliarini, A.; Calzà, L.; et al. In Vivo Nose-to-Brain Delivery of the Hydrophilic Antiviral Ribavirin by Microparticle Agglomerates. Drug Deliv. 2018, 25, 376–387. [Google Scholar] [CrossRef]
- Rinaldi, F.; Oliva, A.; Sabatino, M.; Imbriano, A.; Hanieh, P.N.; Garzoli, S.; Mastroianni, C.M.; De Angelis, M.; Miele, M.C.; Arnaut, M.; et al. Antimicrobial Essential Oil Formulation: Chitosan Coated Nanoemulsions for Nose to Brain Delivery. Pharmaceutics 2020, 12, 678. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Longhi, C.; Carradori, S.; Secci, D.; Zengin, G.; Ammendolia, M.G.; Mattia, E.; Del Favero, E.; Marianecci, C.; et al. Neem Oil Nanoemulsions: Characterisation and Antioxidant Activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. CONTIN: A General Purpose Constrained Regularization Program for Inverting Noisy Linear Algebraic and Integral Equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- De Vos, C.; Deriemaeker, L.; Finsy, R. Quantitative Assessment of the Conditioning of the Inversion of Quasi-Elastic and Static Light Scattering Data for Particle Size Distributions. Langmuir 1996, 12, 2630–2636. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Imbriano, A.; Passeri, D.; Del Favero, E.; Rossi, M.; Marianecci, C.; De Panfilis, S.; Carafa, M. Different Instrumental Approaches to Understand the Chitosan Coated Niosomes/Mucin Interaction. J. Drug Deliv. Sci. Technol. 2020, 55, 101339. [Google Scholar] [CrossRef]
- Zacharzass, K.A. Intramolecular excimer formation with diarylalkanes as a microfluidity probe for sodium dodecyl sulphate micelles. Chem. Phys. Lett. 1978, 57, 429–432. [Google Scholar] [CrossRef]
- Rinaldi, F.; Del Favero, E.; Rondelli, V.; Pieretti, S.; Bogni, A.; Ponti, J.; Rossi, F.; Di Marzio, L.; Paolino, D.; Marianecci, C.; et al. PH-Sensitive Niosomes: Effects on Cytotoxicity and on Inflammation and Pain in Murine Models. J. Enzym. Inhib. Med. Chem. 2017, 32, 538–546. [Google Scholar] [CrossRef]
- Brubacher, J.L.; Bols, N.C. Chemically De-Acetylated 2′,7′-Dichlorodihydrofluorescein Diacetate as a Probe of Respiratory Burst Activity in Mononuclear Phagocytes. J. Immunol. Methods 2001, 251, 81–91. [Google Scholar] [CrossRef]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.P.; Srivastava, P. A Critical Review of Synthesis Procedures, Applications and Future Potential of Nanoemulsions. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar] [CrossRef]
- Stepanovic, S.; Cirkovic, I.; Ranin, L.; Svabic-Vlahovic, M. Biofilm Formation by Salmonella Spp. and Listeria Monocytogenes on Plastic Surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Mahdi, Z.H.; Maraie, N.K. Overview on Nanoemulsion as a Recently Developed Approach in Drug Nanoformulation. Res. J. Pharm. Technol. 2019, 12, 5554. [Google Scholar] [CrossRef]
- Haddick, L.; Zhang, W.; Reinhard, S.; Möller, K.; Engelke, H.; Wagner, E.; Bein, T. Particle-Size-Dependent Delivery of Antitumoral MiRNA Using Targeted Mesoporous Silica Nanoparticles. Pharmaceutics 2020, 12, 505. [Google Scholar] [CrossRef]
- Decreased Drug Resistance of Bladder Cancer Using Phytochemicals Treatment-Cho-2021—The Kaohsiung Journal of Medical Sciences—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/kjm2.12306 (accessed on 9 June 2021).
- Lin, X.; Wu, G.; Huo, W.-Q.; Zhang, Y.; Jin, F.-S. Resveratrol Induces Apoptosis Associated with Mitochondrial Dysfunction in Bladder Carcinoma Cells: Resveratrol Induces Apoptosis. Int. J. Urol. 2012, 19, 757–764. [Google Scholar] [CrossRef]
- Stocco, C. Tissue Physiology and Pathology of Aromatase. Steroids 2012, 77, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kuwajerwala, N.; Cifuentes, E.; Gautam, S.; Menon, M.; Barrack, E.R.; Reddy, G.P.V. Resveratrol Induces Prostate Cancer Cell Entry into S Phase and Inhibits DNA Synthesis. Cancer Res. 2002, 6, 2488–2492. [Google Scholar]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol Scavenges Reactive Oxygen Species and Effects Radical-Induced Cellular Responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Wang, X.-H.; Wang, H.-P.; Hu, L.-Q.; Zheng, X.-M.; Li, S.-W. Capsaicin Mediates Cell Death in Bladder Cancer T24 Cells Through Reactive Oxygen Species Production and Mitochondrial Depolarization. Urology 2010, 75, 735–741. [Google Scholar] [CrossRef]
- Zhou, C.; Ding, J.; Wu, Y. Resveratrol Induces Apoptosis of Bladder Cancer Cells via MiR-21 Regulation of the Akt/Bcl-2 Signaling Pathway. Mol. Med. Rep. 2014, 9, 1467–1473. [Google Scholar] [CrossRef] [PubMed]


| Sample | Neem Oil (mg/mL) | Tw 20 (mg/mL) | RV (mg/mL) | TS (mg/mL) |
|---|---|---|---|---|
| N | 9.2 | 18.4 | - | - |
| NR | 2.5 | - | ||
| NT | - | 2.5 |
| Sample | Hydrodynamic Diameter (nm) ± SD | ζ-Potential (mV) ± SD | PDI ± SD | RV (mg/mL) | TS (mg/mL) | Polarity (I1/I3) | Microviscosity (IE/I3) |
|---|---|---|---|---|---|---|---|
| N | 38.8 ± 0.7 | −15.7 ± 0.4 | 0.21 ± 0.1 | - | - | 1.19 | 0.94 |
| NR | 137.8 ± 0.5 | −23.0 ± 0.7 | 0.22 ± 0.1 | 1.3 | - | 1.64 | 1.15 |
| NT | 156.2 ± 3.2 | −32.6 ± 1.0 | 0.26 ± 0.1 | - | 1.1 | 1.70 | 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, F.; Maurizi, L.; Forte, J.; Marazzato, M.; Hanieh, P.N.; Conte, A.L.; Ammendolia, M.G.; Marianecci, C.; Carafa, M.; Longhi, C. Resveratrol-Loaded Nanoemulsions: In Vitro Activity on Human T24 Bladder Cancer Cells. Nanomaterials 2021, 11, 1569. https://doi.org/10.3390/nano11061569
Rinaldi F, Maurizi L, Forte J, Marazzato M, Hanieh PN, Conte AL, Ammendolia MG, Marianecci C, Carafa M, Longhi C. Resveratrol-Loaded Nanoemulsions: In Vitro Activity on Human T24 Bladder Cancer Cells. Nanomaterials. 2021; 11(6):1569. https://doi.org/10.3390/nano11061569
Chicago/Turabian StyleRinaldi, Federica, Linda Maurizi, Jacopo Forte, Massimiliano Marazzato, Patrizia Nadia Hanieh, Antonietta Lucia Conte, Maria Grazia Ammendolia, Carlotta Marianecci, Maria Carafa, and Catia Longhi. 2021. "Resveratrol-Loaded Nanoemulsions: In Vitro Activity on Human T24 Bladder Cancer Cells" Nanomaterials 11, no. 6: 1569. https://doi.org/10.3390/nano11061569
APA StyleRinaldi, F., Maurizi, L., Forte, J., Marazzato, M., Hanieh, P. N., Conte, A. L., Ammendolia, M. G., Marianecci, C., Carafa, M., & Longhi, C. (2021). Resveratrol-Loaded Nanoemulsions: In Vitro Activity on Human T24 Bladder Cancer Cells. Nanomaterials, 11(6), 1569. https://doi.org/10.3390/nano11061569