Gene-Directed Enzyme Prodrug Therapy by Dendrimer-Like Mesoporous Silica Nanoparticles against Tumor Cells
Abstract
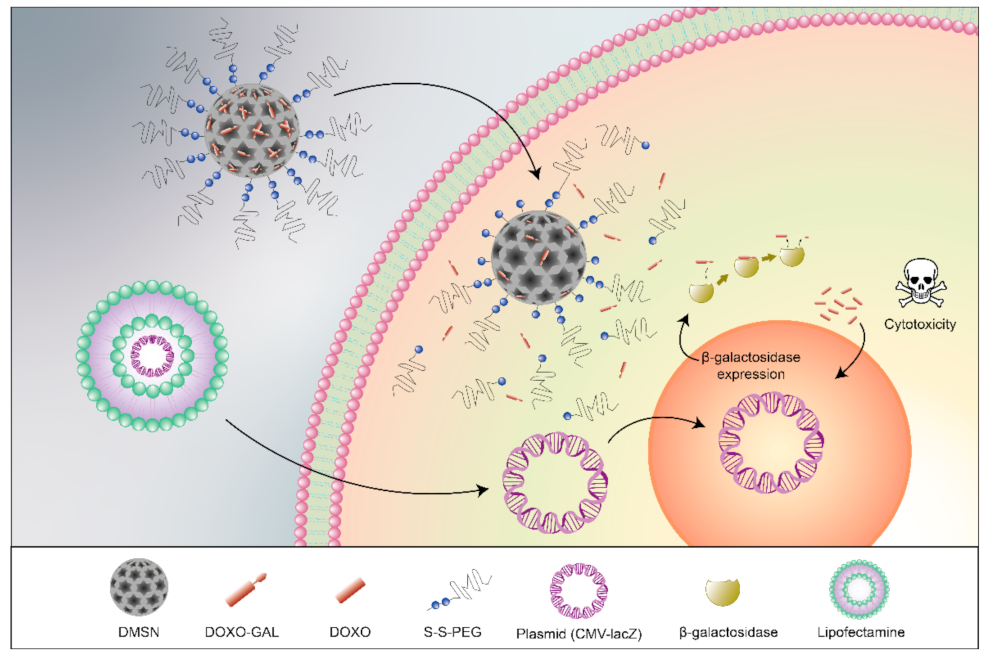
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Techniques
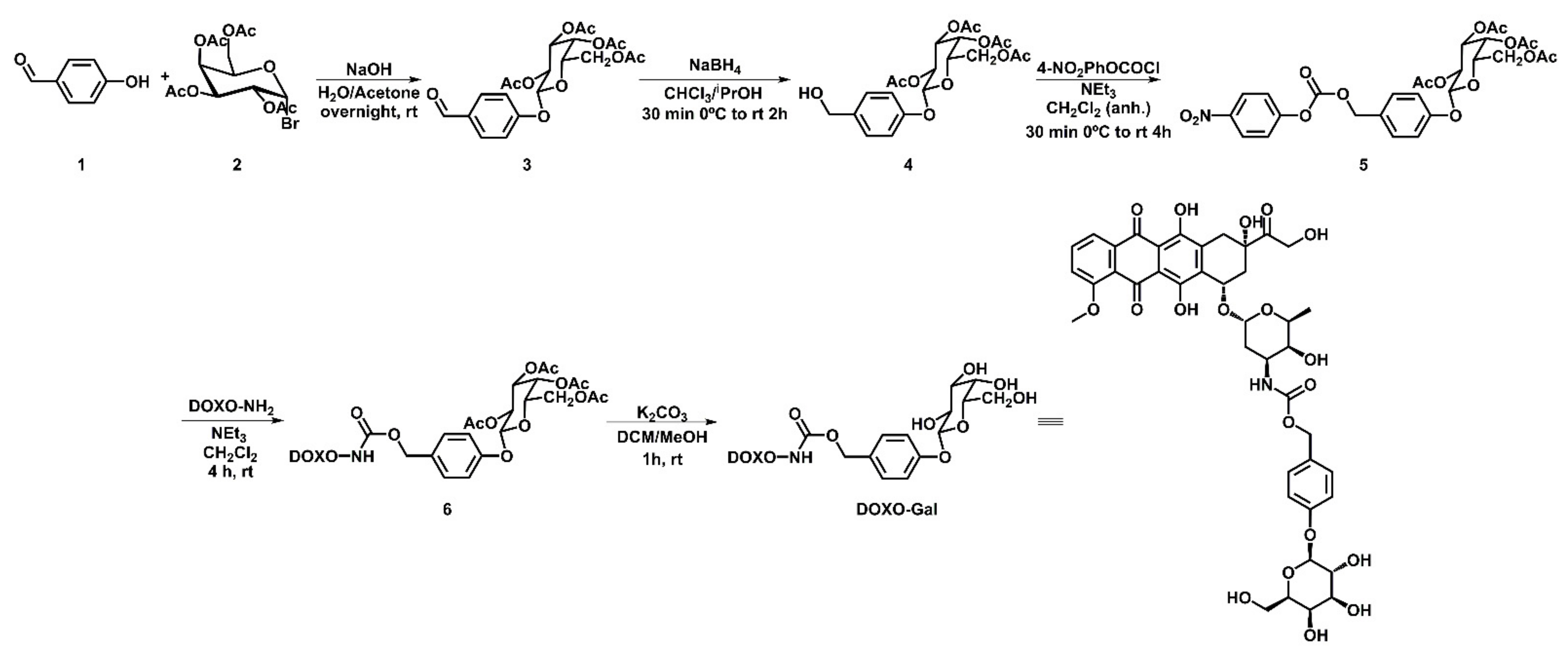
2.3. Synthesis of DOXO-Gal
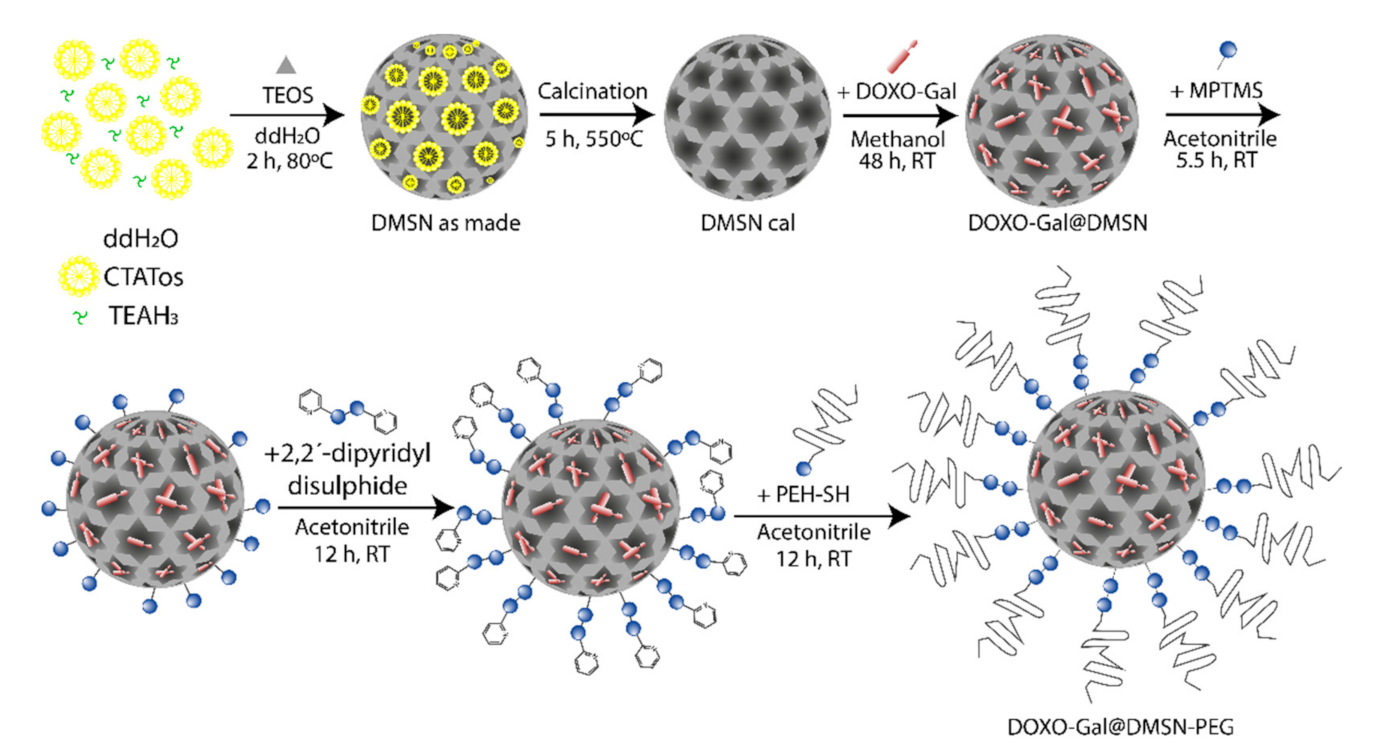
2.4. Synthesis of DMSNs
2.5. Synthesis of DOXO-Gal@DMSN-PEG
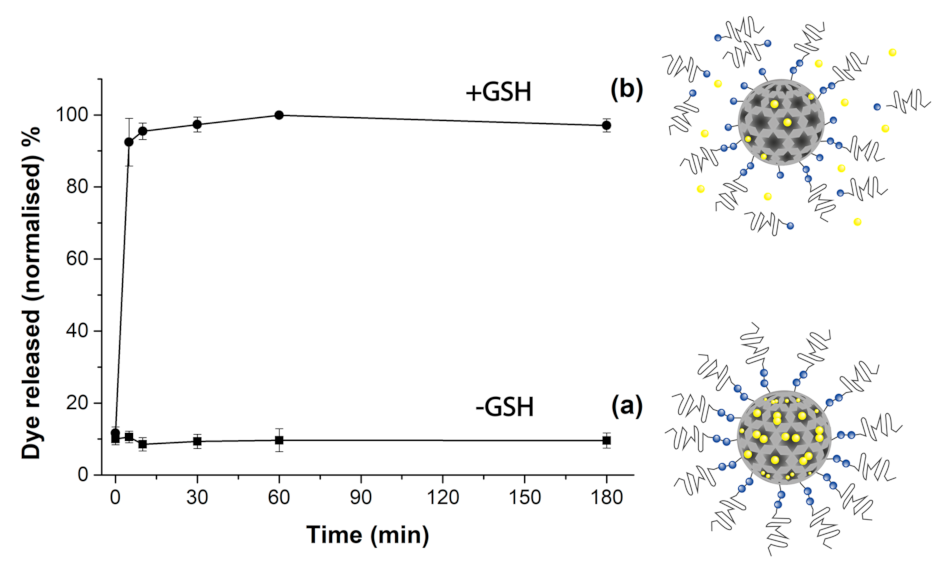
2.6. Release Assays
2.6.1. [Ru(bpy)3]Cl2 Release
2.6.2. Forced Release
2.7. Cells Lines and Maintenance
2.8. Transformation, Cloning, and Extraction
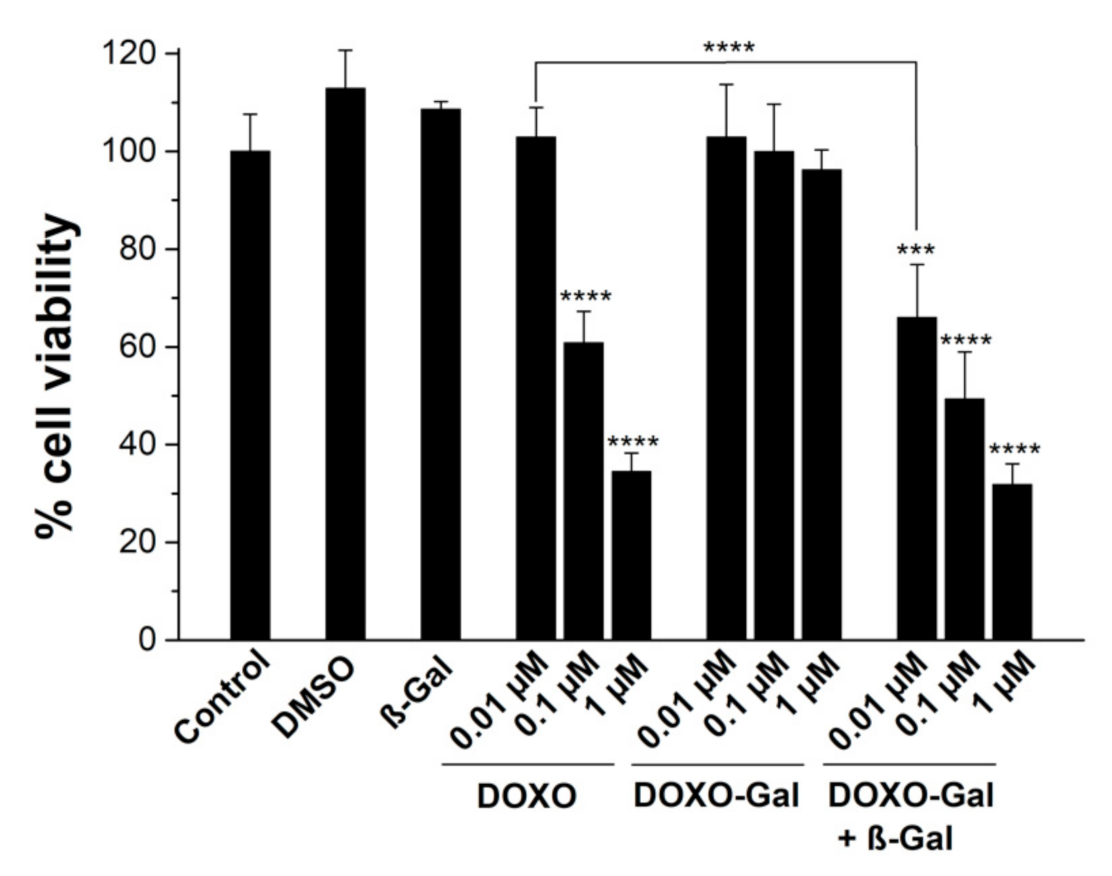
2.9. β-Gal Activity Assay
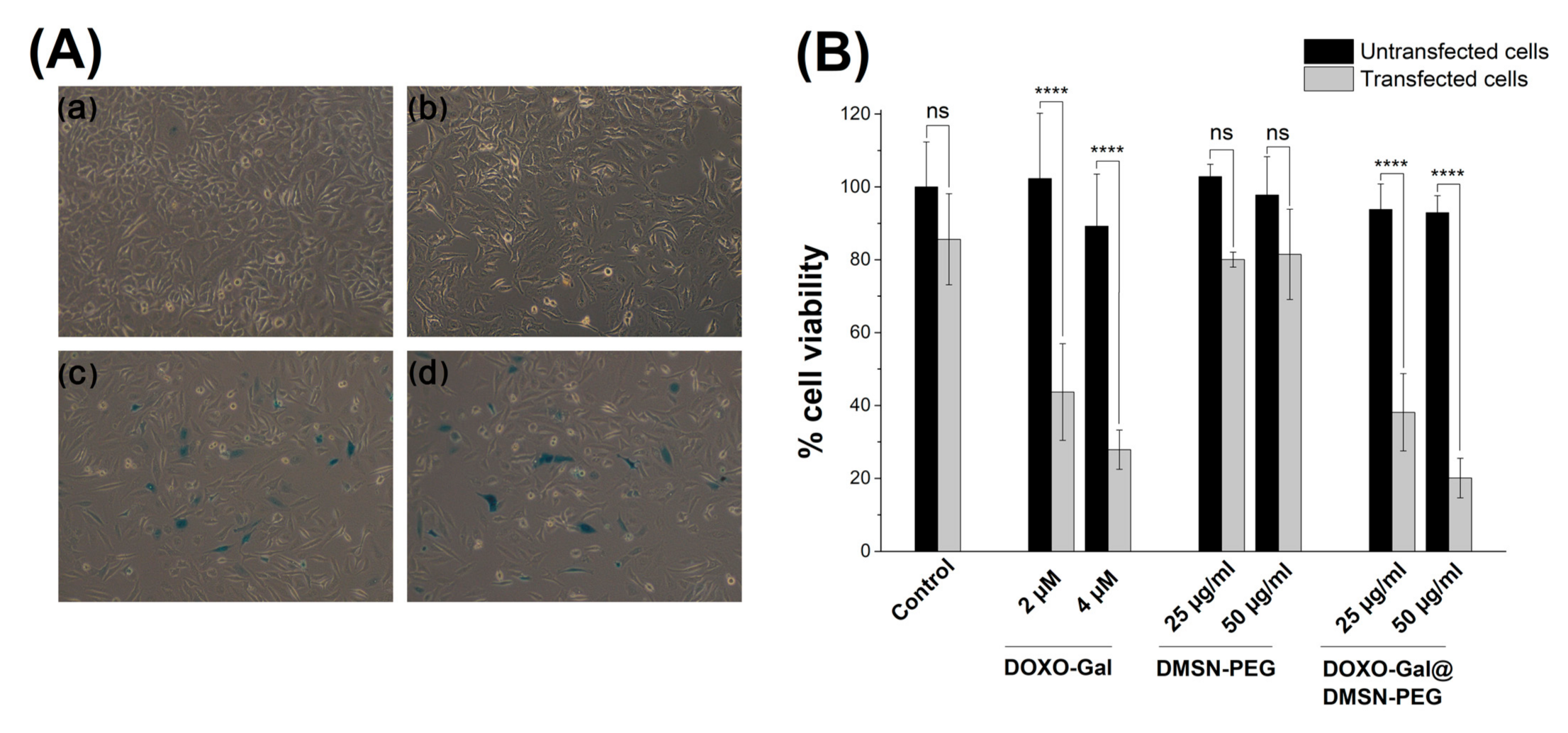
2.10. Transfection Assays
2.11. Treatments with DMSN
2.12. Viability Assays
3. Results and Discussion
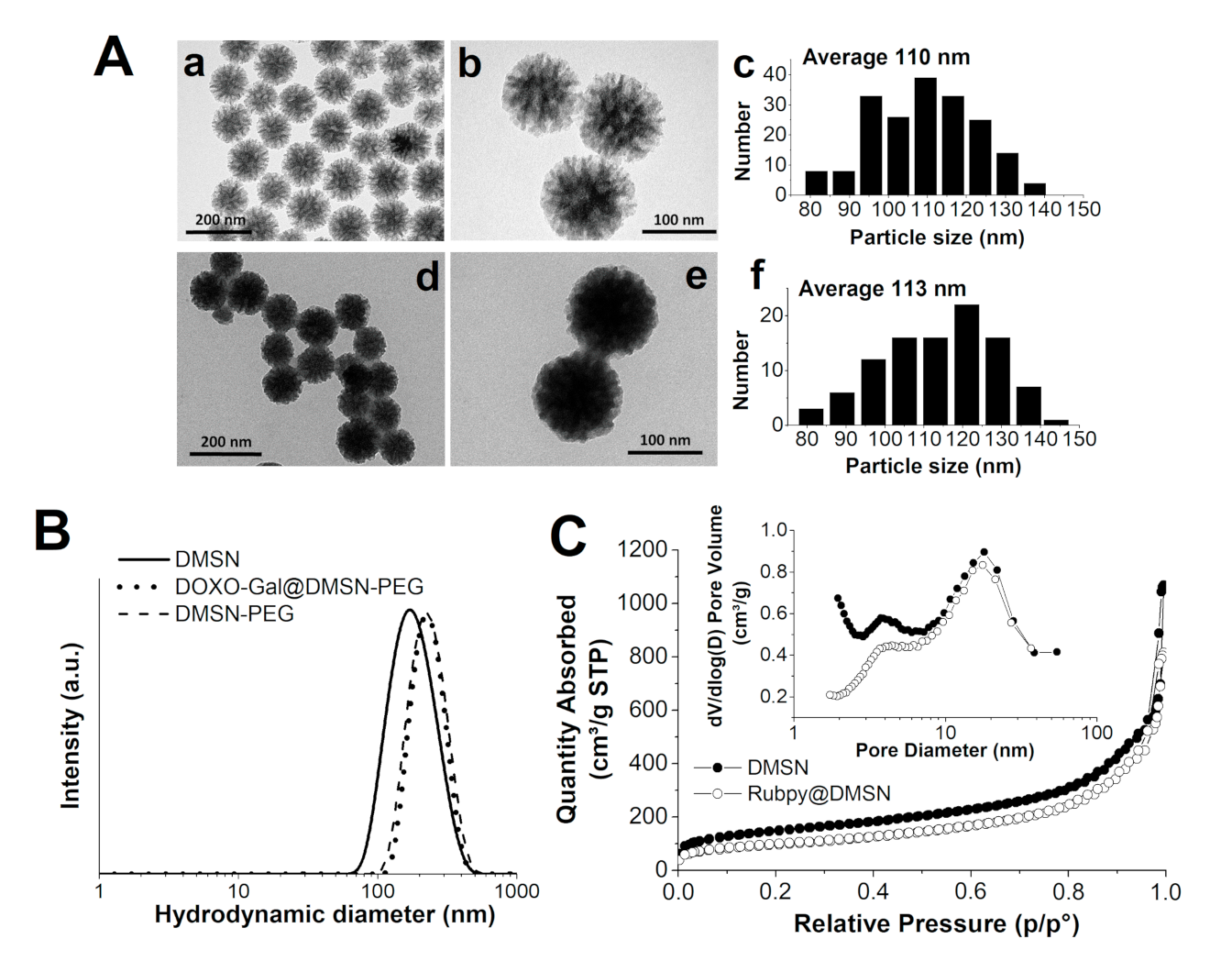
3.1. Synthesis and Characterization
3.2. Cells Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumosulfider Heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef]
- Nguyen, A.; Yoshida, M.; Goodarzi, H.; Tavazoie, S.F. Highly variable cancer subpopulations that exhibit enhanced transcriptome variability and metastatic fitness. Nat. Commun. 2016, 7, 11246. [Google Scholar] [CrossRef] [PubMed]
- Vago, R.; Collico, V.; Zuppone, S.; Prosperi, D.; Colombo, M. Nanoparticle-mediated delivery of suicide genes in cancer therapy. Pharmacol. Res. 2016, 111, 619–641. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kale, V.; Chen, M. Gene-directed enzyme prodrug therapy. AAPS J. 2015, 17, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Yuan, Y.-Y.; Zhang, Y.-M.; Zhao, N.; Zhang, Q.; Meng, F.-X.; Gao, R.-P.; Yu, B.-F.; Zhang, Y.-H.; Guo, R.; et al. HSVtk/GCV system on hepatoma carcinoma cells: Construction of the plasmid PcDNA3.1-PAFP-TK and targeted killing effect. Mol. Med. Rep. 2017, 16, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Trinh, Q.T.; Austin, E.A.; Murray, D.M.; Knick, V.C.; Huber, B.E. Enzyme/prodrug gene therapy: Comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995, 55, 4808–4812. [Google Scholar]
- Chen, C.-S.; Lin, J.T.; Goss, K.A.; He, Y.; Halpert, J.R.; Waxman, D.J. Activation of the anticancer prodrugs cyclophosphamide and ifosfamide: Identification of cytochrome P450 2B enzymes and site-specific mutants with improved enzyme kinetics. Mol. Pharmacol. 2004, 65, 1278–1285. [Google Scholar] [CrossRef]
- Giraud, B.; Hebert, G.; Deroussent, A.; Veal, G.J.; Vassal, G.; Paci, A. Oxazaphosphorines: New therapeutic strategies for an old class of drugs. Expert Opin. Drug Metab. Toxicol. 2010, 6, 919–938. [Google Scholar] [CrossRef]
- Cai, X.-K.; Zhou, J.-L.; Zhou, H.-J.; Zhang, L.; Wu, J.-H.; Lin, J.-S. Killing effect of PNP/MeP-DR suicide gene system driven by an AFP Promoter AF0.3 on AFP-positive hepatoma cells. Ai Zheng 2006, 25, 1334–1339. [Google Scholar] [PubMed]
- Hedley, D.; Ogilvie, L.; Springer, C. Carboxypeptidase G2-based gene-directed enzyme–prodrug therapy: A new weapon in the GDEPT armoury. Nat. Rev. Cancer 2007, 7, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.; Ju, Y.; Yang, Y.; Hua, H.; Chi, J.; Mu, X. Combined antitumor activity of the nitroreductase/CB1954 suicide gene system and γ-rays in hela cells in vitro. Mol. Med. Rep. 2016, 14, 5164–5170. [Google Scholar] [CrossRef] [PubMed]
- Karjoo, Z.; Chen, X.; Hatefi, A. Progress and problems with the use of suicide genes for targeted cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.Y.; Kuthati, Y.; Kankala, R.K.; Kankala, S.; Deng, J.P.; Liu, C.L.; Lee, C.H. Utilization of enzyme-immobilized mesoporous silica nanocontainers (IBN-4) in Prodrug-activated cancer theranostics. Nanomaterials 2015, 5, 2169–2191. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gupta, N.; Shrivastava, A. Silica nanoparticles coencapsulating gadolinium oxide and horseradish peroxidase for imaging and therapeutic applications. Int. J. Nanomed. 2012, 7, 5491. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Li, Y.; Wang, Y.; Ke, W.; Chen, W.; Wang, W.; Ge, Z. Polymer prodrug-based nanoreactors activated by tumor acidity for orchestrated oxidation/chemotherapy. Nano Lett. 2017, 17, 6983–6990. [Google Scholar] [CrossRef]
- Li, J.; Anraku, Y.; Kataoka, K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew. Chem. Int. Ed. 2020, 59, 13526–13530. [Google Scholar] [CrossRef]
- Li, J.; Dirisala, A.; Ge, Z.; Wang, Y.; Yin, W.; Ke, W.; Toh, K.; Xie, J.; Matsumoto, Y.; Anraku, Y.; et al. Therapeutic vesicular nanoreactors with tumor-specific activation and self-destruction for synergistic tumor ablation. Angew. Chem. Int. Ed. 2017, 56, 14025–14030. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Zhou, Q.; Li, X. Recent developments of mesoporous silica nanoparticles in biomedicine. Emergent Mater. 2020, 3, 381–405. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Aznar, E.; Martínez-Máñez, R.; Sancenón, F. New advances in in vivo applications of gated mesoporous silica as drug delivery nanocarriers. Small 2020, 16, 1902242. [Google Scholar] [CrossRef]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous silica-based materials with bactericidal properties. Small 2019, 15, 1900669. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Murugan, B.; Sagadevan, S.; Lett, J.A.; Fatimah, I.; Fatema, K.N.; Oh, W.-C.; Mohammad, F.; Johan, M.R. Role of mesoporous silica nanoparticles for the drug delivery applications. Mater. Res. Express 2020, 7, 102002. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.Y. A Polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef]
- Fontana, F.; Liu, D.; Hirvonen, J.; Santos, H.A. Delivery of therapeutics with nanoparticles: What’s new in cancer immunotherapy? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1421. [Google Scholar] [CrossRef]
- Hao, M.; Chen, B.; Zhao, X.; Zhao, N.; Xu, F.-J. Organic/inorganic nanocomposites for cancer immunotherapy. Mater. Chem. Front. 2020, 4, 2571–2609. [Google Scholar] [CrossRef]
- Pascual, L.; Cerqueira-Coutinho, C.; García-Fernández, A.; de Luis, B.; Bernardes, E.S.; Albernaz, M.S.; Missailidis, S.; Martínez-Máñez, R.; Santos-Oliveira, R.; Orzaez, M.; et al. MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2495–2505. [Google Scholar] [CrossRef]
- Cha, B.G.; Kim, J. Functional Mesoporous silica nanoparticles for bio-imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, 1–22. [Google Scholar] [CrossRef]
- Sancenón, F.; Pascual, L.; Oroval, M.; Aznar, E.; Martínez-Máñez, R. Gated silica mesoporous materials in sensing applications. ChemistryOpen 2015, 4, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Kholafazad Kordasht, H.; Pazhuhi, M.; Pashazadeh-Panahi, P.; Hasanzadeh, M.; Shadjou, N. Multifunctional aptasensors based on mesoporous silica nanoparticles as an efficient platform for bioanalytical applications: Recent advances. Trends Anal. Chem. 2020, 124, 115778. [Google Scholar] [CrossRef]
- Jimenez-Falcao, S.; Parra-Nieto, J.; Pérez-Cuadrado, H.; Martínez-Máñez, R.; Martínez-Ruiz, P.; Villalonga, R. Avidin-gated mesoporous silica nanoparticles for signal amplification in electrochemical biosensor. Electrochem. Commun. 2019, 108, 106556. [Google Scholar] [CrossRef]
- Ribes, À.; Aznar, E.; Santiago-Felipe, S.; Xifre-Perez, E.; Tormo-Mas, M.Á.; Pemán, J.; Marsal, L.F.; Martínez-Máñez, R. Selective and sensitive probe based in oligonucleotide-capped nanoporous alumina for the rapid screening of infection produced by candida albicans. ACS Sens. 2019, 4, 1291–1298. [Google Scholar] [CrossRef]
- Aznar, E.; Reynaldo, V.; Giménez, C.; Sancenón, F.; Marcos, M.D.; Díez, P.; Pingarron, M.; Amorós, P. Glucose-triggered release using enzyme-gated mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 6391–6393. [Google Scholar] [CrossRef]
- Polo, L.; Gómez-Cerezo, N.; Aznar, E.; Vivancos, J.L.; Sancenón, F.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Molecular gates in mesoporous bioactive glasses for the treatment of bone tumors and infection. Acta Biomater. 2017, 50, 114–126. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1573. [Google Scholar] [CrossRef]
- Chen, N.T.; Cheng, S.H.; Souris, J.S.; Chen, C.T.; Mou, C.Y.; Lo, L.W. Theranostic applications of mesoporous silica nanoparticles and their organic/inorganic hybrids. J. Mater. Chem. B 2013, 1, 3128–3135. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiao, X.; Fan, W.; Yang, Z.; Wen, Y.; Chen, X. Controllable synthesis of versatile mesoporous organosilica nanoparticles as precision cancer theranostics. Biomaterials 2020, 256, 120191. [Google Scholar] [CrossRef]
- Giménez, C.; Climent, E.; Aznar, E.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Rurack, K. Towards chemical communication between gated nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 12629–12633. [Google Scholar] [CrossRef] [PubMed]
- Llopis-Lorente, A.; Díez, P.; Sánchez, A.; Marcos, M.D.; Sancenón, F.; Martínez-Ruiz, P.; Villalonga, R.; Martínez-Máñez, R. Interactive models of communication at the nanoscale using nanoparticles that talk to one another. Nat. Commun. 2017, 8, 15511. [Google Scholar] [CrossRef] [PubMed]
- Llopis-Lorente, A.; García-Fernández, A.; Murillo-Cremaes, N.; Hortelão, A.C.; Patiño, T.; Villalonga, R.; Sancenón, F.; Martínez-Máñez, R.; Sánchez, S. Enzyme-powered gated mesoporous silica nanomotors for on-command intracellular payload delivery. ACS Nano 2019, 13, 12171–12183. [Google Scholar] [CrossRef]
- Luis, B.; Llopis-Lorente, A.; Rincón, P.; Gadea, J.; Sancenón, F.; Aznar, E.; Villalonga, R.; Murguía, J.R.; Martínez-Máñez, R. An Interactive model of communication between abiotic nanodevices and microorganisms. Angew. Chem. Int. Ed. 2019, 58, 14986–14990. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: The next generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Lu, J.; Gothard, C.; Yanes, R.; Thomas, C.R.; Olsen, J.-C.; Stoddart, J.F.; Tamanoi, F.; Zink, J.I. Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small 2011, 7, 1816–1826. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Devalapally, H.K.; Navath, R.S.; Yenamandra, V.; Akkinepally, R.R.R.R.; Devarakonda, R.K. β-galactoside prodrugs of doxorubicin for application in antibody directed enzyme prodrug therapy/prodrug monotherapy. Arch. Pharm. Res. 2007, 30, 723–732. [Google Scholar] [CrossRef]
- Ferrari, E.; Lazzari, S.; Marverti, G.; Pignedoli, F.; Spagnolo, F.; Saladini, M. Synthesis, cytotoxic and combined CDDP activity of new stable curcumin derivatives. Bioorg. Med. Chem. 2009, 17, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Xing, J.L.; Pang, J.L.; Jiang, S.H.; Lam, K.F.; Yang, T.Q.; Xue, Q.S.; Zhang, K.; Wu, P. Facile synthesis of size controllable dendritic mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 22655–22665. [Google Scholar] [CrossRef] [PubMed]
- Giménez, C.; De La Torre, C.; Gorbe, M.; Aznar, E.; Sancenón, F.; Murguía, J.R.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P. Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir 2015, 31, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, Q.Q.; Wang, J.; Wang, B.C.; Zhang, S.H.; Yuan, Z. Role of thiol-containing polyethylene glycol (Thiol-PEG) in the modification process of gold nanoparticles (AuNPs): Stabilizer or coagulant? J. Colloid Interface Sci. 2013, 404, 223–229. [Google Scholar] [CrossRef]
- Park, S.S.; Jung, M.H.; Lee, Y.S.; Bae, J.H.; Kim, S.H.; Ha, C.S. Functionalised mesoporous silica nanoparticles with excellent cytotoxicity against various cancer cells for PH-responsive and controlled drug delivery. Mater. Des. 2019, 184, 108187. [Google Scholar] [CrossRef]
- Leenders, R.G.G.; Damen, E.W.P.; Bijsterveld, E.J.A.; Scheeren, H.W.; Houba, P.H.J.; van der Meulen-Muileman, I.H.; Boven, E.; Haisma, H.J. Novel anthracycline-spacer-β-glucuronide, -β-glucoside, and -β-galactoside prodrugs for application in selective chemotherapy. Bioorg. Med. Chem. 1999, 7, 1597–1610. [Google Scholar] [CrossRef]
- Houba, P.H.J.; Boven, E.; van der Meulen-Muileman, I.H.; Leenders, R.G.G.; Scheeren, J.W.; Pinedo, H.M.; Haisma, H.J. A novel doxorubicin-glucuronide Prodrug DOX-GA3 for tumour-selective chemotherapy: Distribution and efficacy in experimental human ovarian cancer. Br. J. Cancer 2001, 84, 550–557. [Google Scholar] [CrossRef]
- González-Gualda, E.; Pàez-Ribes, M.; Lozano-Torres, B.; Macias, D.; Wilson, J.R.; González-López, C.; Ou, H.; Mirón-Barroso, S.; Zhang, Z.; Lérida-Viso, A.; et al. Galacto-conjugation of navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 2020, 19, e13142. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Galiana, I.; Rovira, M.; Garrido, E.; Chaib, S.; Bernardos, A.; Muñoz-Espín, D.; Serrano, M.; Martínez-Máñez, R.; Sancenón, F. An off-on two-photon fluorescent probe for tracking cell senescence in vivo. J. Am. Chem. Soc. 2017, 139, 8808–8811. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Blandez, J.F.; Galiana, I.; Lopez-Dominguez, J.A.; Rovira, M.; Paez-Ribes, M.; González-Gualda, E.; Muñoz-Espín, D.; Serrano, M.; Sancenón, F.; et al. A Two-photon probe based on naphthalimide-styrene fluorophore for the in vivo tracking of cellular senescence. Anal. Chem. 2021, 93, 3052–3060. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Blandez, J.F.; Sancenón, F.; Martínez-Máñez, R. Chromo-fluorogenic probes for β-galactosidase detection. Anal. Bioanal. Chem. 2021, 413, 2361–2388. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Torres, B.; Blandez, J.F.; Galiana, I.; García-Fernández, A.; Alfonso, M.; Marcos, M.D.; Orzáez, M.; Sancenón, F.; Martínez-Máñez, R. Real-time in vivo detection of cellular senescence through the controlled release of the NIR fluorescent dye nile blue. Angew. Chem. Int. Ed. 2020, 59, 15152–15156. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Mondragón, L.; Bernardos, A.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Soto, J.; Costero, A.; Manguan-García, C.; Perona, R.; et al. Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 10556–10560. [Google Scholar] [CrossRef] [PubMed]
- Galiana, I.; Lozano-Torres, B.; Sancho, M.; Alfonso, M.; Bernardos, A.; Bisbal, V.; Serrano, M.; Martínez-Máñez, R.; Orzáez, M. Preclinical antitumor efficacy of senescence-inducing chemotherapy combined with a nanosenolytic. J. Control. Release 2020, 323, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, J.; Wei, M.; Wen, W.; Gao, J.; Zhang, Z.; Qin, W. Selective and augmented β-glucuronidase expression combined with DOX-GA3 application elicits the potent suppression of prostate cancer. Oncol. Rep. 2016, 35, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, S.; Giménez, C.; Aznar, E.; Martínez-Máñez, R.; Sancenón, F.; Licchelli, M. Highly selective and sensitive detection of glutathione using mesoporous silica nanoparticles capped with disulfide-containing oligo (ethylene glycol) chains. Org. Biomol. Chem. 2015, 13, 1017–1021. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Narra, S.; Nair, R.R.; Saini, D.K.; Kondaiah, P.; Govindaraju, T. Stimuli-responsive colorimetric and NIR fluorescence combination probe for selective reporting of cellular hydrogen peroxide. Chem. Sci. 2016, 7, 2832–2841. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, L.; Chen, B.Q.; Zhang, M.; Rodrigues, J.; Sheng, R.; Bao, G.M. A selective cascade reaction-based probe for colorimetric and ratiometric fluorescence detection of benzoyl peroxide in food and living cells. J. Mater. Chem. B 2019, 7, 5775–5781. [Google Scholar] [CrossRef]
- Liu, Z.; Ru, J.; Sun, S.; Teng, Z.; Dong, H.; Song, P.; Yang, Y.; Guo, H. Uniform dendrimer-like mesoporous silica nanoparticles as a nano-adjuvant for foot-and-mouth disease virus-like particle vaccine. J. Mater. Chem. B 2019, 7, 3446–3454. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Deng, W.; Engelke, H.; Helma, J.; Leonhardt, H.; Bein, T. Intracellular chromobody delivery by mesoporous silica nanoparticles for antigen targeting and visualization in real time. Sci. Rep. 2016, 6, 25019. [Google Scholar] [CrossRef]
- Denning, C.; Pitts, J.D. Bystander effects of different enzyme-prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum. Gene Ther. 1997, 8, 1825–1835. [Google Scholar] [CrossRef]
- Glaser, T.; Castro, M.G.; Löwenstein, P.R.; Weller, M. Death receptor-independent cytochrome c release and caspase activation mediate thymidine kinase plus ganciclovir-mediated cytotoxicity in LN-18 and LN-229 human malignant glioma cells. Gene Ther. 2001, 8, 469–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robe, P.A.; Nguyen-Khac, M.; Jolois, O.; Rogister, B.; Merville, M.-P.; Bours, V. Dexamethasone inhibits the HSV-Tk/ganciclovir bystander effect in malignant glioma cells. BMC Cancer 2005, 5, 32. [Google Scholar] [CrossRef]
- Was, H.; Barszcz, K.; Czarnecka, J.; Kowalczyk, A.; Bernas, T.; Uzarowska, E.; Koza, P.; Klejman, A.; Piwocka, K.; Kaminska, B.; et al. Bafilomycin A1 triggers proliferative potential of senescent cancer cells in vitro and in NOD/SCID mice. Oncotarget 2017, 8, 9303–9322. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.; Sahlgren, C.; Lindén, M. Cancer-cell targeting and cell-specific delivery by mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 2707. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Colilla, M.; Vallet-Regí, M. Smart mesoporous nanomaterials for antitumor therapy. Nanomaterials 2015, 5, 1906–1937. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Cai, X.; Ji, J.; He, S.; Zhai, G. Multifunctional mesoporous silica nanocarriers for stimuli-responsive target delivery of anticancer drugs. RSC Adv. 2016, 6, 92073–92091. [Google Scholar] [CrossRef]
- Freitas, L.B.d.O.; Corgosinho, L.d.M.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; de Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candela-Noguera, V.; Vivo-Llorca, G.; Díaz de Greñu, B.; Alfonso, M.; Aznar, E.; Orzáez, M.; Marcos, M.D.; Sancenón, F.; Martínez-Máñez, R. Gene-Directed Enzyme Prodrug Therapy by Dendrimer-Like Mesoporous Silica Nanoparticles against Tumor Cells. Nanomaterials 2021, 11, 1298. https://doi.org/10.3390/nano11051298
Candela-Noguera V, Vivo-Llorca G, Díaz de Greñu B, Alfonso M, Aznar E, Orzáez M, Marcos MD, Sancenón F, Martínez-Máñez R. Gene-Directed Enzyme Prodrug Therapy by Dendrimer-Like Mesoporous Silica Nanoparticles against Tumor Cells. Nanomaterials. 2021; 11(5):1298. https://doi.org/10.3390/nano11051298
Chicago/Turabian StyleCandela-Noguera, Vicente, Gema Vivo-Llorca, Borja Díaz de Greñu, María Alfonso, Elena Aznar, Mar Orzáez, María Dolores Marcos, Félix Sancenón, and Ramón Martínez-Máñez. 2021. "Gene-Directed Enzyme Prodrug Therapy by Dendrimer-Like Mesoporous Silica Nanoparticles against Tumor Cells" Nanomaterials 11, no. 5: 1298. https://doi.org/10.3390/nano11051298
APA StyleCandela-Noguera, V., Vivo-Llorca, G., Díaz de Greñu, B., Alfonso, M., Aznar, E., Orzáez, M., Marcos, M. D., Sancenón, F., & Martínez-Máñez, R. (2021). Gene-Directed Enzyme Prodrug Therapy by Dendrimer-Like Mesoporous Silica Nanoparticles against Tumor Cells. Nanomaterials, 11(5), 1298. https://doi.org/10.3390/nano11051298





