Abstract
“Spinel-layered” Li1+xNi0.5Mn1.5O4 (x = 0, 0.5, 1) materials are considered as a cobalt-free alternative to currently used positive electrode (cathode) materials for Li-ion batteries. In this work, their electrochemical properties and corresponding phase transitions were studied by means of synchrotron X-ray powder diffraction (SXPD) in operando regime. Within the potential limit of 2.2–4.9 V vs. Li/Li+ LiNi0.5Mn1.5O4 with cubic spinel type structure demonstrates the capacity of 230 mAh·g−1 associated with three first-order phase transitions with significant total volume change of 8.1%. The Li2Ni0.5Mn1.5O4 material exhibits similar capacity value and subsequence of the phase transitions of the spinel phase, although the fraction of the spinel-type phase in this material does not exceed 30 wt.%. The main component of Li2Ni0.5Mn1.5O4 is Li-rich layered oxide Li(Li0.28Mn0.64Ni0.08)O2, which provides nearly half of the capacity with very small unit cell volume change of 0.7%. Lower mechanical stress associated with Li (de)intercalation provides better cycling stability of the spinel-layered complex materials and makes them more perspective for practical applications compared to the single-phase LiNi0.5Mn1.5O4 high-voltage cathode material.
Keywords:
Li-ion; cathode material; oxides; spinel; LiNi0.5Mn1.5O4; spinel-layered composite; high-voltage 1. Introduction
Since the discovery of extraordinary electrochemical capacity for so-called “Li-rich” oxides as positive electrodes for Li-ion batteries, materials exploiting anionic redox activity have been the subject of intense studies [1,2,3,4,5]. Mn- and Li-rich NMCs with a typical Li[Li,Mn,Ni,Co]O2 composition were firstly regarded as two-phase composites consisting of the layered rhombohedral (s.g. Rm) phase Li(Mn,Ni,Co)O2 and the layered monoclinic phase Li2MnO3 (s.g. C2/m) as nano-sized domains, while later Li-rich NMCs were recognized as single-phase materials with local cation ordering. It is worth noting that pure Li2MnO3 was considered to be electrochemically inactive for a long time, before demonstration of reversible Li+ extraction/insertion after “activation” at ~4.5–4.7 V vs. Li/Li+ in highly dispersed powder [6]. Unlike the LiMO2 layered oxides (M—transition metal), Li2MnO3 (also represented as Li[Li1/3Mn2/3]O2) exhibits oxygen anion redox activity that has been confirmed by a number of experimental and computational studies [7,8,9]. Single-phase Li2MnO3 suffers from poor cycling stability, low Coulombic efficiency, voltage fading, etc. On the other side, monoclinic Li-rich NMCs exhibit much better capacity retention during cycling demonstrating the capacity up to 300 mAh·g−1. However, in spite of large reversible capacity, Li-rich NMCs still have not found an application because of well-known drawbacks such as irreversible d-cation migration, leading to spinel phase formation and voltage fading [5,10,11,12,13,14].
Another promising direction for the development of oxide materials for Li-ion batteries are a high-voltage spinel LiNi0.5Mn1.5O4 and “spinel-layered” materials demonstrating attractive electrochemical performance with ~700–800 Wh·kg−1 of the cathode energy density [15,16,17,18]. Their nominal composition can be written as Li1+xNiyMn2–yO4 and x value typically determines the spinel to layered phase ratio. When x = 0 and y = 0.5, the well-known high-voltage spinel LiNi0.5Mn1.5O4 is formed [19]. Increasing x by using the excess amount of lithium source for the synthesis leads to monoclinic layered oxide as a second phase. Additionally, an impurity LizNi2−zO2 phase typically associated with a nickel-rich rock-salt derivative is often observed [15,16,17,18]. It should be noted that chemical or electrochemical lithiation of LiNi0.5Mn1.5O4 results in a tetragonal Li2Ni0.5Mn1.5O4 phase (s.g. I41/amd), so the Li1+xNi0.5Mn1.5O2 oxides demonstrate path-dependent phase composition [20,21,22]. Authors who studied electrochemical behavior of the “spinel-layered” composites pointed out high reversible capacity (>200 mAh/g) and excellent cycling stability of these materials, exceeding performance of single-phase LiNi0.5Mn1.5O4. Ex situ X-ray diffraction and microscopy studies revealed the presence of both monoclinic (layered) and cubic (spinel) phases after long-term cycling; however, there is lack of data concerning changes occurring directly during (De)lithiation of “spinel-layered” composite materials [13,14,15,16,17,18].
The study of phase and structural transformations taking place in electrode materials during electrochemical charge–discharge is vital for understanding the processes underlying battery functioning, which, in turn, is necessary for the development of new materials and technologies. Phase transitions occurring during lithiation and de-lithiation of LiNi0.5Mn1.5O4 have already been described [23,24,25,26]. There are two two-phase transitions at the high-voltage part (ca. ~4.7 V vs. Li/Li+), conserving spinel-type structure and cubic-to-tetragonal transition at the low-voltage plateau (ca. 2.8 V vs. Li/Li+). Structural changes in Li-rich NMCs were also studied in a number of papers, including oxygen evolution during first charge, activation of Li in Li-TM (transition metal) layer, migration of TM into Li positions and spinel formation during cycling [5,9,10,11,12,13,14]. On the other hand, the processes occurring in “spinel-layered” Li1+xNi0.5Mn1.5O4 composites during electrochemical cycling are still unclear and have not been studied so far. The main goal of our work was to fill this gap.
Thus, we report here synthesis, electrochemical characterization and detailed study of the phase transitions in Li1+xNi0.5Mn1.5O4 (x = 0, 0.5, 1) cathode materials by means of operando synchrotron X-ray powder diffraction (SXPD).
2. Materials and Methods
The samples of the Li1+xNi0.5Mn1.5O4 (x = 0, 0.5, 1.0) nominal compositions were prepared using hydrothermal synthesis as described previously [27,28]. Briefly, NiSO4·6H2O and MnSO4·H2O were dissolved in water in molar ratio of 1:3. Sodium carbonate solution was added and the obtained suspension was heated to 140 °C and conditioned at this temperature for 12 h. The precipitate was washed, dried and mixed with LiOH taken with 5% excess. The precursor was annealed at 350 °C for 2 h and then at 800 °C for 10 h with intermediate regrinding.
The phase composition of the samples was characterized using powder X-ray diffraction (PXRD, Huber Guinier camera G670, CuKα1 radiation, λ = 1.5406 Å). The particle size and morphology were studied using a JEOL JSM-6490LV (30 kV, W-cathode) scanning electron microscope (SEM).
Samples for the transmission electron microscopy (TEM) study were prepared by crushing the materials in an agate mortar under ethanol and depositing a few drops of suspension onto copper grids covered by a holey carbon layer. High angle annular darkfield scanning transmission electron microscopy (HAADF-STEM) images and energy-dispersive X-ray (EDX) spectra were collected with a probe aberration-corrected FEI Titan G3 microscope operated at 200 kV and equipped with a Super-X EDX system.
Electrode materials for electrochemical testing were prepared by mixing 80 mass.% of active compound, 10 mass.% of carbon black and 10 mass.% of polyvinylidene fluoride (PVDF) binder in N-methylpyrrolidone followed by spreading the obtained mixture on aluminum foil by doctor blade technique. Dried electrodes were calendered, punched to round discs and dried at 110 °C for 3 h under dynamic vacuum. Two-electrode cells were assembled in an Ar-filled glove-box (MBraun). Lithium metal was used as the counter electrode and 1 M solution of LiBF4 in sulfolane was used as the electrolyte. All galvanostatic experiments were carried out using Elins P-20X8 potentiostat-galvanostat (ES8 software) within 2.2–4.9 V vs. Li/Li+.
Synchrotron X-ray powder diffraction (SXPD) in operando regime was performed at the Swiss Norwegian Beamlines (SNBL), BM01 and BM31, at the European Synchrotron Radiation Facility (ESRF, Grenoble, France). The original electrochemical cell with single-crystal sapphire X-ray windows was used [29]. Experiments were conducted in a low intensity beam mode (~40 mA, 4 × 10 filling mode). The PILATUS@SNBL diffractometer was used for SXPD studies (λ = 0.7225 Å). The 2D diffraction data from a Pilatus 2 M detector were processed using the SNBL Toolbox and BUBBLE software [30]. The time of data acquisition was 10 s per pattern. Rietveld refinement using RIETAN-FP software was applied to analyze SXPD patterns [31]. In some cases, Jana 2006 software was applied [32].
3. Results
PXRD revealed the presence of the cubic spinel phase LiNi0.5Mn1.5O4 (sp.gr. Fdm, Z = 8, a = 8.1710(3) Å, V = 545.54(2) Å3) in all synthesized Li1+xNi0.5Mn1.5O4 (x = 0, 0.5, 1.0) samples. In the case of Li1.5Ni0.5Mn1.5O4 and Li2Ni0.5Mn1.5O4, the second phase—layered monoclinic Li1+xM1−xO2 (M = Mn, Ni, sp.gr. C2/m, a = 4.941(1) Å, b = 8.542(2) Å, c = 5.037(1)Å, β= 109.29(2)o, V = 200.6(1)Å3)—was observed with the content increasing with x (Figure 1). In addition, an increase in x was accompanied by the appearance of and increase in intensity of a few diffraction maxima, which may be assigned to the disordered LizM2−zO2 phase (M = Mn, Ni, sp.gr. Rm, Z = 3, a = 2.930(1) Å, c = 14.41(3) Å, V = 107.1(1)Å3) [33,34]. The morphology of all the samples was found to be the same: relatively large (~2–5 µm) spherical aggregates consisting of small (~200–700 nm) crystallites (example for the LiNi0.5Mn1.5O4 material is given in Figure 1).
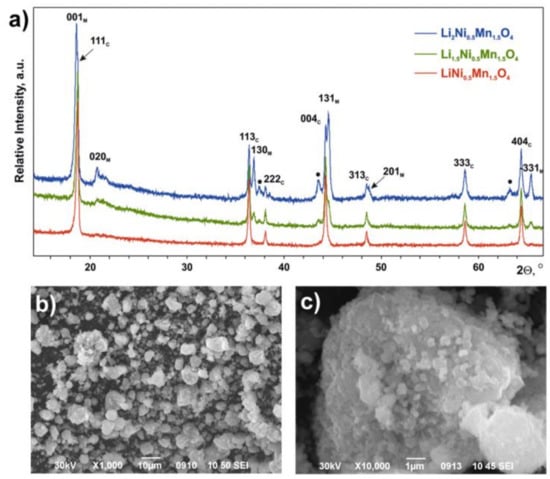
Figure 1.
(a) Powder X-ray diffraction (PXRD) patterns of the initial samples. The reflections of cubic LiNi0.5Mn1.5O4 (C) and monoclinic Li1+xM1−xO2 (M) phase are indexed. The reflections of the LizM2−zO2 phase are marked by dots. (b,c) Scanning electron microscopy (SEM) images for the LiNi0.5Mn1.5O4 sample.
Atomic-resolution HAADF-STEM images clearly demonstrate co-existence of three different phases in the Li2Ni0.5Mn1.5O4 sample (Figure 2). All three are based on the rock-salt structure with the cubic close packing of the oxygen atoms, but differ in the ordering pattern of the Li and d-metal cations. The crystals with the spinel-type ordering (the LiNi0.5Mn1.5O4 phase, Figure 2a), layered ordering (the Li1+xM1−xO2 phase, Figure 2b) and partially ordered rock-salt phase in which the transition metal cations are preferentially located at the {111} planes (the LizM2−zO2 phase, Figure 2c) were observed. EDX compositional maps (Figure 2d) also clearly demonstrates three phases with different Ni:Mn ratio. Quantification of the EDX spectra provides the Ni:Mn ratio of 24(1):76(1), 11(2):89(2) and 82.1(8):17.9(8) for the LiNi0.5Mn1.5O4 phase, Li1+xM1−xO2 phase and LizM2−zO2 phase, respectively.
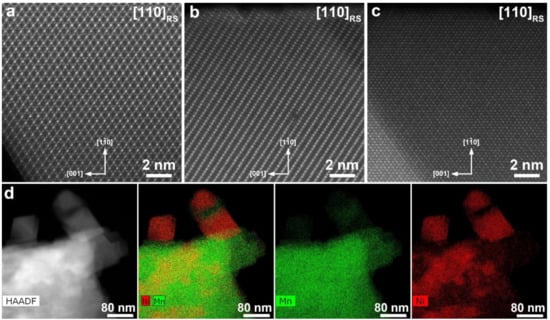
Figure 2.
High angle annular darkfield scanning transmission electron microscopy (HAADF-STEM) images of the LiNi0.5Mn1.5O4 (a), Li1+xM1−xO2 (b) and LizM2−zO2 (c) phases in the Li2Ni0.5Mn1.5O4 sample. The images are taken along the [110] direction of the parent rock-salt (RS) structure. HAADF-STEM image, the color-coded Mn and Ni compositional map and individual energy-dispersive X-ray (EDX) maps of Mn and Ni (d) demonstrating three phases with different Ni:Mn atomic ratio.
The phase compositions of the samples were confirmed by Rietveld refinement of PXRD data (Figures S1–S3, the details of the refinement are given in SI) using the values of Ni:Mn ratio for each phase obtained by EDX. The structure of the layered monoclinic phase Li1+xM1−xO2 can be described as a derivative of the Li2MnO3structure formed by an alternation of the Li3 and Li1−xM2+x layers (M = Mn, Ni) along the c axis [5,6,7,8,9,10,11,12,35]. The phase composition used in the refinement corresponds to the formula of Li(Li0.28Mn0.64Ni0.08)O2 (it can also be written as Li1.92Mn0.96Ni0.12O3, so the composition is close enough to the Li2MnO3 monoclinic phase). The composition of the third, rhombohedral LizM2−zO2 phase can be written as Li0.5Ni1.21Mn0.26O2. In the Li2Ni0.5Mn1.5O4 sample, the weight fractions of the LiNi0.5Mn1.5O4, Li1+xM1−xO2 and LizM2−zO2 phases were found to be 28(1), 63(1) and 9.0(4) wt.%, respectively.
Electrochemical characterization of the samples (Figure 3) revealed that the cubic spinel LiNi0.5Mn1.5O4 is characterized by two high-voltage plateaus at the first charge, with an average potential of ~4.7 V vs. Li/Li+. An additional plateau at ~2.7 V vs. Li/Li+ appears at discharge, providing ca. 230 mAh·g−1 of the reversible capacity within 2.2–4.9 V potential interval. As it was reported earlier, high-voltage part of the charge–discharge curve is associated with Ni2+/Ni3+/Ni4+ redox transitions, and the low-voltage part with the Mn3+/Mn4+ transition [22,23,24,25]. Increasing x in Li1+xNi0.5Mn1.5O4 leads to a decrease in length of the high-voltage and low-voltage plateaus and to appearance of additional capacity within sloping part of the charge–discharge curve situated between 2.8 and 4.7 V vs. Li/Li+. Total capacity is approximately the same for all the samples (~230 mAh·g−1); however, it grows during several primary cycles in case of Li1.5Ni0.5Mn1.5O4 and especially in Li2Ni0.5Mn1.5O4. Similar electrochemical behavior was shown earlier for Li1+xNi0.5Mn1.5O4 and materials of close compositions [14,15,16,17]. At the moment, there is no unambiguous understanding this phenomenon; authors consider it as higher utilization of the layered phase achieved during cycling. Most likely, it is associated with transformation of the domain structure of the materials following the formation of higher amount of grain boundaries enhancing electrochemical activity. Besides this, the process of oxygen sublattice activation (taking place in the Li-rich cathodes at the fist charge) may be hindered by the presence of spinel phase and therefore several cycles are needed to complete the process. Elongation of the lower part of the sloping plateau region between 3 and 4 V corresponding to Mn3+/Mn4+ redox in Li-rich oxides, which appears after oxygen evolution, favors such an assumption. Short-term cycling at C/3 charge and discharge rate revealed ca. 10% degradation after 50 cycles for LiNi0.5Mn1.5O4 and Li1.5Ni0.5Mn1.5O4 and almost steady capacity for Li2Ni0.5Mn1.5O4. The voltage curve of Li2Ni0.5Mn1.5O4 undergoes a certain transformation during cycling (Figure 3f). However, it almost does not affect the specific energy density of the material (680 vs. 660 Wh kg−1 at 10th and 50th cycles, respectively). The samples demonstrate attractive C-rate capacity retention even after cycling: increasing the current from C/10 to 3C almost does not reduce the capacity associated with the high-voltage part of the charge–discharge curve (Figure 3g–i). However, the lower part (below 3 V vs. Li/Li+) definitely demonstrates more sluggish kinetics of Li+ incorporation.
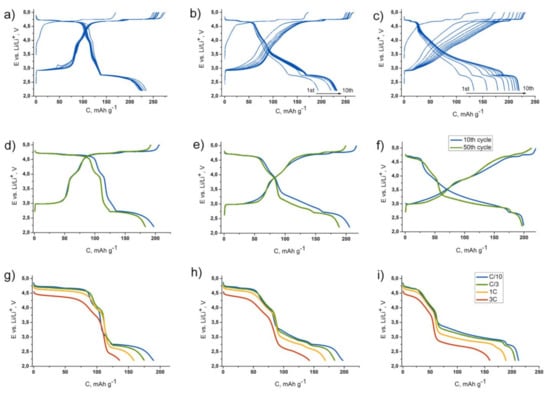
Figure 3.
1st–10th charge–discharge curves for the LiNi0.5Mn1.5O4 (a), Li1.5Ni0.5Mn1.5O4 (b) and Li2Ni0.5Mn1.5O4 (c) samples at C/10 rate; 10th and 50th charge–discharge curves for the same samples at C/3 rate (d–f); the discharge curves at C/10-3C rate for the same samples after cycling (g–i).
To analyze structural transitions responsible for the observed electrochemical signatures, we performed SXPD study of LiNi0.5Mn1.5O4 and Li2Ni0.5Mn1.5O4 materials in operando regime. Initially, the cells were charged up to 4.9 V and discharged to 2.2 V vs. Li/Li+. The obtained diffraction patterns and corresponding E–x curves for the second charge–discharge cycle (2.2–4.9–2.2 V vs. Li/Li+) are shown in Figure 4.
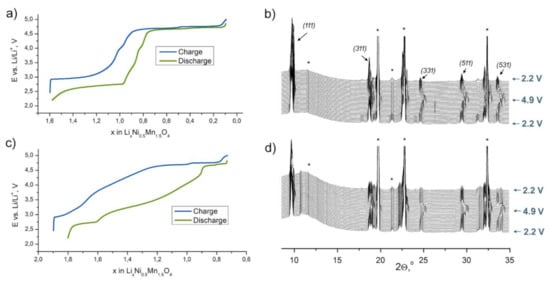
Figure 4.
E–x curves and operando synchrotron X-ray powder diffraction (SXPD) patterns of the second charge–discharge cycle for the LiNi0.5Mn1.5O4 (a,b) and Li2Ni0.5Mn1.5O4 (c,d) materials. The most intense cubic spinel reflections are marked with corresponding hkl indices. Asterisks belong to the reflections caused by the cell components.
The data on LiNi0.5Mn1.5O4 are consistent with those previously published [23,24,25,26]. During the second charge, three first-order phase transitions with intermediate solid-solution regions (Figure 5) occur: (1) “Tetragonal” ↔ “Cubic 1”at ca. 2.8 V vs. Li/Li+; (2) “Cubic 1” ↔ “Cubic 2” at ca. 4.7 V and (3) “Cubic 2” ↔ “Cubic 3” at ca. 4.75 V.
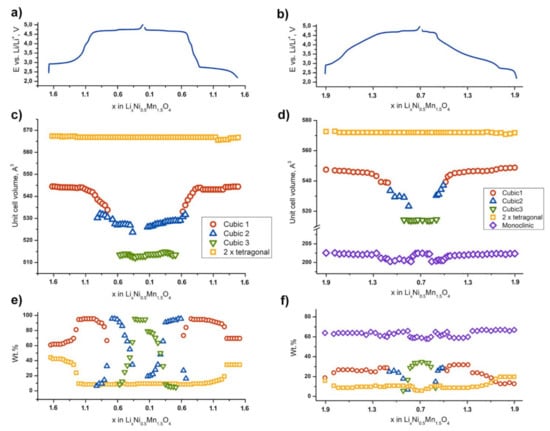
Figure 5.
E–x curves and variation of the unit cell volumes and weight fractions of the phases for the LiNi0.5Mn1.5O4 (a,c,e) and Li2Ni0.5Mn1.5O4 (b,d,f) samples.
“Tetragonal” phase corresponds to Li1+αNi0.5Mn1.5O4 (α ≈ 0.6) (s.g. I41/amd) formed during the first discharge [36]. The structure of Li1+αNi0.5Mn1.5O4 represents a tetragonally distorted spinel, where the deformation is caused by axial elongation of the MnO6 octahedra due to the Jahn–Teller effect, induced by partial reduction of Mn4+ to Mn3+. At the beginning of the second charge (2.7 V), the LiNi0.5Mn1.5O4 cathode material contains about 41 wt.% of Li1+αNi0.5Mn1.5O4 (sp.gr. I41/amd, Z = 4, a = 5.7317(6) Å, c = 8.637(2) Å, V = 283.7(1) Å3) and 59 wt.% of the “Cubic 1” phase (sp. gr. Fdm, Z = 8, a = 8.180(2) Å, V = 547.4(1) Å3); the latter is similar to the initial spinel. The amount of the tetragonal phase gradually decreases up to ca. 4.0 V vs. Li/Li+. The tetragonal phase is characterized by an absence of notable solid solution region. According to our data, some amount of the tetragonal phase (about 5–6 wt.%) remains in the material at the higher potentials and does not participate in redox processes.
The composition of the “Cubic 1” phase can be written as Li1−yNi0.5Mn1.5O4, where 0 ≤ y < 0.5 [23,24,25,26]. Upon the “Tetragonal” ↔ “Cubic 1” transition, a smooth shrinkage of the cubic spinel cell is observed. Further Li+ extraction between 4.0 and 4.7 V and corresponding oxidation of Ni2+ to Ni3+ forces more drastic “Cubic 1” phase cell volume reduction. The shrinkage of the “Cubic 1” cell is also observed in the “Cubic 1” ↔ “Cubic 2” two-phase region (4.7–4.75 V). In should be noted that the “Cubic 1” phase undergoes the most remarkable cell volume change comparing to other phases in the system: the volume decrease is 5 Å3 per one transition metal atom (nearly 2%).
The region of the existence of the “Cubic 2” phase is significantly shorter and relates to the potential range of 4.7–4.8 V. The “Cubic 2” phase preserves the initial cubic spinel structure with the formula of Li0.5−yNi0.5Mn1.5O4 [23,24,25,26], where 0.5 ≤ y< 0 (sp.gr. Fdm, Z = 8, at y ≈ 0 a = 8.091(6) Å, V = 529.5(7) Å3). The volume decrease in the “Cubic 2” unit cell upon Li+ extraction is also much smaller compared to that of the “Cubic 1” phase and has been found to be 3.5Å3 per one transition metal atom (nearly 1.5%). Complete oxidation of Ni3+ to Ni4+ leads to the “Cubic 3” phase Ni0.5Mn1.5O4. This phase is first revealed as a second phase in the “Cubic 2”–“Cubic 3” two-phase region (in the vicinity of 4.8 V). At 5 V, only the “Cubic 3” phase exists. The lattice constant of the “Cubic 3” phase (sp.gr. Fdm, Z = 8, a = 7.9955(3) Å, V = 511.1(1) Å3) does not vary notably; the volume change is as small as 0.2%. It is worth noting that at the end of the “Cubic 1”–“Cubic 2” two-phase region the lattice parameters of both phases are very close to each other while there is no such a behavior for the “Cubic 2”–“Cubic 3” transition.
The described phase transitions demonstrate excellent reproducibility upon the second discharge. As it can be seen in Figure 5a,c, the cell volumes of the phases are similar to the ones upon charge within the value of 3σ.
The behavior of the Li2Ni0.5Mn1.5O4-based electrode differs significantly from that of LiNi0.5Mn1.5O4. After the first charge–discharge cycle at the starting point (2.2 V vs. Li/Li+), the material consists of cubic spinel “Cubic 1”, tetragonally distorted spinel “Tetragonal” and layered monoclinic Li(Li0.28Mn0.64Ni0.08)O2 phase with the phase fraction of more than 60 wt.%. It should be noted that there is no hint of the participation of the rhombohedral Li0.5Ni1.21Mn0.26O2 phase in redox processes. Considering the significant overlap of the diffraction maxima of Li0.5Ni1.21Mn0.26O2 with the maxima of Al current collector, Li0.5Ni1.21Mn0.26O2 was excluded from the refinement of SXPD data and all mass ratio presented hereinafter corresponds to 91% of the active composite fraction.
It should be emphasized that no evidence of the degradation/content decrease in the layered monoclinic phase within the Li2Ni0.5Mn1.5O4 sample after the first cycle was found using both laboratory (ex situ) and synchrotron (operando) XRD techniques (Figure 6). Recent HRTEM studies of the electrode material with close Li[Ni1/3Mn2/3]O2 composition after cycling also revealed the presence of both monoclinic and spinel phases [17]. In contrast, pure Li2MnO3 demonstrates different behavior. The irreversible transformation of Li2MnO3 to the tetragonal spinel-related Li2Mn2O4 phase at the first charge has been reported by Amalraj et al. [37].
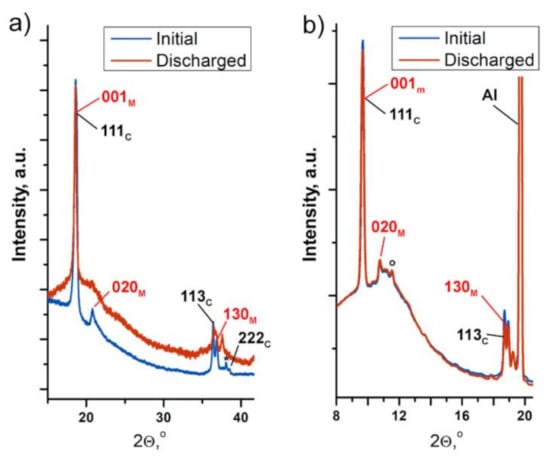
Figure 6.
PXRD patterns of the Li2Ni0.5Mn1.5O4 electrode at the initial state and after the first charge–discharge cycle collected by means of laboratory (a) and synchrotron (b) XRD. Reflections of the monoclinic Li(Li0.28Mn0.64Ni0.08)O2 phase are marked in red; those for the cubic spinel are in black.
Concerning the experiment, at the beginning of the second charge–discharge cycle, the contents of the tetragonal spinel and “Cubic 1” phases are 16 and 19 wt.%, respectively. The phase transitions intrinsic in LiNi0.5Mn1.5O4 are conserved, however, with the dramatic shortening of two-phase transition regions for the cubic phases. There is no two-phase “Cubic 1”—“Cubic 2” region either on charge or on discharge at the second cycle. The two-phase “Cubic 2”—“Cubic 3” transition becomes narrower than that for the LiNi0.5Mn1.5O4 sample. However, the observed cell volume differences for both “Cubic 1” and “Cubic 2” phases are approximately the same, as in the case of LiNi0.5Mn1.5O4. This shortening of two-phase areas may be explained by the higher specific charge–discharge rate due to the lower absolute amount of the cubic phases.
In contrast, the weight fraction of Li(Li0.28Mn0.64Ni0.08)O2 as well as its unit cell parameters are nearly stable during the whole charge–discharge cycle except in the high-voltage region (at ca. 4.75 V), where tiny reversible changes can be seen (Figure 5b). In this potential region, the cell volume of Li(Li0.28Mn0.64Ni0.08)O2 reduces by 2Å3 per one transition metal atom (1%).
Phase transformations taking place during charge–discharge of the Li1.5Ni0.5Mn1.5O4 sample are right between the two described materials. However, the monoclinic phase fraction in the Li1.5Ni0.5Mn1.5O2 sample is significantly lower than in the Li2Ni0.5Mn1.5O2 sample, which hampers the precise determination of its lattice constants taking into account the remarkable reflections overlap. However, the refinement has been done and the results are listed in Tables S1–S3. Variation of unit cell volumes and weight fractions of the involved phases are shown in Figure S4.
4. Discussion
As it can be seen from Figure 3a,c, reversible capacities of the LiNi0.5Mn1.5O4 spinel and spinel-layered Li2Ni0.5Mn1.5O4 materials at the second charge–discharge cycle are ~220 and ~170 mAh·g−1, respectively. Since the mass fraction of the spinel-type phases in the Li2Ni0.5Mn1.5O4-based electrode is almost steady during charge–discharge process and corresponds to 35–40%, we can assume that appr. 50% of the capacity obtained during the second cycle belongs to the monoclinic layered oxide Li(Li0.28Mn0.64Ni0.08)O2. The oxidation state of Mn in this oxide is +4, as in Li2MnO3, so we may conclude that anionic redox is predominantly responsible for the electrochemical activity of this component.
Better cycling stability of the Li2Ni0.5Mn1.5O4 material compared to LiNi0.5Mn1.5O4 is most probably related to improved mechanical stability: the lower volume fraction of the spinel-type phases with high specific volume change on the charge/discharge leads to a decrease in total volume variation. This finding is in a good agreement with the earlier studies on LiNi0.5Mn1.5O4 and LiNi0.5Mn1.5O4-Li2MnO3 composites by means of HRTEM [38]. Lu et al. observed significant pulverization of LiNi0.5Mn1.5O4 after cycling within the extended voltage range of 2.0–5.0 V vs. Li/Li+. Microstructure of the electrode consisting of LiNi0.5Mn1.5O4 and monoclinic domains was found to be much more stable, as well as its electrochemical characteristics upon cycling [38].
Based on the data presented in Figure 5 and literature data on the redox activity of Ni and Mn cations, we suggest a tentative scheme of the electrochemical transitions occurring at (De)lithiation of LiNi0.5Mn1.5O4 and Li2Ni0.5Mn1.5O4, and corresponding volume changes of the overall electrode composite (determined taking into account the volumetric changes during phase transitions and the mass fractions of the involved phases). The scheme is illustrated in Figure 7. The overall estimated volume change of the LiNi0.5Mn1.5O4 electrode is twice larger than that for Li2Ni0.5Mn1.5O4, as can be seen from the Figure 6, and mainly caused by Mn3+/Mn4+ and Ni3+/Ni4+ transitions at 2.8 and 4.75 V, respectively. Since the mass fraction of the spinel-type phases in Li2Ni0.5Mn1.5O4 does not exceed 30%, these transitions do not strongly affect overall volume change. Therefore, this composite material appears to be mechanically sustainable, which gives another reason to consider it promising for next-generation LIBs.
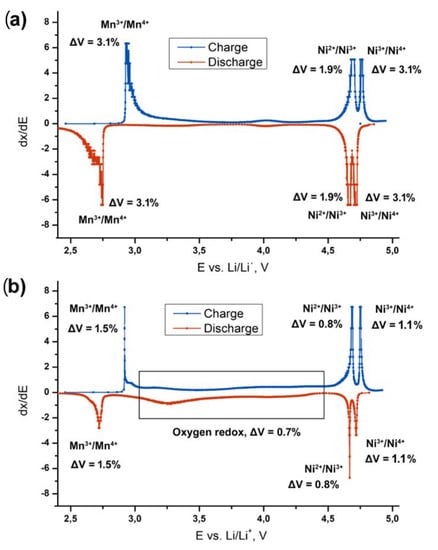
Figure 7.
Differential capacity curves for the (a) LiNi0.5Mn1.5O4 and (b) Li2Ni0.5Mn1.5O4 electrodes, the corresponding redox transitions [23,24,25,26] and volume changes.
5. Conclusions
Cobalt-free “spinel-layered” composites of the Li1+xNi0.5Mn1.5O2” (x = 0, 0.5, 1) composition demonstrate attractive electrochemical performance with capacity exceeding 200 mAh·g−1 within 2.2–4.9 V vs. Li/Li+. Operando synchrotron X-ray diffraction studies revealed complex phase transition mechanisms including tetragonal, three cubic and monoclinic phases. Although phase transitions for the spinel-type phases in the Li2Ni0.5Mn1.5O4 cathode material remain almost the same as in the case of LiNi0.5Mn1.5O4, they are associated with only half of the observed capacity, which means that the layered Li-rich Li(Li0.28Mn0.64Ni0.08)O2 phase significantly contributes to the electrochemical activity of the whole composite. The redox process associated with Li(Li0.28Mn0.64Ni0.08)O2 is characterized by very small unit cell volume change of 0.7% and therefore provides better mechanical stability of the “spinel-layered” composites during electrochemical operation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11061368/s1, Figures S1–S3; Tables S1–S3.
Author Contributions
Conceptualization, O.A.D., A.M.A. (Anastasia M. Alekseeva); methodology, O.A.D., A.M.A. (Anastasia M. Alekseeva); investigation, V.A.S.; resources, D.C.; writing—original draft preparation, O.A.D., A.M.A. (Anastasia M. Alekseeva); writing—review and editing, D.C., A.M.A. (Artem M. Abakumov), E.V.A.; supervision, A.M.A. (Artem M. Abakumov), E.V.A.; project administration, O.A.D.; funding acquisition, O.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (grant No. 19-73-10078).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work was performed according to the Development program of the Interdisciplinary Scientific and Educational School of Lomonosov Moscow State University—“The future of the planet and global environmental change”. AICF of Skoltech is acknowledged for providing access to the TEM facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Lu, Z.; Dahn, J.R. Understanding the Anomalous Capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 Cells Using In Situ X-Ray Diffraction and Electrochemical Studies. J. Electrochem. Soc. 2002, 149, A815. [Google Scholar] [CrossRef]
- Lu, Z.; Beaulieu, L.Y.; Donaberger, R.A.; Thomas, C.L.; Dahn, J.R. Synthesis, Structure, and Electrochemical Behavior of Li[NixLi1/3−2x/3Mn2/3−x/3]O2. J. Electrochem. Soc. 2002, 149, A778–A791. [Google Scholar] [CrossRef]
- Kim, J.-S.; Johnson, C.S.; Vaughey, J.; Thackeray, M.M.; Hackney, S.A.; Yoon, W.-S.; Grey, C.P. Electrochemical and Structural Properties of xLi2M‘O3(1−x)LiMn0.5Ni0.5O2 Electrodes for Lithium Batteries (M‘ = Ti, Mn, Zr; 0 ≤ x ≤ 0.3). Chem. Mater. 2004, 16, 1996–2006. [Google Scholar] [CrossRef]
- Rozier, P.; Tarascon, J.M. Review—Li-Rich Layered Oxide Cathodes for Next-Generation Li-Ion Batteries: Chances and Challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- And, A.D.R.; Bruce, P.G. Mechanism of Electrochemical Activity in Li2MnO3. Chem. Mater. 2003, 15, 1984–1992. [Google Scholar] [CrossRef]
- Rana, J.; Papp, J.K.; Lebens-Higgins, Z.; Zuba, M.J.; Kaufman, L.A.; Goel, A.; Schmuch, R.; Winter, M.; Whittingham, M.S.; Yang, W.; et al. Quantifying the Capacity Contributions during Activation of Li2MnO3. ACS Energy Lett. 2020, 5, 634–641. [Google Scholar] [CrossRef]
- Cho, E.; Kim, K.; Jung, C.; Seo, S.-W.; Min, K.; Lee, H.S.; Park, G.-S.; Shin, J. Overview of the Oxygen Behavior in the Degradation of Li2MnO3 Cathode Material. J. Phys. Chem. C 2017, 121, 21118–21127. [Google Scholar] [CrossRef]
- McCalla, E.; Abakumov, A.M.; Saubanère, M.; Foix, D.; Berg, E.J.; Rousse, G.; Doublet, M.-L.; Gonbeau, D.; Novak, P.; Van Tendeloo, G.; et al. Visualization of O-O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 2015, 350, 1516–1521. [Google Scholar] [CrossRef]
- Gent, W.E.; Lim, K.; Liang, Y.; Li, Q.; Barnes, T.; Ahn, S.-J.; Stone, K.H.; McIntire, M.; Hong, J.; Song, J.H.; et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat. Commun. 2017, 8, 2091. [Google Scholar] [CrossRef]
- Seo, D.-H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R.; Holzapfel, M.; Novák, P.; Johnson, C.S.; Kang, S.-H.; Thackeray, M.M.; Bruce, P.G. Demonstrating Oxygen Loss and Associated Structural Reorganization in the Lithium Battery Cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the Spinel Phase in the Layered Composite Cathode Used in Li-Ion Batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223–2233. [Google Scholar] [CrossRef]
- Lee, E.-S.; Huq, A.; Chang, H.-Y.; Manthiram, A. High-Voltage, High-Energy Layered-Spinel Composite Cathodes with Superior Cycle Life for Lithium-Ion Batteries. Chem. Mater. 2011, 24, 600–612. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Johnson, C.; Amine, K.; Thackeray, M. Lithium–manganese–nickel-oxide electrodes with integrated layered–spinel structures for lithium batteries. Electrochem. Commun. 2007, 9, 262–268. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, M.D.; Haik, O.; Levi, E.; Talianker, M.; Markovsky, B.; Sun, Y.-K.; Aurbach, D. Electrochemical Performance of a Layered-Spinel Integrated Li[Ni1/3Mn2/3]O2 as a High Capacity Cathode Material for Li-Ion Batteries. Chem. Mater. 2015, 27, 2600–2611. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, R.; Li, L.; Yu, C.; Ren, Y.; Belharouak, I. A Study of High-Voltage LiNi0.5Mn1.5O4and High-Capacity Li1.5Ni0.25Mn0.75O2.5 Blends. J. Electrochem. Soc. 2013, 160, A1079–A1083. [Google Scholar] [CrossRef]
- Zhong, Q.; Bonakdarpour, A.; Zhang, M.; Gao, Y.; Dahn, J.R. Synthesis and Electrochemistry of LiNixMn2 − xO 4. J. Electrochem. Soc. 1997, 144, 205–213. [Google Scholar] [CrossRef]
- Mancini, M.; Axmann, P.; Gabrielli, G.; Kinyanjui, M.; Kaiser, U.; Wohlfahrt-Mehrens, M. A High-Voltage and High-Capacity Li1+xNi0.5Mn1.5O4Cathode Material: From Synthesis to Full Lithium-Ion Cells. ChemSusChem 2016, 9, 1843–1849. [Google Scholar] [CrossRef]
- Betz, J.; Nowak, L.; Winter, M.; Placke, T.; Schmuch, R. An Approach for Pre-Lithiation of Li1+xNi0.5Mn1.5O4 Cathodes Mitigating Active Lithium Loss. J. Electrochem. Soc. 2019, 166, A3531–A3538. [Google Scholar] [CrossRef]
- Mancini, M.; Gabrielli, G.; Axmann, P.; Wohlfahrt-Mehrens, M. Electrochemical Performance and Phase Transitions between 1.5 and 4.9 V of Highly-Ordered LiNi0.5Mn1.5O4with Tailored Morphology: Influence of the Lithiation Method. J. Electrochem. Soc. 2016, 164, A6229–A6235. [Google Scholar] [CrossRef]
- Arai, H.; Sato, K.; Orikasa, Y.; Murayama, H.; Takahashi, I.; Koyama, Y.; Uchimoto, Y.; Ogumi, Z. Phase transition kinetics of LiNi0.5Mn1.5O4 electrodes studied by in situ X-ray absorption near-edge structure and X-ray diffraction analysis. J. Mater. Chem. A 2013, 1, 10442–10449. [Google Scholar] [CrossRef]
- Komatsu, H.; Arai, H.; Koyama, Y.; Sato, K.; Kato, T.; Yoshida, R.; Murayama, H.; Takahashi, I.; Orikasa, Y.; Fukuda, K.; et al. Solid Solution Domains at Phase Transition Front of LixNi0.5Mn1.5O4. Adv. Energy Mater. 2015, 5, 1500638. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yoon, C.S.; Myung, S.-T.; Prakash, J.; Sun, Y.-K. Phase Transitions in Li1−δNi0.5Mn1.5O4 during Cycling at 5 V. Electrochem. Solid State Lett. 2004, 7, A216–A220. [Google Scholar] [CrossRef]
- Saravanan, K.; Jarry, A.; Kostecki, R.; Chen, G. A study of room-temperature LixMn1.5Ni0.5O4 solid solutions. Sci. Rep. 2015, 5, 8027. [Google Scholar] [CrossRef]
- Cheng, J.; Li, X.; Wang, Z.; Guo, H. Hydrothermal synthesis of LiNi0.5Mn1.5O4 sphere and its performance as high-voltage cathode material for lithium ion batteries. Ceram. Int. 2016, 42, 3715–3719. [Google Scholar] [CrossRef]
- Drozhzhin, O.; Shevchenko, V.; Zakharkin, M.; Gamzyukov, P.; Yashina, L.; Abakumov, A.; Stevenson, K.; Antipov, E. Improving salt-to-solvent ratio to enable high-voltage electrolyte stability for advanced Li-ion batteries. Electrochim. Acta 2018, 263, 127–133. [Google Scholar] [CrossRef]
- Drozhzhin, O.A.; Tereshchenko, I.V.; Emerich, H.; Antipov, E.V.; Abakumov, A.M.; Chernyshov, D. An electrochemical cell with sapphire windows foroperandosynchrotron X-ray powder diffraction and spectroscopy studies of high-power and high-voltage electrodes for metal-ion batteries. J. Synchrotron Radiat. 2018, 25, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Dyadkin, V.; Pattison, P.; Dmitriev, V.; Chernyshov, D. A new multipurpose diffractometer PILATUS@SNBL. J. Synchrotron Radiat. 2016, 23, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Izumi, F.; Ikeda, T. A Rietveld-Analysis Programm RIETAN-98 and its Applications to Zeolites. Mater. Sci. Forum 2000, 321-324, 198–205. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Betz, J.; Nowak, L.; Brinkmann, J.-P.; Bärmann, P.; Diehl, M.; Winter, M.; Placke, T.; Schmuch, R. Understanding the impact of calcination time of high-voltage spinel Li1+xNi0.5Mn1.5O4 on structure and electrochemical behavior. Electrochim. Acta 2019, 325, 134901. [Google Scholar] [CrossRef]
- Li, W.; Reimers, J.N.; Dahn, J.R. Crystal structure ofLixNi2−xO2and a lattice-gas model for the order-disorder transition. Phys. Rev. B 1992, 46, 3236–3246. [Google Scholar] [CrossRef]
- Sathiya, M.; Abakumov, A.M.; Foix, D.; Rousse, G.; Ramesha, K.; Saubanere, M.; Doublet, M.L.; Vezin, H.; Laisa, C.P.; Prakash, A.S.; et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat. Mater. 2015, 14, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, K.; Iwakoshi, Y.; Nakayama, N.; Ohzuku, T. Topotactic Two-Phase Reactions of Li[Ni1/2Mn3/2]O4 (P4332) in Nonaqueous Lithium Cells. J. Electrochem. Soc. 2004, 151, A296–A303. [Google Scholar] [CrossRef]
- Amalraj, S.F.; Burlaka, L.; Julien, C.M.; Mauger, A.; Kovacheva, D.; Talianker, M.; Markovsky, B.; Aurbacha, D. Phase Transitions in Li2MnO3 Electrodes at Various States-of-Charge. Electrochim. Acta 2014, 123, 395–404. [Google Scholar] [CrossRef]
- Lu, J.; Chang, Y.-L.; Song, B.; Xia, H.; Yang, J.-R.; Lee, K.S.; Lu, L. High energy spinel-structured cathode stabilized by layered materials for advanced lithium-ion batteries. J. Power Sources 2014, 271, 604–613. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).