Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical
Abstract
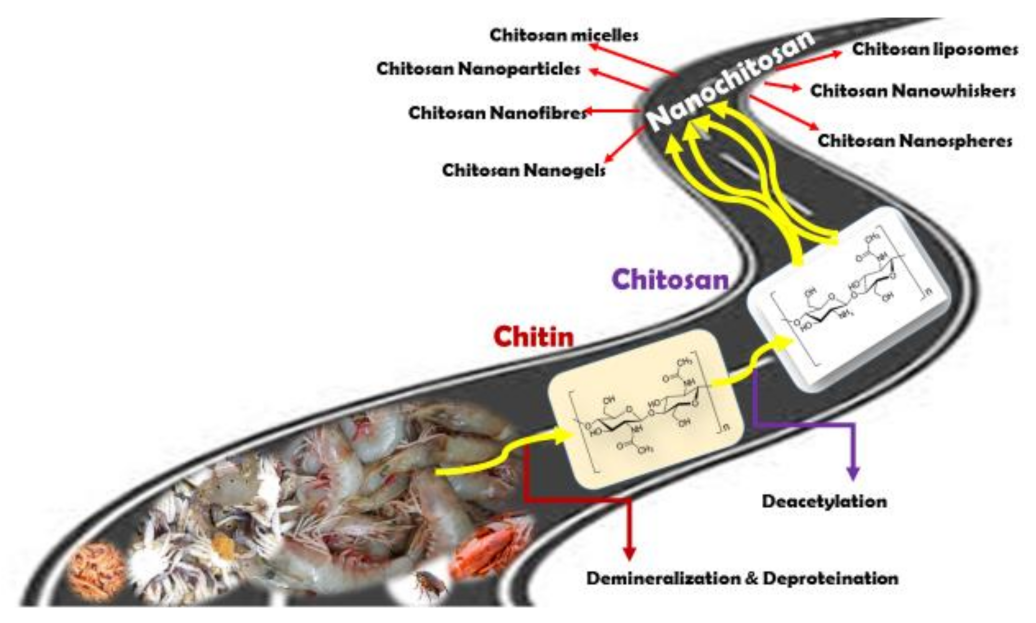
1. Introduction
2. Preparation of Nanochitosan
Methods of Nanochitosan Preparations
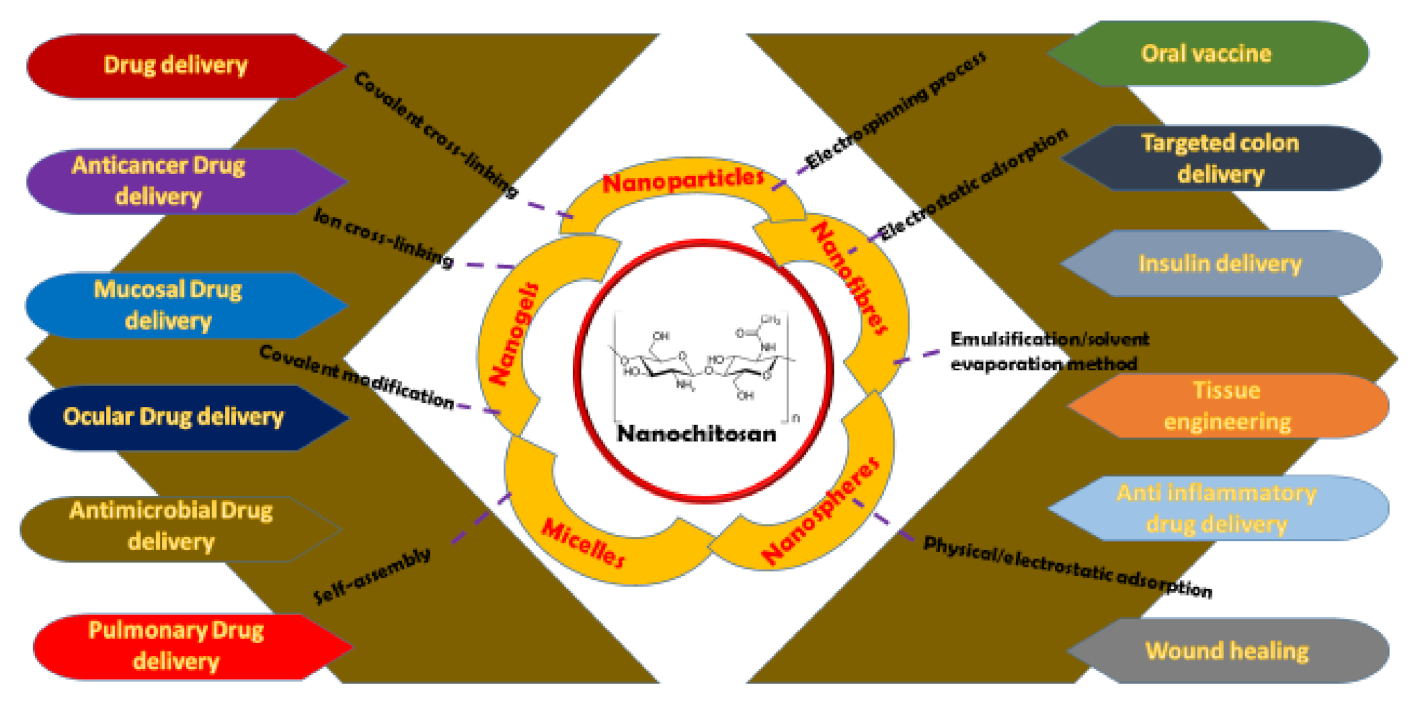
3. Biomedical Milestones of Nanochitosan
3.1. Antitumor Applications of Nanochitosan
3.2. Drug Delivery Applications of Nanochitosan
3.3. Miscellaneous Applications of Nanochitosan
4. Challenges Facing Nanochitosan Applications and Future Perspectives
4.1. Clinical Challenges of Nanochitosan
4.2. Limiting Challenges in Nanomodifications of Chitosan
4.3. Limitations in Biomedical Applications of Nanochitosan
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stocker, H.; Kaiser, S.; Niederschlag, E.; Gartner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Vilsinski, B.H.; Bonafe, E.G.; Monteiro, J.P.; Kipper, M.J.; Martins, A.F. Chitosan content modulates durability and structural homogeneity of chitosan-gellan gum assemblies. Int. J. Biol. Macromol. 2019, 128, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Kurita, K. Chemistry and application of chitin and chitosan. Polym. Degrad. Stabil. 1998, 59, 117–120. [Google Scholar] [CrossRef]
- Hudson, S.M.; Smith, C. Polysaccharides: Chitin and chitosan: Chemistry and technology of their use as structural materials. In Biopolymers from renewable resources; Kaplan, D.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 96–118. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.H.; Liu, J.J.; Zhou, Q.F.; Huang, L.; Sun, T. Synthesis of Modified Chitosan Superplasticizer by Amidation and Sulfonation and Its Application Performance and Working Mechanism. Ind. Eng. Chem. Res. 2014, 53, 3908–3916. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Vauthier, C.; Farabollini, A.; Palmieri, G.F.; Ponchel, G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials 2007, 28, 2233–2243. [Google Scholar] [CrossRef]
- Dunnhaupt, S.; Barthelmes, J.; Rahmat, D.; Leithner, K.; Thurner, C.C.; Friedl, H.; Bernkop-Schnurch, A. S-Protected Thiolated Chitosan for Oral Delivery of Hydrophilic Macromolecules: Evaluation of Permeation Enhancing and Efflux Pump Inhibitory Properties. Mol. Pharm. 2012, 9, 1331–1341. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Needleman, I.G.; Smales, F.C.; Martin, G.P. An investigation of bioadhesion for periodontal and oral mucosal drug delivery. J. Clin. Periodontol. 1997, 24, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Felt, O.; Furrer, P.; Mayer, J.M.; Plazonnet, B.; Buri, P.; Gurny, R. Topical use of chitosan in ophthalmology: Tolerance assessment and evaluation of precorneal retention. Int. J. Pharmaceut. 1999, 180, 185–193. [Google Scholar] [CrossRef]
- Magetsari Ph, D.R.; Dewo Ph, D.P.; Saputro Md, B.K.; Lanodiyu Md, Z. Cinnamon Oil and Chitosan Coating on Orthopaedic Implant Surface for Prevention of Staphylococcus Epidermidis Biofilm Formation. Malays. Orthop. J. 2014, 8, 11–14. [Google Scholar] [CrossRef]
- Fu, S.; Xia, J.; Wu, J. Functional Chitosan Nanoparticles in Cancer Treatment. J. Biomed. Nanotechnol. 2016, 12, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Xu, F.; Han, Z.; Wei, D.; Jia, D.; Zhou, Y. Tough Magnetic Chitosan Hydrogel Nanocomposites for Remotely Stimulated Drug Release. Biomacromolecules 2018, 19, 3351–3360. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 28796. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Li, Q.; Zou, Q.; Du, C. Biomimetic mineralization of carboxymethyl chitosan nanofibers with improved osteogenic activity in vitro and in vivo. Carbohydr. Polym. 2018, 195, 225–234. [Google Scholar] [CrossRef]
- Yan, L.; Crayton, S.H.; Thawani, J.P.; Amirshaghaghi, A.; Tsourkas, A.; Cheng, Z. A pH-Responsive Drug-Delivery Platform Based on Glycol Chitosan-Coated Liposomes. Small 2015, 11, 4870–4874. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.K.; Frisch, S.; Biehl, A.; Terriac, E.; De Rossi, C.; Schwarzkopf, K.; Lautenschlager, F.; Loretz, B.; Murgia, X.; Lehr, C.M. Farnesylated Glycol Chitosan as a Platform for Drug Delivery: Synthesis, Characterization, and Investigation of Mucus-Particle Interactions. Biomacromolecules 2018, 19, 3489–3501. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Bio. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Martinez-Martinez, M.; Rodriguez-Berna, G.; Gonzalez-Alvarez, I.; Hernandez, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic Hydrogel Based on Chitosan Cross-Linked with 6-Phosphogluconic Trisodium Salt as a Drug Delivery System. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.X.; Chen, X.M.; Gong, H.Z.; Qiu, P.; Xiao, X.; Dang, S.Y.; Hong, A.; Ma, Y. Delivery of a TNF--derived peptide by nanoparticles enhances its antitumor activity by inducing cell-cycle arrest and caspase-dependent apoptosis. Faseb J. 2018, 32, 6948–6964. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, Q.B.; Zhang, Z.; Wang, L.X.; Kang, Y.J.; Denning, T.; Merlin, D. TNF alpha gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control. Release 2018, 287, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitin, 1st ed.; Pergamon: Oxford, UK, 1977; pp. 51–55. [Google Scholar]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Marine Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Mao, S.R.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliver. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Wang, S.H.; Xu, T.; Yang, Y.H.; Shao, Z.Z. Colloidal Stability of Silk Fibroin Nanoparticles Coated with Cationic Polymer for Effective Drug Delivery. ACS Appl. Mater. Inter. 2015, 7, 21254–21262. [Google Scholar] [CrossRef]
- Lin, J.Y.; Li, Y.; Li, Y.X.; Wu, H.J.; Yu, F.; Zhou, S.F.; Xie, L.Y.; Luo, F.H.; Lin, C.J.; Hou, Z.Q. Drug/Dye-Loaded, Multifunctional PEG-Chitosan-Iron Oxide Nanocomposites for Methotraxate Synergistically Self-Targeted Cancer Therapy and Dual Model Imaging. Acs. Appl. Mater. Inter. 2015, 7, 11908–11920. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Bio. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Dobhal, A.; Bangde, P.; Dey, A.; Dandekar, P.; Jain, R. Chitosan-Based Nanoparticulate Systems: Implication Towards Therapeutics Application. In Particulate Technology for Delivery of Therapeutics; Jana, S., Ed.; Springer: Singapore, 2017; pp. 167–225. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Li, J.H.; Cai, C.; Li, J.R.; Li, J.; Li, J.; Sun, T.T.; Wang, L.H.; Wu, H.T.; Yu, G.L. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloid Surface B 2006, 53, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Martins, S.; Ribeiro, A.; Veiga, F.; Neufeld, R.; Ferreira, D. Development and comparison of different nanoparticulate polyelectrolyte complexes as insulin carriers. Int. J. Pept. Res. Ther. 2006, 12, 131–138. [Google Scholar] [CrossRef]
- Yang, S.J.; Lin, F.H.; Tsai, H.M.; Lin, C.F.; Chin, H.C.; Wong, J.M.; Shieh, M.J. Alginate-folic acid-modified chitosan nanoparticles for photodynamic detection of intestinal neoplasms. Biomaterials 2011, 32, 2174–2182. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed.-Nanotechnol. Biol. Med. 2010, 6, 58–63. [Google Scholar] [CrossRef]
- Du, J.; Sun, R.; Zhang, S.; Govender, T.; Zhang, L.F.; Xiong, C.D.; Peng, Y.X. Novel polyelectrolyte carboxymethyl konjac glucomannan-chitosan nanoparticles for drug delivery. Macromol. Rapid Commun. 2004, 25, 954–958. [Google Scholar] [CrossRef]
- Kaihara, S.; Suzuki, Y.; Fujimoto, K. In situ synthesis of polysaccharide nanoparticles via polyion complex of carboxymethyl cellulose and chitosan. Colloid Surface B 2011, 85, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.K.; Cheng, K.M.; Hu, C.S.; Huang, Y.C.; Young, J.J. Novel protein-loaded chondroitin sulfate-chitosan nanoparticles: Preparation and characterization. Acta. Biomater. 2011, 7, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- Grabnar, P.A.; Kristl, J. Physicochemical characterization of protein-loaded pectin-chitosan nanoparticles prepared by polyelectrolyte complexation. Pharmazie 2010, 65, 851–852. [Google Scholar] [CrossRef]
- Liu, Z.G.; Jiao, Y.P.; Liu, F.N.; Zhang, Z.Y. Heparin/chitosan nanoparticle carriers prepared by polyelectrolyte complexation. J. Biomed. Mater. Res. Part A 2007, 83, 806–812. [Google Scholar] [CrossRef]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest. Ophthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef]
- Elsayed, A.; Al-Remawi, M.; Qinna, N.; Farouk, A.; Al-Sou’od, K.A.; Badwan, A.A. Chitosan-Sodium Lauryl Sulfate Nanoparticles as a Carrier System for the In Vivo Delivery of Oral Insulin. Aaps. Pharmscitech. 2011, 12, 958–964. [Google Scholar] [CrossRef]
- Teijeiro-Osorio, D.; Remunan-Lopez, C.; Alonso, M.J. New Generation of Hybrid Poly/Oligosaccharide Nanoparticles as Carriers for the Nasal Delivery of Macromolecules. Biomacromolecules 2009, 10, 243–249. [Google Scholar] [CrossRef]
- Lin, Y.H.; Mi, F.L.; Chen, C.T.; Chang, W.C.; Peng, S.F.; Liang, H.F.; Sung, H.W. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules 2007, 8, 146–152. [Google Scholar] [CrossRef]
- Lin, Y.H.; Sonaje, K.; Lin, K.M.; Juang, J.H.; Mi, F.L.; Yang, H.W.; Sung, H.W. Multi-ion-crosslinked nanoparticles with pH-responsive characteristics for oral delivery of protein drugs. J. Control. Release 2008, 132, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, X.; Ding, Y.; Ge, H.; Yuan, Y.; Yang, C. Synthesis and characterization of chitosan-poly(acrylic acid) nanoparticles. Biomaterials 2002, 23, 3193–3201. [Google Scholar] [CrossRef]
- Bayat, A.; Larijani, B.; Ahmadian, S.; Junginger, H.E.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and its quaternized derivatives. Nanomed.-Nanotechnol. Biol. Med. 2008, 4, 115–120. [Google Scholar] [CrossRef]
- Erbacher, P.; Zou, S.; Bettinger, T.; Steffan, A.M.; Remy, J.S. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm Res 1998, 15, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dai, W.; Wang, Z.; Chen, B.; Li, Z.; Fan, X. Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae. Clin. Vaccine Immunol. 2011, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M.; Lollo, G.; Remunan-Lopez, C.; Quaglia, F.; Alonso, M.J. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef]
- Alonso-Sande, M.; Cuna, M.; Remunan-Lopez, C. Formation of new glucomannan-chitosan nanoparticles and study of their ability to associate and deliver proteins. Macromolecules 2006, 39, 4152–4158. [Google Scholar] [CrossRef]
- Ohya, Y.; Shiratani, M.; Kobayashi, H.; Ouchi, T. Release Behavior of 5-Fluorouracil from Chitosan-Gel Nanospheres Immobilizing 5-Fluorouracil Coated with Polysaccharides and Their Cell-Specific Cytotoxicity. J. Macromol. Sci.-Pure Appl. Chem. 1994, 31, 629–642. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Ichikawa, H.; Fukumori, Y.; Block, L.H. Preparation of gadopentetic acid-loaded chitosan microparticles for gadolinium neutron-capture therapy of cancer by a novel emulsion-droplet coalescence technique. Chem. Pharm. Bull. 1999, 47, 838–842. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharmaceut. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control. Release 2001, 74, 317–323. [Google Scholar] [CrossRef]
- Calvo, P.; RemunanLopez, C.; VilaJato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Tian, X.X.; Groves, M.J. Formulation and biological activity of antineoplastic proteoglycans derived from Mycobacterium vaccae in chitosan nanoparticles. J. Pharm. Pharmacol. 1999, 51, 151–157. [Google Scholar] [CrossRef]
- Chan, H.K.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef]
- Wang, J.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. More Effective Nanomedicines through Particle Design. Small 2011, 7, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.M.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef]
- Kawashima, Y.; Handa, T.; Kasai, A.; Takenaka, H.; Lin, S.Y.; Ando, Y. Novel method for the preparation of controlled-release theophylline granules coated with a polyelectrolyte complex of sodium polyphosphate-chitosan. J. Pharm. Sci. 1985, 74, 264–268. [Google Scholar] [CrossRef]
- Bhattarai, N.; Ramay, H.R.; Chou, S.H.; Zhang, M. Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int. J. Nanomedicine 2006, 1, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef]
- Zhang, S.J.; Wu, L.X. Amyloid-Beta Associated with Chitosan Nano-Carrier has Favorable Immunogenicity and Permeates the BBB. Aaps. Pharmscitech. 2009, 10, 900–905. [Google Scholar] [CrossRef]
- Ngan, L.T.K.; Wang, S.L.; Hiep, D.M.; Luong, P.M.; Vui, N.T.; Dinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Sinsuebpol, C.; Chatchawalsaisin, J.; Kulvanich, P. Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery. Drug. Des. Dev. Ther. 2013, 7, 861–873. [Google Scholar] [CrossRef]
- Li, F.Q.; Ji, R.R.; Chen, X.; You, B.M.; Pan, Y.H.; Su, J.C. Cetirizine Dihydrochloride Loaded Microparticles Design Using Ionotropic Cross-linked Chitosan Nanoparticles by Spray-drying Method. Arch. Pharm. Res. 2010, 33, 1967–1973. [Google Scholar] [CrossRef]
- Mehrotra, A.; Nagarwal, R.C.; Pandit, J.K. Fabrication of Lomustine Loaded Chitosan Nanoparticles by Spray Drying and in Vitro Cytostatic Activity on Human Lung Cancer Cell Line L132. J. Nanomed. Nanotechnol. 2010, 1, 1–7. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Ichikawa, H.; Fukumori, Y. Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: Preparation by novel emulsion-droplet coalescence technique and characterization. Pharm. Res. 1999, 16, 1830–1835. [Google Scholar] [CrossRef]
- Reddy, Y.D.; Dhachinamoorthi, D. Formulation and in vitro evaluation of antineoplastic drug loaded nanoparticles as drug delivery system. Afr. J. Pharm. Pharmacol. 2013, 7, 1592–1604. [Google Scholar] [CrossRef]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. Preparations of Biodegradable Nanospheres of Water-Soluble and Insoluble Drugs with D,L-Lactide Glycolide Copolymer by a Novel Spontaneous Emulsification Solvent Diffusion Method, and the Drug Release Behavior. J. Control. Release 1993, 25, 89–98. [Google Scholar] [CrossRef]
- Luque-Alcaraz, A.G.; Lizardi-Mendoza, J.; Goycoolea, F.M.; Higuera-Ciapara, I.; Arguelles-Monal, W. Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. Rsc Adv 2016, 6, 59250–59256. [Google Scholar] [CrossRef]
- Melo, E.P.; Aires-Barros, M.R.; Cabral, J.M. Reverse micelles and protein biotechnology. Biotechnol. Annu. Rev. 2001, 7, 87–129. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse micelles used as templates: A new understanding in nanocrystal growth. J. Exp. Nanosci. 2006, 1, 13–27. [Google Scholar] [CrossRef]
- Banerjee, T.; Mitra, S.; Singh, A.K.; Sharma, R.K.; Maitra, A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int. J. Pharmaceut. 2002, 243, 93–105. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Khorram, M.; Mansouri, M.; Samimi, A.; Osfouri, S. Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran Polym. J. 2012, 21, 99–107. [Google Scholar] [CrossRef]
- Alonso, M.J. Nanoparticulate drug carrier technology. In Microparticulate systems for the delivery of proteins and vaccines; Cohen, S., Bernstein, H., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 203–242. [Google Scholar]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliver. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef]
- Poncelet, D. Microencapsulation: Fundamentals, methods and applications. In Surface chemistry in biomedical and environmental science; Blitz, J.P., Gun’ko, V.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 23–24. [Google Scholar]
- Sajeesh, S.; Sharma, C.P. Cyclodextrin-insulin complex encapsulated polymethacrylic acid based nanoparticles for oral insulin delivery. Int. J. Pharm. 2006, 325, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, S.; Sharma, C.P. Novel pH responsive polymethacrylic acid-chitosan-polyethylene glycol nanoparticles for oral peptide delivery. J. Biomed. Mater. Res. B. Appl. Biomater. 2006, 76, 298–305. [Google Scholar] [CrossRef]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Terbojevich, M.; Muzzarelli, R.A.A. Chitosan. In Handbook of hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2009; pp. 367–378. [Google Scholar]
- Mao, H.Q.; Roy, K.; Troung-Le, V.L.; Janes, K.A.; Lin, K.Y.; Wang, Y.; August, J.T.; Leong, K.W. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J. Control. Release 2001, 70, 399–421. [Google Scholar] [CrossRef]
- Li, P.W.; Wang, G.; Yang, Z.M.; Duan, W.; Peng, Z.; Kong, L.X.; Wang, Q.H. Development of drug-loaded chitosan-vanillin nanoparticles and its cytotoxicity against HT-29 cells. Drug Deliv. 2016, 23, 30–35. [Google Scholar] [CrossRef]
- Wang, G.; Li, P.; Peng, Z. Formulation of vanillin cross-linked chitosan nanoparticles and its characterization. Adv. Mater. Res. 2011, 2, 474–477. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Wang, Y.; Chen, H.; Zhang, H.; Gao, F.; Liu, L. Self-aggregated nanoparticles from methoxy poly(ethylene glycol)-modified chitosan: Synthesis; characterization; aggregation and methotrexate release in vitro. Colloids Surf. B Biointerfaces 2008, 61, 125–131. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, L.R.; Jiang, Q.; Zhang, Q.Q. Self-aggregated nanoparticles of cholesterol-modified chitosan conjugate as a novel carrier of epirubicin. Eur. Polym. J. 2007, 43, 43–51. [Google Scholar] [CrossRef]
- Anitha, A.; Deepagan, V.G.; Rani, V.V.D.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Curcumin-Loaded N,O-Carboxymethyl Chitosan Nanoparticles for Cancer Drug Delivery. J. Biomater. Sci.-Polym. Ed. 2012, 23, 1381–1400. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S.; Dong, L.; Wu, L.; Shen, X. Nanoparticles based on the complex of chitosan and polyaspartic acid sodium salt: Preparation, characterization and the use for 5-fluorouracil delivery. Eur. J. Pharm. Biopharm. 2007, 67, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. 5-Flourouracil Loaded N,O-Carboxymethyl Chitosan Nanoparticles as an Anticancer Nanomedicine for Breast Cancer. J. Biomed. Nanotechnol. 2012, 8, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Lee, W.B.; Park, C.R.; Cho, Y.W.; Ahn, C.H.; Kwon, I.C. Preparation and characterization of cisplatin-incorporated chitosan hydrogels, microparticles, and nanoparticles. Macromol. Res. 2006, 14, 573–578. [Google Scholar] [CrossRef]
- Bilensoy, E.; Sarisozen, C.; Esendagli, G.; Dogan, A.L.; Aktas, Y.; Sen, M.; Mungan, N.A. Intravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumors. Int. J. Pharmaceut. 2009, 371, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.N.; Wen, X.J.; Zhou, S.H.; Tong, X.W.; Su, P.P.; Li, H.; Shi, D.L. Anti-tumor activity of paclitaxel-loaded chitosan nanoparticles: An in vitro study. Mat. Sci. Eng. C-Mater. 2009, 29, 2392–2397. [Google Scholar] [CrossRef]
- Vivek, R.; Babu, V.N.; Thangam, R.; Subramanian, K.S.; Kannan, S. pH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloid Sur. B 2013, 111, 117–123. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Kim, I.S.; Kwon, I.C.; Kim, Y.H. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2008, 128, 23–31. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhang, H.; Yin, L.Q.; Hu, R.; Qiu, T.; Yin, Y.H.; Xiong, X.; Zheng, H.; Wang, Q. A pH-Sensitive Nanosystem Based on Carboxymethyl Chitosan for Tumor-Targeted Delivery of Daunorubicin. J. Biomed. Nanotechnol. 2016, 12, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yin, L.Q.; Zhang, X.Q.; Zhang, H.; Hu, R.; Yin, Y.H.; Qiu, T.; Xiong, X.; Wang, Q. Redox Sensitive Shell and Core Crosslinked Hyaluronic Acid Nanocarriers for Tumor-Targeted Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zheng, H.; Cao, J.; Davoudi, Z.; Wang, Q. Synthesis and In Vitro Characterization of Carboxymethyl Chitosan-CBA-Doxorubicin Conjugate Nanoparticles as pH-Sensitive Drug Delivery Systems. J. Biomed. Nanotechnol. 2017, 13, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Lavelle, E. Targeted mucosal delivery of drugs and vaccines. Expert Opin. Ther. Pat. 2000, 10, 179–190. [Google Scholar] [CrossRef]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan for mucosal vaccination. Adv. Drug Deliver. Rev. 2001, 52, 139–144. [Google Scholar] [CrossRef]
- Vila, A.; Sanchez, A.; Tobio, M.; Calvo, P.; Alonso, M.J. Design of biodegradable particles for protein delivery. J. Control. Release 2002, 78, 15–24. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sanchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharmaceut. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Roy, K.; Mao, H.Q.; Huang, S.K.; Leong, K.W. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999, 5, 387–391. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohl, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Baltzley, S.; Mohammad, A.; Malkawi, A.H.; Al-Ghananeem, A.M. Intranasal Drug Delivery of Olanzapine-Loaded Chitosan Nanoparticles. Aaps. Pharmscitech. 2014, 15, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghananeem, A.M.; Saeed, H.; Florence, R.; Yokel, R.A.; Malkawi, A.H. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by AIDS viruses. J. Drug Target. 2010, 18, 381–388. [Google Scholar] [CrossRef]
- Park, I.K.; Park, Y.H.; Shin, B.A.; Choi, E.S.; Kim, Y.R.; Akaike, T.; Cho, C.S. Galactosylated chitosan-graft-dextran as hepatocyte-targeting DNA carrier. J. Control. Release 2000, 69, 97–108. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliver. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Amidi, M.; Romeijn, S.G.; Verhoef, J.C.; Junginger, H.E.; Bungener, L.; Huckriede, A.; Crommelin, D.J.A.; Jiskoot, W. N-Trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: Biological properties and immunogenicity in a mouse model. Vaccine 2007, 25, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Boonyo, W.; Junginger, H.E.; Waranuch, N.; Polnok, A.; Pitaksuteepong, T. Chitosan and trimethyl chitosan chloride (TMC) as adjuvants for inducing immune responses to ovalbumin in mice following nasal administration. J. Control. Release 2007, 121, 168–175. [Google Scholar] [CrossRef]
- Svirshchevskaya, E.V.; Alekseeva, L.G.; Reshetov, P.D.; Phomicheva, N.N.; Parphenyuk, S.A.; Ilyina, A.V.; Zueva, V.S.; Lopatin, S.A.; Levov, A.N.; Varlamov, V.P. Mucoadjuvant properties of lipo- and glycoconjugated derivatives of oligochitosans. Eur. J. Med. Chem. 2009, 44, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Sayin, B.; Somavarapu, S.; Li, X.W.; Thanou, M.; Sesardic, D.; Alpar, H.O.; Senel, S. Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. Int. J. Pharmaceut. 2008, 363, 139–148. [Google Scholar] [CrossRef]
- Borges, O.; Cordeiro-da-Silva, A.; Tavares, J.; Santarem, N.; De Sousa, A.; Borchard, G.; Junginger, H.E. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 69, 405–416. [Google Scholar] [CrossRef]
- Klas, S.D.; Petrie, C.R.; Warwood, S.J.; Williams, M.S.; Olds, C.L.; Stenz, J.P.; Cheff, A.M.; Hinchcliffe, M.; Richardson, C.; Wimer, S. A single immunization with a dry powder anthrax vaccine protects rabbits against lethal aerosol challenge. Vaccine 2008, 26, 5494–5502. [Google Scholar] [CrossRef]
- Frank, M.; Schloissnig, S. Bioinformatics and molecular modeling in glycobiology. Cell Mol. Life Sci. 2010, 67, 2749–2772. [Google Scholar] [CrossRef]
- Baudner, B.C.; Giuliani, M.M.; Verhoef, J.C.; Rappuoli, R.; Junginger, H.E.; Del Giudice, G. The concomitant use of the LTK63 mucosal adjuvant and of chitosan-based delivery system enhances the immunogenicity and efficacy of intranasally administered vaccines. Vaccine 2003, 21, 3837–3844. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Cui, Z.; Mumper, R.J. Chitosan-based nanoparticles for topical genetic immunization. J. Control. Release 2001, 75, 409–419. [Google Scholar] [CrossRef]
- Madrigal-Carballo, S.; Esquivel, M.; Sibaja, M. Protein-loaded chitosan nanoparticles modulate uptake and antigen presentation of hen egg-white lysozyme by murine peritoneal macrophages. Int. J. Nanoparticles 2010, 3, 179–191. [Google Scholar] [CrossRef]
- Xu, W.; Shen, Y.; Jiang, Z.; Wang, Y.; Chu, Y.; Xiong, S. Intranasal delivery of chitosan-DNA vaccine generates mucosal SIgA and anti-CVB3 protection. Vaccine 2004, 22, 3603–3612. [Google Scholar] [CrossRef]
- Smitha, K.T.; Sreelakshmi, M.; Nisha, N.; Jayakumar, R.; Biswas, R. Amidase encapsulated O-carboxymethyl chitosan nanoparticles for vaccine delivery. Int. J. Biol. Macromol. 2014, 63, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Pattani, A.; Patravale, V.B.; Panicker, L.; Potdar, P.D. Immunological Effects and Membrane Interactions of Chitosan Nanoparticles. Mol. Pharmaceut. 2009, 6, 345–352. [Google Scholar] [CrossRef]
- Jamil, B.; Habib, H.; Abbasi, S.; Nasir, H.; Rahman, A.; Rehman, A.; Bokhari, H.; Imran, M. Cefazolin loaded chitosan nanoparticles to cure multi drug resistant Gram-negative pathogens. Carbohydr. Polym. 2016, 136, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Reynolds, J.L.; Law, W.C.; Maponga, C.C.; Prasad, P.N.; Morse, G.D. Multimodal nanoparticles that provide immunomodulation and intracellular drug delivery for infectious diseases. Nanomed.-Nanotechnol. Biol. Med. 2014, 10, 831–838. [Google Scholar] [CrossRef]
- Garg, T.; Rath, G.; Goyal, A.K. Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis. Artif. Cell. Nanomed. Biotechnol. 2016, 44, 997–1001. [Google Scholar] [CrossRef]
- Moretton, M.A.; Chiappetta, D.A.; Andrade, F.; Das Neves, J.; Ferreira, D.; Sarmento, B.; Sosnik, A. Hydrolyzed Galactomannan-Modified Nanoparticles and Flower-Like Polymeric Micelles for the Active Targeting of Rifampicin to Macrophages. J. Biomed. Nanotechnol. 2013, 9, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Junise, V.; Saraswathi, R. Development and characterization of inhaled chitosan nanoparticles loaded with Isoniazid. J. pharm. technol. res. manag. 2014, 2, 159–170. [Google Scholar] [CrossRef]
- Maya, S.; Indulekha, S.; Sukhithasri, V.; Smitha, K.T.; Nair, S.V.; Jayakumar, R.; Biswas, R. Efficacy of tetracycline encapsulated O-carboxymethyl chitosan nanoparticles against intracellular infections of Staphylococcus aureus. Int. J. Biol. Macromol. 2012, 51, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Gupta, A.; Pawar, V.K.; Asthana, S.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Chitosan-assisted immunotherapy for intervention of experimental leishmaniasis via amphotericin B-loaded solid lipid nanoparticles. Appl. Biochem. Biotechnol. 2014, 174, 1309–1330. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Jian, J.; Song, S. Self-aggregated nanoparticles based on amphiphilic poly(lactic acid)-grafted-chitosan copolymer for ocular delivery of amphotericin B. Int. J. Nanomedicine 2013, 8, 3715–3728. [Google Scholar] [CrossRef]
- Chhonker, Y.S.; Prasad, Y.D.; Chandasana, H.; Vishvkarma, A.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int. J. Biol. Macromol. 2015, 72, 1451–1458. [Google Scholar] [CrossRef]
- Kong, Z.Q.; Yu, M.F.; Cheng, K.; Weng, W.J.; Wang, H.M.; Lin, J.; Du, P.Y.; Han, G.R. Incorporation of chitosan nanospheres into thin mineralized collagen coatings for improving the antibacterial effect. Colloid Surf. B 2013, 111, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.M.; Zhang, L.F. Bacterial Toxin-Triggered Drug Release from Gold Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection. J. Am. Chem. Soc. 2011, 133, 4132–4139. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Ustundag-Okur, N.; Gokce, E.H.; Bozbiyik, D.I.; Egrilmez, S.; Ozer, O.; Ertan, G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur. J. Pharm. Sci. 2014, 63, 204–215. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tsai, S.C.; Lai, C.H.; Lee, C.H.; He, Z.S.; Tseng, G.C. Genipin-cross-linked fucose-chitosan/heparin nanoparticles for the eradication of Helicobacter pylori. Biomaterials 2013, 34, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; Van Meijgaarden, K.E.; Franken, K.L.; Junginger, H.E.; Borchard, G.; Ottenhoff, T.H.; Geluk, A. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 2004, 22, 1609–1615. [Google Scholar] [CrossRef]
- Cota-Arriola, O.; Cortez-Rocha, M.O.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: Development of new strategies for microbial control in agriculture. J. Sci. Food Agric. 2013, 93, 1525–1536. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed.-Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharmaceut. 2013, 456, 31–40. [Google Scholar] [CrossRef]
- Tozaki, H.; Odoriba, T.; Okada, N.; Fujita, T.; Terabe, A.; Suzuki, T.; Okabe, S.; Muranishi, S.; Yamamoto, A. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J. Control. Release 2002, 82, 51–61. [Google Scholar] [CrossRef]
- Li, P.W.; Wang, Y.C.; Zeng, F.B.; Chen, L.J.; Peng, Z.; Kong, L.X. Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29 cells. Carbohyd. Res. 2011, 346, 801–806. [Google Scholar] [CrossRef]
- Saboktakin, M.R.; Tabatabaie, R.M.; Maharramov, A.; Ramazanov, M.A. Synthesis and in vitro evaluation of carboxymethyl starch-chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2011, 48, 381–385. [Google Scholar] [CrossRef]
- Kadiyala, I.; Loo, Y.; Roy, K.; Rice, J.; Leong, K.W. Transport of chitosan-DNA nanoparticles in human intestinal M-cell model versus normal intestinal enterocytes. Eur. J. Pharm. Sci. 2010, 39, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K.; Ganesh, N.; Barve, J.; Beg, A.M. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine 2010, 6, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Shieh, M.J.; Lin, F.H.; Lou, P.J.; Peng, C.L.; Wei, M.F.; Yao, C.J.; Lai, P.S.; Young, T.H. Colorectal cancer cell detection by 5-aminolaevulinic acid-loaded chitosan nano-particles. Cancer Lett. 2009, 273, 210–220. [Google Scholar] [CrossRef]
- Park, J.S.; Koh, Y.S.; Bang, J.Y.; Jeong, Y.I.; Lee, J.J. Antitumor effect of all-trans retinoic acid-encapsulated nanoparticles of methoxy poly(ethylene glycol)-conjugated chitosan against CT-26 colon carcinoma in vitro. J. Pharm. Sci. 2008, 97, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur. J. Pharm. Sci. 2008, 35, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.B.; Li, H.; Yuan, Y.B. Preparation of cholesterol-modified chitosan self-aggregated nanoparticles for delivery of drugs to ocular surface. Carbohyd. Polym. 2006, 65, 337–345. [Google Scholar] [CrossRef]
- De Salamanca, A.E.; Diebold, Y.; Calonge, M.; Garcia-Vazquez, C.; Callejo, S.; Vila, A.; Alonso, M.J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: Toxicity, uptake mechanism and in vivo tolerance. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1416–1425. [Google Scholar] [CrossRef]
- Diebold, Y.; Jarrin, M.; Saez, V.; Carvalho, E.L.S.; Orea, M.; Calonge, M.; Seijo, B.; Alonso, M.J. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP). Biomaterials 2007, 28, 1553–1564. [Google Scholar] [CrossRef]
- Calvo, P.; VilaJato, J.L.; Alonso, M.J. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. J. Appl. Polym. Sci. 1997, 153, 41–50. [Google Scholar] [CrossRef]
- Calvo, P.; Alonso, M.J.; VilaJato, J.L.; Robinson, J.R. Improved ocular bioavailability of indomethacin by novel ocular drug carriers. J. Pharm. Pharmacol. 1996, 48, 1147–1152. [Google Scholar] [CrossRef]
- Kao, H.J.; Lin, H.R.; Lo, Y.L.; Yu, S.P. Characterization of pilocarpine-loaded chitosan/carbopol nanoparticles. J. Pharm. Pharmacol. 2006, 58, 179–186. [Google Scholar] [CrossRef]
- Lin, H.R.; Yu, S.P.; Kuo, C.J.; Kao, H.J.; Lo, Y.L.; Lin, Y.J. Pilocarpine-loaded chitosan-PAA nanosuspension for ophthalmic delivery. J. Biomater. Sci.-Polym. Ed. 2007, 18, 205–221. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.Z.; Khutoryanskiy, V.V.; Stewart, A.; Rahman, S.; Papahadjopoulos-Sternberg, B.; Dufes, C.; McCarthy, D.; Wilson, C.G.; Lyons, R.; Carter, K.C.; et al. Carbohydrate-based micelle clusters which enhance hydrophobic drug bioavailability by up to 1 order of magnitude. Biomacromolecules 2006, 7, 3452–3459. [Google Scholar] [CrossRef]
- Tanner, T.; Marks, R. Delivering drugs by the transdermal route: Review and comment. Skin Res. Technol. 2008, 14, 249–260. [Google Scholar] [CrossRef]
- Al-Kassas, R.; Wen, J.Y.; Cheng, A.E.M.; Kim, A.M.J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef]
- Hafner, A.; Lovric, J.; Pepic, I.; Filipovic-Grcic, J. Lecithin/chitosan nanoparticles for transdermal delivery of melatonin. J. Microencapsul. 2011, 28, 807–815. [Google Scholar] [CrossRef]
- Shah, H.A.; Patel, R.P. Statistical modeling of zaltoprofen loaded biopolymeric nanoparticles: Characterization and anti-inflammatory activity of nanoparticles loaded gel. Int. J. Pharm. Investig. 2015, 5, 20–27. [Google Scholar] [CrossRef]
- Lopez-Leon, T.; Carvalho, E.L.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-Gonzalez, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interf. Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef]
- Fardet, L.; Flahault, A.; Kettaneh, A.; Tiev, K.P.; Genereau, T.; Toledano, C.; Lebbe, C.; Cabane, J. Corticosteroid-induced clinical adverse events: Frequency, risk factors and patient’s opinion. Br. J. Dermatol. 2007, 157, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, D.M.; Marcato, P.D.; Justo, G.Z.; Cordi, L.; Machado, D.; Duran, N. Chitosan-solid lipid nanoparticles as carriers for topical delivery of tretinoin. Colloid Surf. B 2012, 93, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohyd. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef]
- Dahmane, E.; Rhazi, M.; Taourirte, M. Chitosan Nanoparticles as a New Delivery System for the Anti-HIV Drug Zidovudine. Bull. Korean Chem. Soc. 2013, 34, 1333–1338. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, S.; Chakraborty, S.P.; Sahu, S.K.; Pramanik, P.; Roy, S. Synthesis, characterization of chitosan-tripolyphosphate conjugated chloroquine nanoparticle and its in vivo anti-malarial efficacy against rodent parasite: A dose and duration dependent approach. Int. J. Pharmaceut. 2012, 434, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.K.; Patil, S.S.; Navathar, D.A. Chiotsan Nanoparticles Loaded with Thiocolchicoside. Der. Pharma. Chemica. 2012, 4, 1619–1625. [Google Scholar]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur. J. Pharm. Sci. 2011, 42, 445–451. [Google Scholar] [CrossRef]
- Katas, H.; Alpar, H.O. Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release 2006, 115, 216–225. [Google Scholar] [CrossRef]
- Zhi, J.; Wang, Y.J.; Luo, G.S. Adsorption of diuretic furosemide onto chitosan nanoparticles prepared with a water-in-oil nanoemulsion system. React. Funct. Polym. 2005, 65, 249–257. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloid Surf. B-Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Cetin, M.; Aktas, Y.; Vural, I.; Capan, Y.; Dogan, L.A.; Duman, M.; Dalkara, T. Preparation and in vitro evaluation of bFGF-loaded chitosan nanoparticles. Drug Deliv. 2007, 14, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bozkir, A.; Saka, O.M. Chitosan nanoparticles for plasmid DNA delivery: Effect of chitosan molecular structure on formulation and release characteristics. Drug Deliv. 2004, 11, 107–112. [Google Scholar] [CrossRef]
- Xu, F.; Burg, K.J.L. Three-dimensional polymeric systems for cancer cell studies. Cytotechnology 2007, 54, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Urrusuno, R.; Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesaro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.M.; He, M.; Yin, L.C.; Bao, J.M.; Shi, L.L.; Wang, B.Q.; Tang, C.; Yin, C.H. Biodegradable Nanoparticles Based on Linoleic Acid and Poly(beta-malic acid) Double Grafted Chitosan Derivatives as Carriers of Anticancer Drugs. Biomacromolecules 2009, 10, 565–572. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Jin, S.G.; Kim, I.Y.; Pei, J.; Wen, M.; Jung, T.Y.; Moon, K.S.; Jung, S. Doxorubicin-incorporated nanoparticles composed of poly(ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloids Surf. B Biointerfaces 2010, 79, 149–155. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, C.N.; Wang, X.H.; Wang, W.; Huang, W.; Cha, R.T.; Wang, C.H.; Yuan, Z.; Liu, M.; Wan, H.Y.; et al. Glycyrrhetinic acid-modified chitosan/poly(ethylene glycol) nanoparticles for liver-targeted delivery. Biomaterials 2010, 31, 4748–4756. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.S.; Kim, S.; Park, J.H.; Kim, K.; Choi, K.; Chung, H.; Jeong, S.Y.; Park, R.W.; Kim, I.S.; et al. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release 2006, 111, 228–234. [Google Scholar] [CrossRef]
- Trickler, W.J.; Khurana, J.; Nagvekar, A.A.; Dash, A.K. Chitosan and glyceryl monooleate nanostructures containing gemcitabine: Potential delivery system for pancreatic cancer treatment. Aaps. Pharmscitech. 2010, 11, 392–401. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Yang, D.Z.; Chen, X.M.; Xu, Q.; Lu, F.M.; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Yang, H.J.; Liu, X.; Mao, J.; Gu, S.J.; Xu, W.L. Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2013, 53, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.M.; El-Aassar, M.R.; Al-Deyab, S.S. Antimicrobial activity of carboxymethyl chitosan/polyethylene oxide nanofibers embedded silver nanoparticles. Carbohydr. Polym. 2013, 92, 1012–1017. [Google Scholar] [CrossRef]
- Kossovich, L.Y.; Salkovskiy, Y.; Kirillova, Y. Electrospun Chitosan Nanofiber Materials as Burn Dressing. In Proceedings of the 6th World Congress of Biomechanics, Singapore, 1–6 August 2010; pp. 1212–1214. [Google Scholar]
- Zhao, Y.H.; Zhou, Y.; Wu, X.M.; Wang, L.; Xu, L.; Wei, S.C. A facile method for electrospinning of Ag nanoparticles/poly (vinyl alcohol)/carboxymethyl-chitosan nanofibers. Appl. Surf. Sci. 2012, 258, 8867–8873. [Google Scholar] [CrossRef]
- Toshkova, R.; Manolova, N.; Gardeva, E.; Ignatova, M.; Yossifova, L.; Rashkov, I.; Alexandrov, M. Antitumor activity of quaternized chitosan-based electrospun implants against Graffi myeloid tumor. Int. J. Pharm. 2010, 400, 221–233. [Google Scholar] [CrossRef]
- Alipour, S.M.; Nouri, M.; Mokhtari, J.; Bahrami, S.H. Electrospinning of poly(vinyl alcohol)-water-soluble quaternized chitosan derivative blend. Carbohydr. Res. 2009, 344, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Starbova, K.; Markova, N.; Manolova, N.; Rashkov, I. Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly(vinyl alcohol). Carbohydr. Res. 2006, 341, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I. Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur. Polym. J. 2007, 43, 1112–1122. [Google Scholar] [CrossRef]
- Ignatova, M.G.; Manolova, N.E.; Toshkova, R.A.; Rashkov, I.B.; Gardeva, E.G.; Yossifova, L.S.; Alexandrov, M.T. Electrospun nanofibrous mats containing quaternized chitosan and polylactide with in vitro antitumor activity against HeLa cells. Biomacromolecules 2010, 11, 1633–1645. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef]
- Bai, B.; Mi, X.; Xiang, X.; Heiden, P.A.; Heldt, C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr. Res. 2013, 380, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.B.; Lin, P.H.; Xin, S.J.; Huang, R.; Li, W.; Du, Y.M.; Zhou, X.; Yang, J.H. Quaternized chitosan-layered silicate intercalated composites based nanofibrous mats and their antibacterial activity. Carbohydr. Polym. 2012, 89, 307–313. [Google Scholar] [CrossRef]
- Jiang, H.L.; Fang, D.F.; Hsiao, B.J.; Chu, B.J.; Chen, W.L. Preparation and characterization of ibuprofen-loaded poly(lactide-co-glycolide)/poly(ethylene glycol)-g-chitosan electrospun membranes. J. Biomater. Sci.-Polym. Ed. 2004, 15, 279–296. [Google Scholar] [CrossRef]
- Chen, H.L.; Huang, J.; Yu, J.H.; Liu, S.Y.; Gu, P. Electrospun chitosan-graft-poly (epsilon-caprolactone)/poly (epsilon-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2011, 48, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nawalakhe, R.G.; Hudson, S.M.; Seyam, A.F.M.; Waly, A.I.; Abou-Zeid, N.Y.; Ibrahim, H.M. Development of Electrospun Iminochitosan for Improved Wound Healing Application. J. Eng. Fiber Fabr. 2012, 7, 47–55. [Google Scholar] [CrossRef]
- Seyam, A.F.M.; Hudson, S.M.; Ibrahim, H.M.; Waly, A.I.; Abou-Zeid, N.Y. Healing performance of wound dressing from cyanoethyl chitosan electrospun fibres. Indian J. Fibre Text. 2012, 37, 205–210. [Google Scholar]
- Datta, P.; Dhara, S.; Chatterjee, J. Hydrogels and electrospun nanofibrous scaffolds of N-methylene phosphonic chitosan as bioinspired osteoconductive materials for bone grafting. Carbohydr. Polym. 2012, 87, 1354–1362. [Google Scholar] [CrossRef]
- Deng, H.; Lin, P.; Li, W.; Xin, S.; Zhou, X.; Yang, J. Hydroxypropyl chitosan/organic rectorite-based nanofibrous mats with intercalated structure for bacterial inhibition. J. Biomater. Sci. Polym. Ed. 2013, 24, 485–496. [Google Scholar] [CrossRef]
- Venkatesan, P.; Puvvada, N.; Dash, R.; Prashanth Kumar, B.N.; Sarkar, D.; Azab, B.; Pathak, A.; Kundu, S.C.; Fisher, P.B.; Mandal, M. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 2011, 32, 3794–3806. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.L.; Zheng, J.J.; Wang, K.; Tang, Y.; Zhang, X.F.; Zhang, H.S.; Huang, F.P.; Pei, Y.Y.; Jiang, Y.Y. Cationic core-shell nanoparticles with carmustine contained within O-6-benzylguanine shell for glioma therapy. Biomaterials 2013, 34, 8968–8978. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Hu, Y.W.; Du, Y.Z.; Liu, N.; Liu, X.; Meng, T.T.; Cheng, B.L.; He, J.B.; You, J.; Yuan, H.; Hu, F.Q. Selective redox-responsive drug release in tumor cells mediated by chitosan based glycolipid-like nanocarrier. J. Control. Release 2015, 206, 91–100. [Google Scholar] [CrossRef]
- Tekade, R.K.; Youngren-Ortiz, S.R.; Yang, H.N.; Haware, R.; Chougule, M.B. Albumin-chitosan hybrid onconase nanocarriers for mesothelioma therapy. Cancer Res. 2015, 75. [Google Scholar] [CrossRef]
- Butt, A.M.; Amin, M.C.; Katas, H.; Abdul Murad, N.A.; Jamal, R.; Kesharwani, P. Doxorubicin and siRNA Codelivery via Chitosan-Coated pH-Responsive Mixed Micellar Polyplexes for Enhanced Cancer Therapy in Multidrug-Resistant Tumors. Mol. Pharm. 2016, 13, 4179–4190. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, Z.K.; Zhu, Q.L.; Rong, X.H.; Liang, C.L.; Wang, J.; Ma, D.; Sun, J.; Wang, G.H. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surf. B Biointerfaces 2017, 155, 41–50. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jiang, Y.; Liu, T.; Luo, Y.; Diao, E.; Cao, Y.; Chen, L.; Zhang, L.; Gu, Q.; et al. Enhanced cytotoxic and apoptotic potential in hepatic carcinoma cells of chitosan nanoparticles loaded with ginsenoside compound K. Carbohydr. Polym. 2018, 198, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhuang, Q.; Ji, T.; Zhang, Y.; Li, C.; Wang, Y.; Li, H.; Jia, H.; Liu, Y.; Du, L. Multi-functionalized chitosan nanoparticles for enhanced chemotherapy in lung cancer. Carbohydr. Polym. 2018, 195, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Stigliano, C.; Aryal, S.; De Tullio, M.D.; Nicchia, G.P.; Pascazio, G.; Svelto, M.; Decuzzi, P. siRNA-Chitosan Complexes in Poly(lactic-co-glycolic acid) Nanoparticles for the Silencing of Aquaporin-1 in Cancer Cells. Mol. Pharmaceut. 2013, 10, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Saboktakin, M.R.; Tabatabaie, R.M.; Maharramov, A.; Ramazanov, M.A. Synthesis and in vitro studies of biodegradable modified chitosan nanoparticles for photodynamic treatment of cancer. Int. J. Biol. Macromol. 2011, 49, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
| Nanochitosan composites | Biomedical Application | Associated Drug | Reference |
|---|---|---|---|
| Chitosan + alginate + Pluronic | Drug delivery | Curcumin | [191] |
| Poly(β-malic acid)-γ-chitosan-Linoleic acid | Drug delivery | Paclitaxel | [192] |
| Poly(ethylene-glycol)-γ carboxymethyl chitosan | Drug delivery | Dox | [193] |
| Chitosan/poly(ethylene glycol)– glycyrrhetinic acid | Drug delivery | Dox | [194] |
| Chitosan-Cholanic acid | Drug delivery | Paclitaxel | [195] |
| Chitosan Glyceryl monooleate | Drug delivery | Paclitaxel | [196] |
| Chitosan Cholesterol | Drug delivery | Epirubicin | [98] |
| Carboxymethyl Chitosan-PVA | wound dressings | NA | [197] |
| Carboxymethyl Chitosan-PVA/silk fibroin | wound dressings | NA | [198] |
| Carboxymethyl Chitosan-PEO | Antimicrobial | NA | [199] |
| Chitosan/PEO nanofibers | wound dressings | NA | [200] |
| Carboxymethyl Chitosan-PVA/Ag nanoparticles | antibacterial | NA | [201] |
| Quaternized chitosan-coPLA | antitumor | DOX | [202] |
| Quaternized chitosan-PVA | Antibacterial | NA | [203,204] |
| Quaternized chitosan-PVP | Antibacterial | NA | [205] |
| Quaternized chitosan-PLA | Antitumor Wound dressing | NA | [206,207] |
| Quaternized chitosan-Graphene | Virus removal | NA | [208] |
| Quaternized chitosan-Organic rectorite | Antibacterial | NA | [209] |
| PEG-graft chitosan | Drug release | PLGA | [210] |
| Poly-ε-caprolactone-graft chitosan | Skin tissue engineering | [211] | |
| Iminochitosan | Wound healing | NA | [212] |
| Cyanoethyl chitosan | Wound dressing | NA | [213] |
| N-Methylene phosphonic chitosan | Bone grafting | NA | [214] |
| Hydroxypropyl Chitosan-Organic rectorite | Antibacterial | NA | [215] |
| Hydroxyapatite-chitosan nanocomposite | Colon cancer theraphy | Celecoxib | [216] |
| PGLA-chitosan | Rat glioblastoma | Carmustine (BCNU), O(6)-benzylguanine (BG) – therapeutic agents | [217] |
| Hyaluronic acid (HA)-CS nanoparticles | Breast cancer | miR-34a and doxorubicin (DOX) | [218] |
| Chitosan based glycolipid-like | Human ovarian cancer cells | Paclitaxel (PTX) | [219] |
| Albumin-chitosan | Mesothelioma therapy | Onconase (ONC) | [220] |
| Chitosan coated mixed micelles | Multidrug resistant cancer cells | siRNA and Doxorubicin | [221] |
| Stearic acid-grafted chitosan oligosaccharide (CSO-SA) | Cancer therapy | Polymer–drug conjugate of doxorubicin | [222] |
| Deoxycholic acid-O carboxymethyl chitosan | Liver cancer | Ginsenoside compound K (CK) | [223] |
| Cholesterol conjugated chitosan | Human lung carcinoma cells | Curcumin | [224] |
| Fluorescent gold nanocluster-conjugated chitosan | Lung cancer | Methotrexate | [225] |
| Glycol chitosan nanopolymers (psi-TGC) | knockdown of tumour Protein for cancer gene therapy | Poly siRNA | [226] |
| Glycol chitosan | In vivo inhibition of tumour via Gene therapy | Poly siRNA | [226] |
| Chitosan: poly(lactic-co-glycolic acid) nanoparticles | Silencing of aquaporin-1 cancer cells via Gene therapy | siRNA | [227] |
| N-sulfonato-N,O-carboxymethylchitosan (NOCCS) | In vivo cancer cells via Photodynamic therapy | mTHPP. | [228] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivanesan, I.; Muthu, M.; Gopal, J.; Hasan, N.; Kashif Ali, S.; Shin, J.; Oh, J.-W. Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical. Nanomaterials 2021, 11, 821. https://doi.org/10.3390/nano11030821
Sivanesan I, Muthu M, Gopal J, Hasan N, Kashif Ali S, Shin J, Oh J-W. Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical. Nanomaterials. 2021; 11(3):821. https://doi.org/10.3390/nano11030821
Chicago/Turabian StyleSivanesan, Iyyakkannu, Manikandan Muthu, Judy Gopal, Nazim Hasan, Syed Kashif Ali, Juhyun Shin, and Jae-Wook Oh. 2021. "Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical" Nanomaterials 11, no. 3: 821. https://doi.org/10.3390/nano11030821
APA StyleSivanesan, I., Muthu, M., Gopal, J., Hasan, N., Kashif Ali, S., Shin, J., & Oh, J.-W. (2021). Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical. Nanomaterials, 11(3), 821. https://doi.org/10.3390/nano11030821








