Thin Films of Metal-Organic Framework Interfaces Obtained by Laser Evaporation
Abstract
1. Introduction
2. Materials and Methods
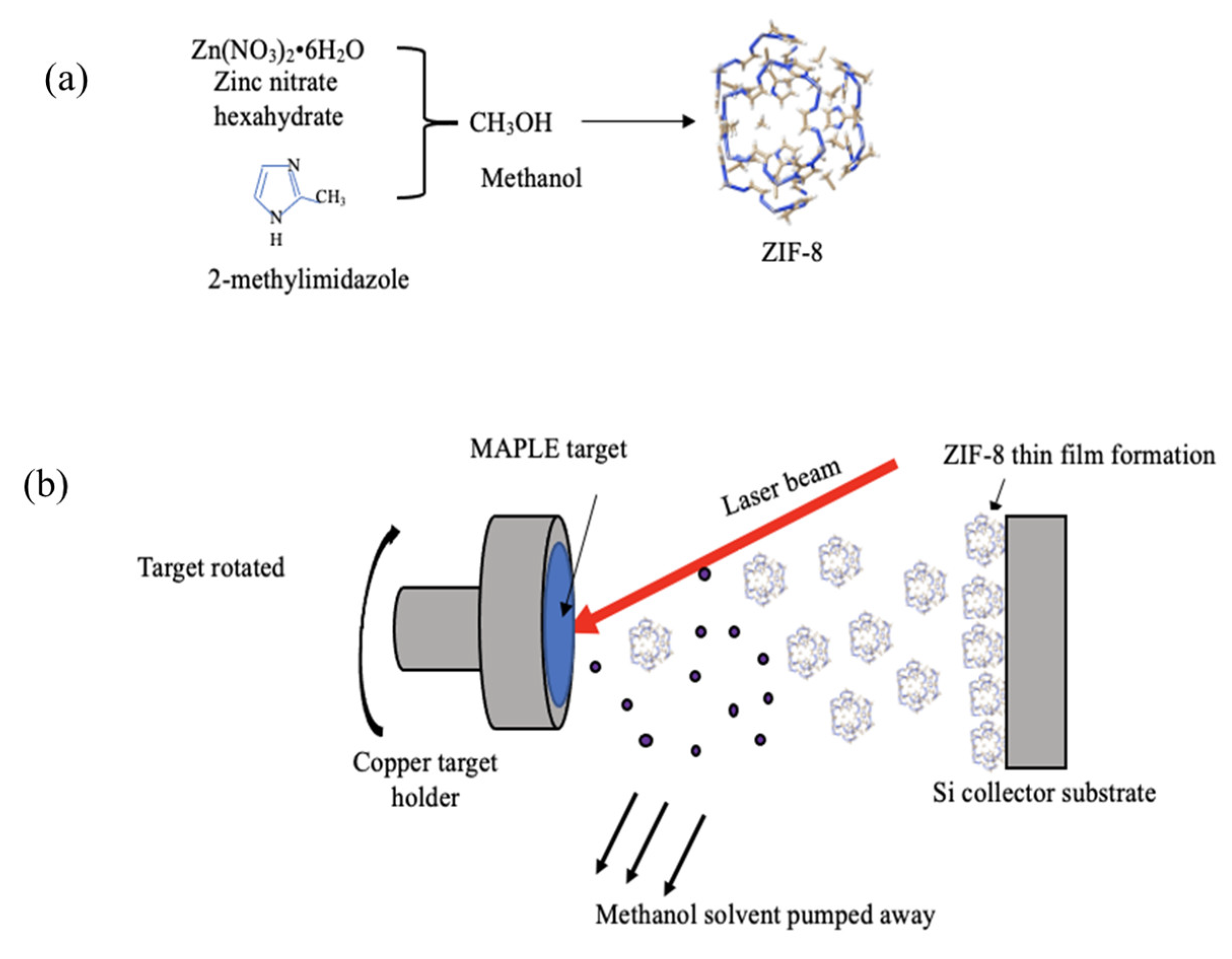
2.1. Preparation of ZIF-8 Particles
2.2. Target Preparation
2.3. Matrix-Assisted Pulsed Laser Evaporation (MAPLE) for Film Formation
2.4. Characterization of the MAPLE Deposited Films and of Controls of ZIF-8
3. Results and Discussion
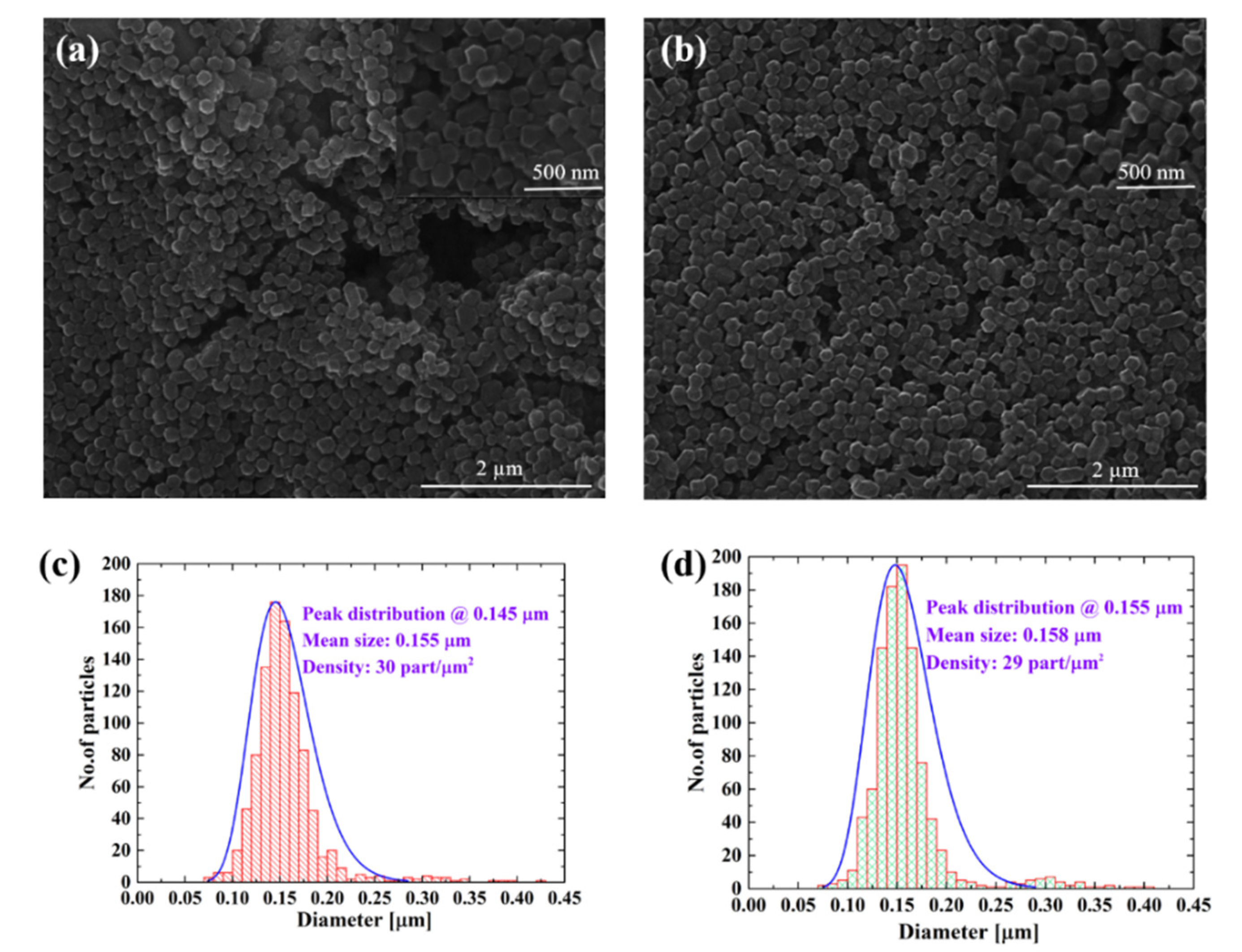
3.1. Morphological Analysis of Deposited Samples Relative to Controls
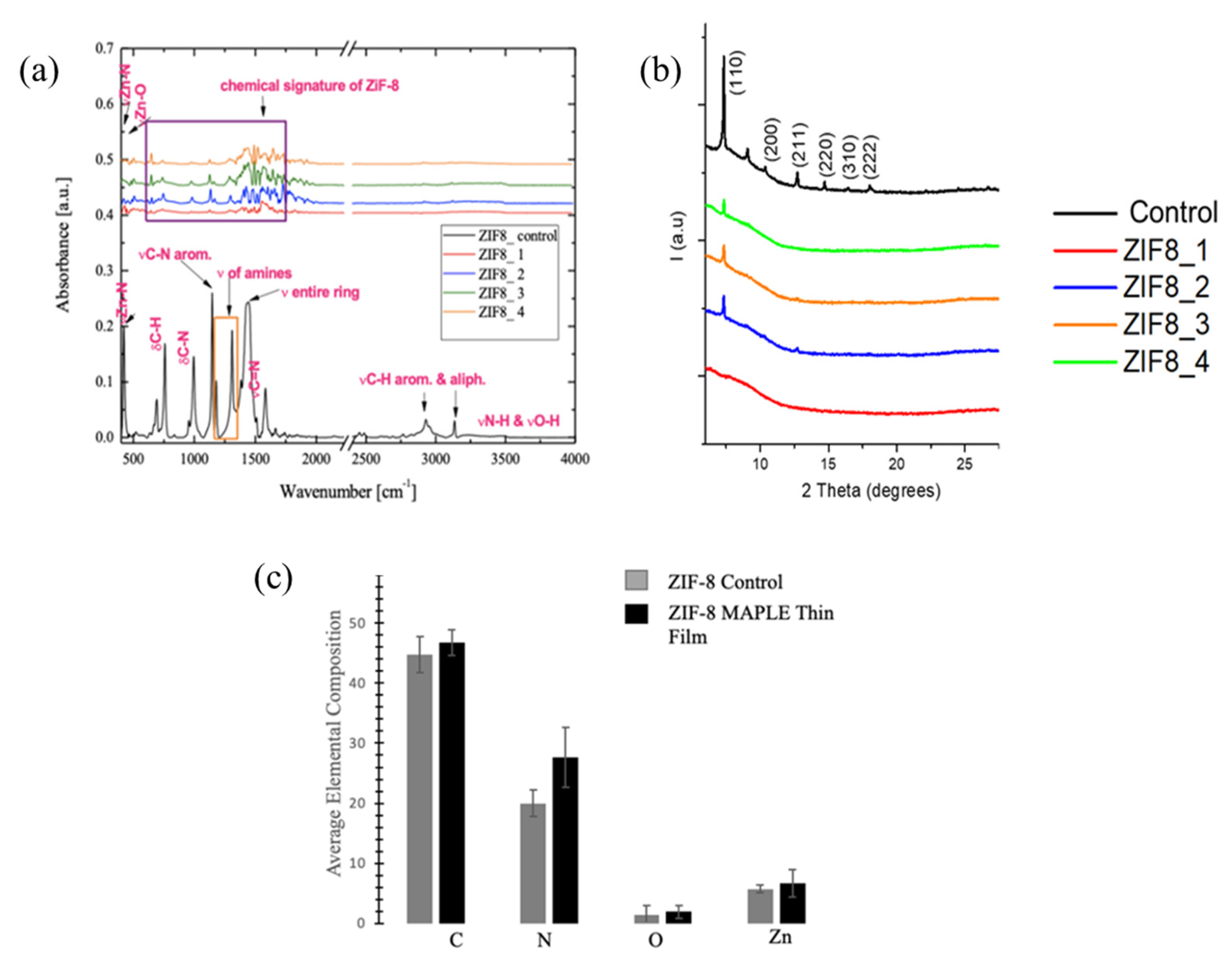
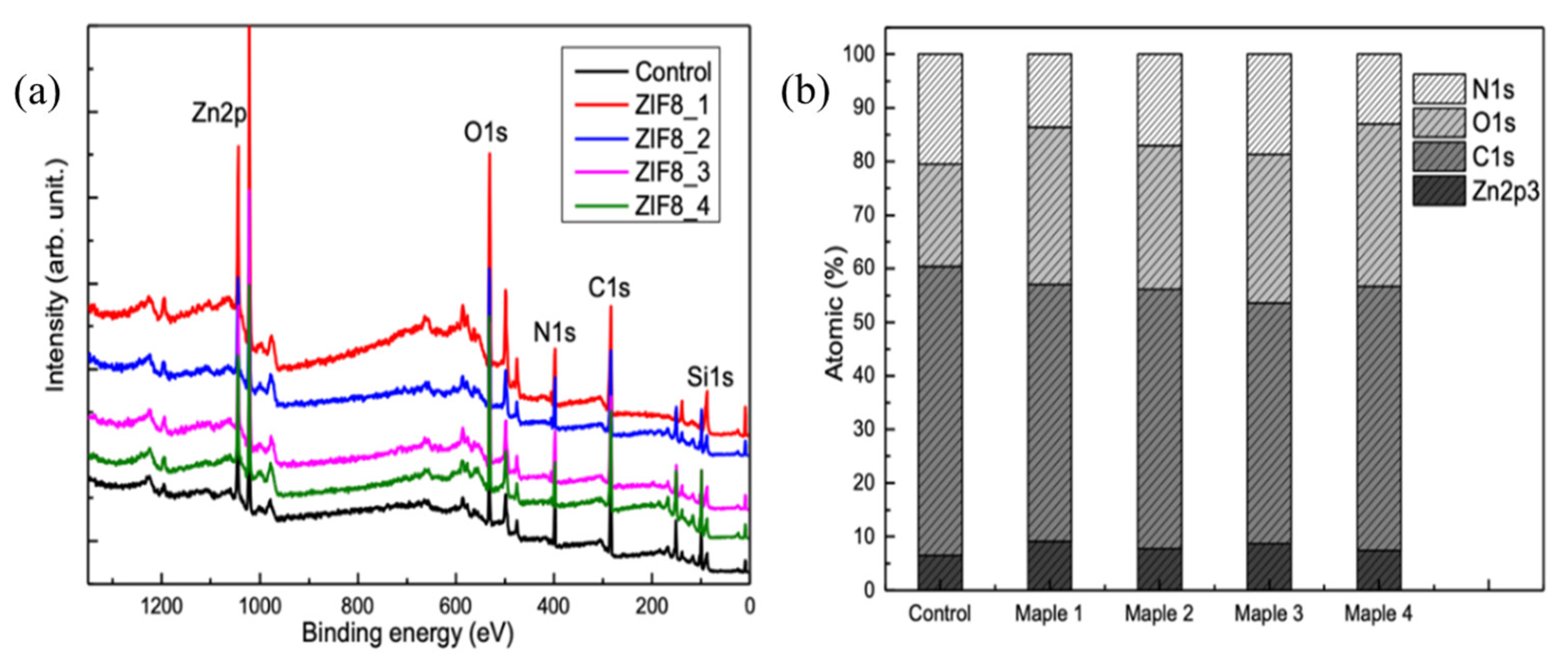
3.2. Chemical and Structural Analyses of the Deposited Samples Relative to Controls
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, W.; Hu, Q.; Yu, J.; Jiang, K.; Yang, Y.; Xiang, S.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. Low Cytotoxic Metal-Organic Frameworks as Temperature-Responsive Drug Carriers. ChemPlusChem 2016, 81, 804–810. [Google Scholar] [CrossRef]
- Chen, G.; Leng, X.; Luo, J.; You, L.; Qu, C.; Dong, X.; Huang, H.; Yin, X.; Ni, J. In Vitro Toxicity Study of a Porous Iron(III) Metal‒Organic Framework. Molecules 2019, 24, 1211. [Google Scholar] [CrossRef]
- Lu, G.; Hupp, J.T. Metal−Organic Frameworks as Sensors: A ZIF-8 Based Fabry−Pérot Device as a Selective Sensor for Chemical Vapors and Gases. J. Am. Chem. Soc. 2010, 132, 7832–7833. [Google Scholar] [CrossRef]
- Hou, C.; Peng, J.; Xu, Q.; Ji, Z.; Hu, X. Elaborate fabrication of MOF-5 thin films on a glassy carbon electrode (GCE) for photoelectrochemical sensors. RSC Adv. 2012, 2, 12696–12698. [Google Scholar] [CrossRef]
- Pan, Q.-S.; Chen, T.-T.; Nie, C.-P.; Yi, J.-T.; Liu, C.; Hu, Y.-L.; Chu, X. In Situ Synthesis of Ultrathin ZIF-8 Film-Coated MSNs for Codelivering Bcl 2 siRNA and Doxorubicin to Enhance Chemotherapeutic Efficacy in Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 33070–33077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Begum, S.; Wang, Z.; Krolla, P.; Wagner, D.; Bräse, S.; Wöll, C.; Tsotsalas, M. High Antimicrobial Activity of Metal–Organic Framework-Templated Porphyrin Polymer Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 1528–1533. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.M.; Zhang, Y.T.; Uliana, A.; Zhu, J.Y.; Liu, J.D.; Van der Bruggen, B. Zeolitic Imidazolate Framework/Graphene Oxide Hybrid Nanosheets Functionalized Thin Film Nanocomposite Membrane for Enhanced Antimi-crobial Performance. ACS Appl. Mater. Interfaces 2016, 8, 25508–25519. [Google Scholar] [CrossRef] [PubMed]
- Abednejad, A.; Ghaee, A.; Nourmohammadi, J.; Mehrizi, A.A. Hyaluronic acid/carboxylated Zeolitic Imidazolate Framework film with improved mechanical and antibacterial properties. Carbohydr. Polym. 2019, 222, 115033. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Zhang, X.; Huang, C.; Cai, H.; Hu, S.S.; Wan, Q.B.; Pei, X.B.; Wang, J. Osteogenic activity and anti-bacterial effect of porous titanium modified with metal-organic framework films. J. Biomed. Mater. Res. Part A 2017, 105, 834–846. [Google Scholar] [CrossRef]
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-Sieve Membrane with Hydrogen Permselectivity: ZIF-22 in LTA Topology Prepared with 3-Aminopropyltriethoxysilane as Covalent Linker. Angew. Chem. Int. Ed. 2010, 49, 4958–4961. [Google Scholar] [CrossRef]
- Huang, A.; Dou, W.; Caro, J. Steam-Stable Zeolitic Imidazolate Framework ZIF-90 Membrane with Hydrogen Selectivity through Covalent Functionalization. J. Am. Chem. Soc. 2010, 132, 15562–15564. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Von Mankowski, A.; Ranft, A.; Vasa, S.K.; Linser, R.; Mannhart, J.; Lotsch, B.V. ZIF-8 Films Prepared by Femtosecond Pulsed-Laser Deposition. Chem. Mater. 2017, 29, 5148–5155. [Google Scholar] [CrossRef]
- Fischer, D.; Meyer, L.V.; Jansen, M.; Muller-Buschbaum, K. Highly Luminescent Thin Films of the Dense Framework (3)(infinity) EuIm(2) with Switchable Transparency Formed by Scanning Femtosecond-Pulse Laser Deposition. Angew. Chem. Int. Ed. 2014, 53, 706–710. [Google Scholar] [CrossRef]
- Marti, A.M.; Perera, S.D.; McBeath, L.D.; Balkus, K.J. Fabrication of Oriented Silver-Functionalized RPM3 Films for the Selective Detection of Olefins. Langmuir 2013, 29, 5927–5936. [Google Scholar] [CrossRef] [PubMed]
- Salmi, L.D.; Heikkilä, M.; Puukilainen, E.; Sajavaara, T.; Grosso, D.; Ritala, M. Studies on atomic layer deposition of MOF-5 thin films. Microporous Mesoporous Mater. 2013, 182, 147–154. [Google Scholar] [CrossRef]
- Khaletskaya, K.; Turner, S.; Tu, M.; Wannapaiboon, S.; Schneemann, A.; Meyer, R.; Ludwig, A.; Van Tendeloo, G.; Fischer, R.A. Self-Directed Localization of ZIF-8 Thin Film Formation by Conversion of ZnO Nanolayers. Adv. Funct. Mater. 2014, 24, 4804–4811. [Google Scholar] [CrossRef]
- Lausund, K.B.; Olsen, M.S.; Hansen, P.-A.; Valen, H.; Nilsen, O. MOF thin films with bi-aromatic linkers grown by molecular layer deposition. J. Mater. Chem. A 2020, 8, 2539–2548. [Google Scholar] [CrossRef]
- Lausund, K.B.; Petrovic, V.; Nilsen, O. All-gas-phase synthesis of amino-functionalized UiO-66 thin films. Dalton Trans. 2017, 46, 16983–16992. [Google Scholar] [CrossRef] [PubMed]
- Streit, H.C.; Adlung, M.; Shekhah, O.; Stammer, X.; Arslan, H.K.; Zybaylo, O.; Ladnorg, T.; Gliemann, H.; Franzreb, M.; Wöll, C.; et al. Surface-Anchored MOF-Based Photonic Antennae. ChemPhysChem 2012, 13, 2699–2702. [Google Scholar] [CrossRef]
- Wannapaiboon, S.; Sumida, K.; Dilchert, K.; Tu, M.; Kitagawa, S.; Furukawa, S.; Fischer, R.A. Enhanced properties of metal–organic framework thin films fabricated via a coordination modulation-controlled layer-by-layer process. J. Mater. Chem. A 2017, 5, 13665–13673. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Kowarik, S.; Schreiber, F.; Paulus, M.; Tolan, M.; Sternemann, C.; Evers, F.; Zacher, D.; Fischer, A.R.A.; et al. Step-by-Step Route for the Synthesis of Metal−Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 15118–15119. [Google Scholar] [CrossRef] [PubMed]
- Demessence, A.; Boissière, C.; Grosso, D.; Horcajada, P.; Serre, C.; Férey, G.; Soler-Illia, G.J.A.A.; Sanchez, C. Adsorption properties in high optical quality nanoZIF-8 thin films with tunable thickness. J. Mater. Chem. 2010, 20, 7676–7681. [Google Scholar] [CrossRef]
- Sarango, L.; Paseta, L.; Navarro, M.; Zornoza, B.; Coronas, J. Controlled deposition of MOFs by dip-coating in thin film nanocomposite membranes for organic solvent nanofiltration. J. Ind. Eng. Chem. 2018, 59, 8–16. [Google Scholar] [CrossRef]
- Motoyama, S.; Makiura, R.; Sakata, O.; Kitagawa, H. Highly Crystalline Nanofilm by Layering of Porphyrin Metal−Organic Framework Sheets. J. Am. Chem. Soc. 2011, 133, 5640–5643. [Google Scholar] [CrossRef]
- Makiura, R.; Motoyama, S.; Umemura, Y.; Yamanaka, H.; Sakata, O.; Kitagawa, H. Surface nano-architecture of a metal–organic framework. Nat. Mater. 2010, 9, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Greer, H.F.; Liu, Y.; Greenaway, A.; Wright, P.A.; Zhou, W. Synthesis and Formation Mechanism of Textured MOF-5. Cryst. Growth Des. 2016, 16, 2104–2111. [Google Scholar] [CrossRef]
- Mihaiescu, D.E.; Cristescu, R.; Dorcioman, G.E.; Popescu, C.; Nita, C.; Socol, G.; Mihailescu, I.N.; Grumezescu, A.M.; Tamas, D.; Enculescu, M.; et al. Functionalized magnetite silica thin films fabricated by MAPLE with antibiofilm properties. Biofabrication 2012, 5, 015007. [Google Scholar] [CrossRef]
- Piqué, A.; McGill, R.; Chrisey, D.; Leonhardt, D.; Mslna, T.; Spargo, B.; Callahan, J.; Vachet, R.; Chung, R.; Bucaro, M. Growth of organic thin films by the matrix assisted pulsed laser evaporation (MAPLE) technique. Thin Solid Films 1999, 355–356, 536–541. [Google Scholar] [CrossRef]
- Caricato, A.P.; Epifani, M.; Martino, M.; Romano, F.; Rella, R.; Taurino, A.; Tunno, T.; Valerini, D. MAPLE deposi-tion and characterization of SnO2 colloidal nanoparticle thin films. J. Phys. D Appl. Phys. 2009, 42, 5. [Google Scholar] [CrossRef]
- Caricato, A.P.; Capone, S.; Ciccarella, G.; Martino, M.; Rella, R.; Romano, F.; Spadavecchia, J.; Taurino, A.; Tunno, T.; Valerini, D. TiO2 nanoparticle thin film deposition by matrix assisted pulsed laser evaporation for sensing appli-cations. Appl. Surf. Sci. 2007, 253, 7937–7941. [Google Scholar] [CrossRef]
- Grumezescu, V.; Andronescu, E.; Holban, A.M.; Mogoantă, L.; Mogoşanu, G.D.; Grumezescu, A.M.; Stănculescu, A.; Socol, G.; Iordache, F.; Maniu, H.; et al. MAPLE fabrication of thin films based on kanamycin functionalized magnetite nanoparticles with anti-pathogenic properties. Appl. Surf. Sci. 2015, 336, 188–195. [Google Scholar] [CrossRef]
- Constantinescu, C.; Scarisoreanu, N.; Moldovan, A.; Dinescu, M.; Vasiliu, C. Thin films of polyaniline deposited by MAPLE technique. Appl. Surf. Sci. 2007, 253, 7711–7714. [Google Scholar] [CrossRef]
- Wagner, A.; Liu, Q.; Rose, O.L.; Eden, A.; Vijay, A.; Rojanasakul, Y.; Dinu, C.Z. Toxicity screening of two prevalent metal organic frameworks for therapeutic use in human lung epithelial cells. Int. J. Nanomed. 2019, 14, 7583–7591. [Google Scholar] [CrossRef]
- Hoop, M.; Walde, C.F.; Riccò, R.; Mushtaq, F.; Terzopoulou, A.; Chen, X.-Z.; Demello, A.J.; Doonan, C.J.; Falcaro, P.; Nelson, B.J.; et al. Biocompatibility characteristics of the metal organic framework ZIF-8 for therapeutical applications. Appl. Mater. Today 2018, 11, 13–21. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bansal, V.; Paul, A.K.; Bharadwaj, L.M.; Deep, A.; Kim, K.-H. Biological applications of zinc imidazole framework through protein encapsulation. Appl. Nanosci. 2016, 6, 951–957. [Google Scholar] [CrossRef]
- Bétard, A.; Fischer, R.A. Metal–Organic Framework Thin Films: From Fundamentals to Applications. Chem. Rev. 2011, 112, 1055–1083. [Google Scholar] [CrossRef]
- Zacher, D.; Shekhah, O.; Woll, C.; Fischer, R.A. Thin films of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1418–1429. [Google Scholar] [CrossRef]
- Liu, Q.; Chapman, J.; Huang, A.S.; Williams, K.C.; Wagner, A.; Garapati, N.; Sierros, K.A.; Dinu, C.Z. User-Tailored Metal Organic Frameworks as Supports for Carbonic Anhydrase. ACS Appl. Mater. Int. 2018, 10, 41326–41337. [Google Scholar] [CrossRef]
- Pan, Y.C.; Liu, Y.Y.; Zeng, G.F.; Zhao, L.; Lai, Z.P. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nano-crystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Casey, C.N.; Campbell, S.E.; Gibson, U.J. Phenylalanine detection using matrix assisted pulsed laser evaporation of molecularly imprinted amphiphilic block copolymer films. Biosens. Bioelectron. 2010, 26, 703–709. [Google Scholar] [CrossRef]
- Shepard, K.B.; Priestley, R.D. MAPLE Deposition of Macromolecules. Macromol. Chem. Phys. 2013, 214, 862–872. [Google Scholar] [CrossRef]
- Werner, D.; Hashimoto, S.; Uwada, T. Remarkable Photothermal Effect of Interband Excitation on Nanosecond La-ser-Induced Reshaping and Size Reduction of Pseudospherical Gold Nanoparticles in Aqueous Solution. Langmuir 2010, 26, 9956–9963. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Hashimoto, S. Improved Working Model for Interpreting the Excitation Wavelength-and Fluence-Dependent Response in Pulsed Laser-Induced Size Reduction of Aqueous Gold Nanoparticles. J. Phys. Chem. C 2010, 115, 5063–5072. [Google Scholar] [CrossRef]
- Pyatenko, A.; Yamaguchi, M.; Suzuki, M. Mechanisms of Size Reduction of Colloidal Silver and Gold Nanoparticles Irradiated by Nd:YAG Laser. J. Phys. Chem. C 2009, 113, 9078–9085. [Google Scholar] [CrossRef]
- Wu, P.; Ringeisen, B.; Callahan, J.; Brooks, M.; Bubb, D.; Wu, H.; Piqué, A.; Spargo, B.; McGill, R.; Chrisey, D. The deposition, structure, pattern deposition, and activity of biomaterial thin-films by matrix-assisted pulsed-laser evaporation (MAPLE) and MAPLE direct write. Thin Solid Films 2001, 398, 607–614. [Google Scholar] [CrossRef]
- Lock, N.; Wu, Y.; Christensen, M.; Cameron, L.J.; Peterson, V.K.; Bridgeman, A.J.; Kepert, C.J.; Iversen, B.B. Elu-cidating Negative Thermal Expansion in MOF-5. J. Phys. Chem. C 2010, 114, 16181–16186. [Google Scholar] [CrossRef]
- Wee, L.H.; Lohe, M.R.; Janssens, N.; Kaskel, S.; Martens, J.A. Fine tuning of the metal–organic framework Cu3(BTC)2 HKUST-1 crystal size in the 100 nm to 5 micron range. J. Mater. Chem. 2012, 22, 13742–13746. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Jilani, A.; Ismail, A.; Hashim, H.; Jaafar, J.; Rahman, M.A.; Rehman, G.U. Economical, environmental friendly synthesis, characterization for the production of zeolitic imidazolate framework-8 (ZIF-8) nanoparticles with enhanced CO2 adsorption. Arab. J. Chem. 2018, 11, 1072–1083. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Hou, L. Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM2.5 removal. RSC Adv. 2018, 8, 31471–31477. [Google Scholar] [CrossRef]
- Shahrak, M.N.; Ghahramaninezhad, M.; Eydifarash, M. Zeolitic imidazolate framework-8 for efficient adsorption and removal of Cr(VI) ions from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 9624–9634. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.A.H.M.; Racha, S.M.; Matsuura, T.; Misdan, N.; Sani, N.A.A.; Ismail, A.F.; Mustafa, A. Facile modification of ZIF-8 mixed matrix membrane for CO2/CH4 separation: Synthesis and preparation. RSC Adv. 2015, 5, 43110–43120. [Google Scholar] [CrossRef]
- Yu, Z.; Bernstein, E.R. Experimental and theoretical studies of the decomposition of new imidazole based energetic materials: Model systems. J. Chem. Phys. 2012, 137, 114303. [Google Scholar] [CrossRef]
- Ribar, A.; Fink, K.; Li, Z.; Ptasińska, S.; Carmichael, I.; Feketeová, L.; Denifl, S. Stripping off hydrogens in imidazole triggered by the attachment of a single electron. Phys. Chem. Chem. Phys. 2017, 19, 6406–6415. [Google Scholar] [CrossRef]
- Lancok, J.; Garapon, C.; Martinet, C.; Mugnier, J.; Brenier, R. Influence of the PLD parameters on the crystalline phases and fluorescence of Eu:Y2O3 planar waveguides. Appl. Phys. A 2004, 79, 1263–1265. [Google Scholar] [CrossRef]
- Suda, Y.; Kawasaki, H.; Ueda, T.; Ohshima, T. Preparation of high quality nitrogen doped TiO2 thin film as a photocatalyst using a pulsed laser deposition method. Thin Solid Films 2004, 453, 162–166. [Google Scholar] [CrossRef]
- Chin, M.; Cisneros, C.; Araiza, S.M.; Vargas, K.M.; Ishihara, K.M.; Tian, F. Rhodamine B degradation by nanosized zeolitic imidazolate framework-8 (ZIF-8). RSC Adv. 2018, 8, 26987–26997. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Cerro, A.M.; Mosier, A.M.; Wayment-Steele, H.K.; Shine, R.S.; Park, A.; Webster, E.R.; Johnson, L.E.; Johal, M.S.; Benz, L. Surface and Stability Characterization of a Nanoporous ZIF-8 Thin Film. J. Phys. Chem. C 2014, 118, 14449–14456. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Figueiredo, F.M.L. High Surface Proton Conduction in Nanostructured ZIF-8. Nanomaterials 2019, 9, 1369. [Google Scholar] [CrossRef]
- Si, Y.; Li, X.; Yang, G.; Mie, X.; Ge, L. Fabrication of a novel core–shell CQDs@ZIF-8 composite with enhanced photocatalytic activity. J. Mater. Sci. 2020, 55, 13049–13061. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Zhang, G.; Sheng, L. Matrix-assisted pulsed laser evaporation of polyimide thin films and the XPS study. Sci. China Ser. B Chem. 2008, 51, 983–989. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Varela-Guerrero, V.; Barnett, G.V.; Jeong, H.-K. Synthesis of Zeolitic Imidazolate Framework Films and Membranes with Controlled Microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Burrows, A.D.; Jaber, R.; Edler, K. Facile synthesis of metal-organic framework films via in situ seeding of nanoparticles. Chem. Commun. 2012, 48, 4965–4967. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rose, O.L.; Bonciu, A.; Marascu, V.; Matei, A.; Liu, Q.; Rusen, L.; Dinca, V.; Dinu, C.Z. Thin Films of Metal-Organic Framework Interfaces Obtained by Laser Evaporation. Nanomaterials 2021, 11, 1367. https://doi.org/10.3390/nano11061367
Rose OL, Bonciu A, Marascu V, Matei A, Liu Q, Rusen L, Dinca V, Dinu CZ. Thin Films of Metal-Organic Framework Interfaces Obtained by Laser Evaporation. Nanomaterials. 2021; 11(6):1367. https://doi.org/10.3390/nano11061367
Chicago/Turabian StyleRose, Olivia L., Anca Bonciu, Valentina Marascu, Andreea Matei, Qian Liu, Laurentiu Rusen, Valentina Dinca, and Cerasela Zoica Dinu. 2021. "Thin Films of Metal-Organic Framework Interfaces Obtained by Laser Evaporation" Nanomaterials 11, no. 6: 1367. https://doi.org/10.3390/nano11061367
APA StyleRose, O. L., Bonciu, A., Marascu, V., Matei, A., Liu, Q., Rusen, L., Dinca, V., & Dinu, C. Z. (2021). Thin Films of Metal-Organic Framework Interfaces Obtained by Laser Evaporation. Nanomaterials, 11(6), 1367. https://doi.org/10.3390/nano11061367







