Stable Coloured Micrometric Films from Highly Concentrated Nano-Silver Sols: The Role of the Stabilizing Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Diluted and Concentrated Ag Nanoparticles (NPs)
2.2. Ag NPs Physico-Chemical Characterizations
3. Results and Discussion
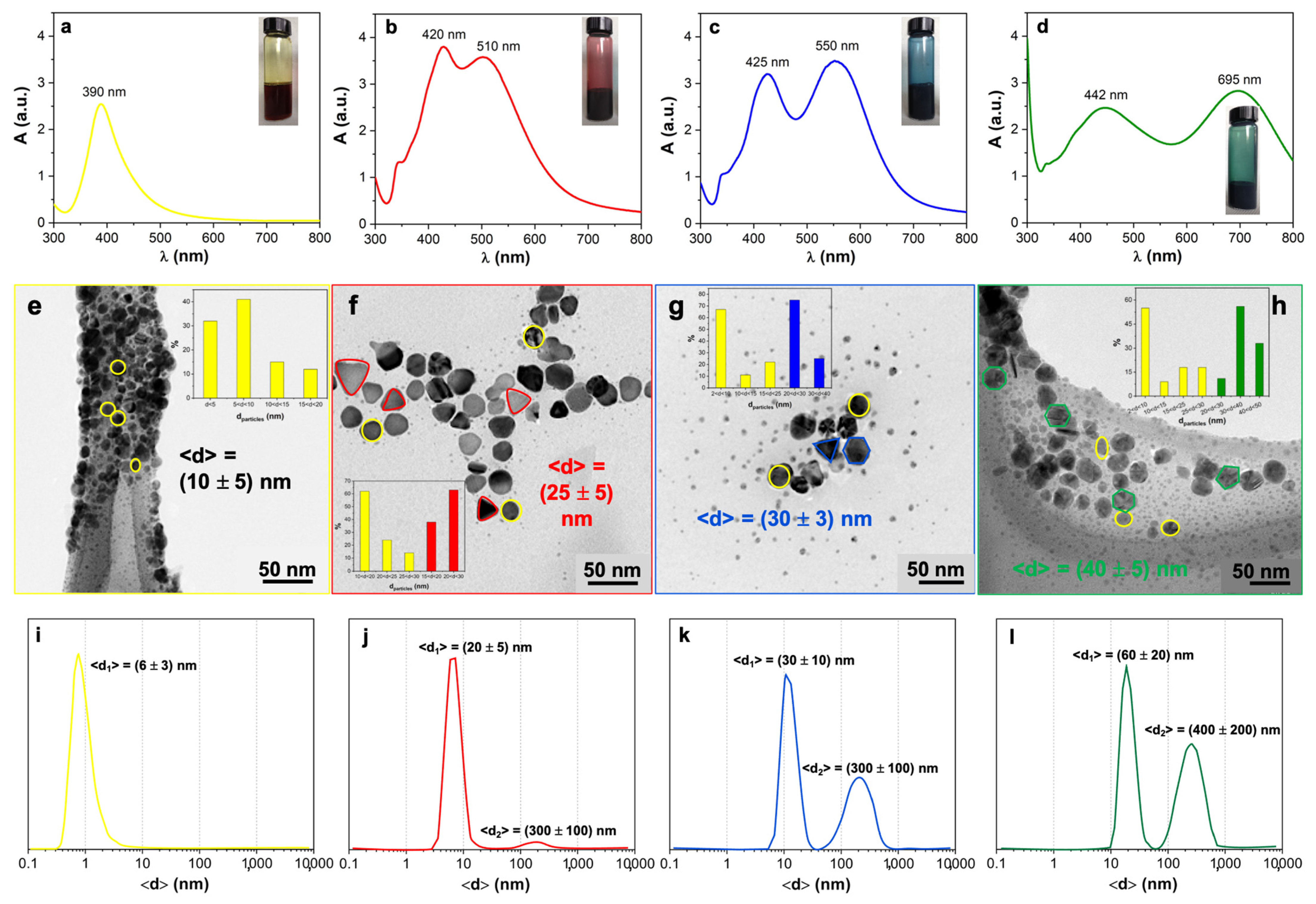
3.1. Diluted and Concentrated Ag Sols
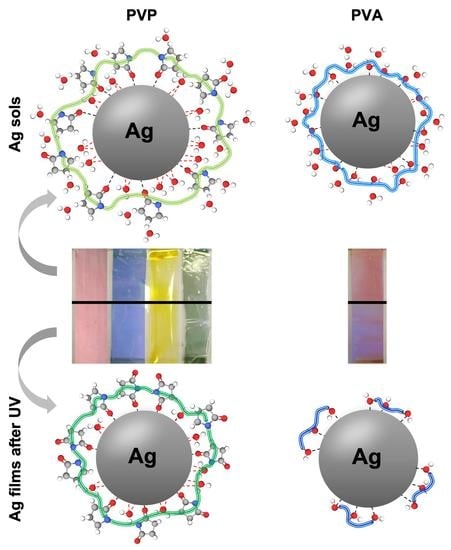
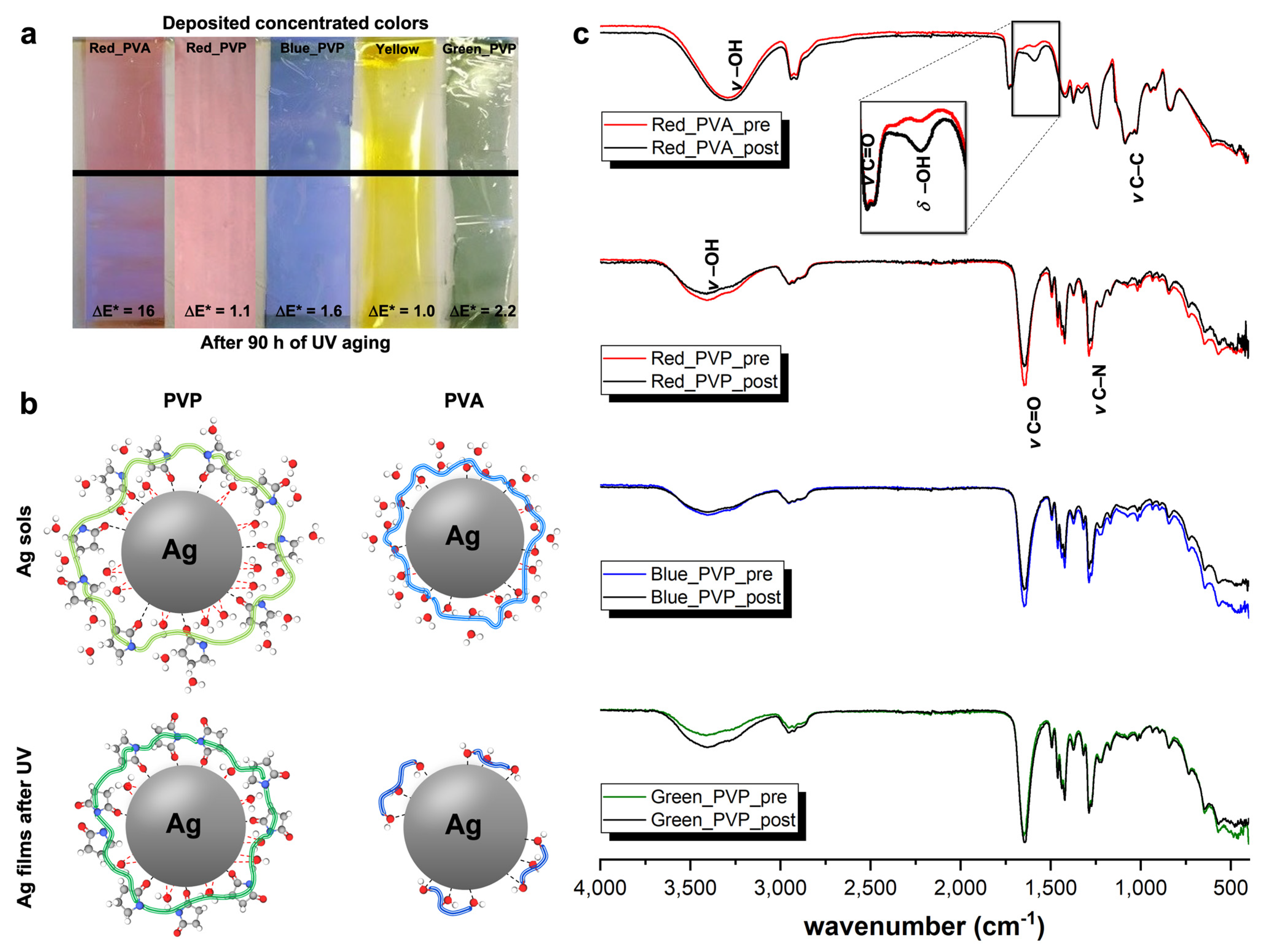
3.2. Coloured Ag Micrometric Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Sun, M.; Lan, B.; Lin, T.; Cheng, G.; Ye, F.; Yu, L.; Cheng, X.; Zheng, X. Controlled synthesis of nanostructured manganese oxide: Crystalline evolution and catalytic activities. Cryst. Eng. Comm. 2013, 15, 7010. [Google Scholar] [CrossRef]
- Shahzad, A.; Kim, W.S.; Yu, T. Synthesis, stabilization, growth behavior, and catalytic activity of highly concentrated silver nanoparticles using a multifunctional polymer in an aqueous-phase. RSC Adv. 2015, 5, 28652–28661. [Google Scholar] [CrossRef]
- Ajitha, B.; Divya, A.; Harish, G.; Sreedhara Reddy, P. The influence of silver precursor concentration on size of silver nanoparticles grown by soft chemical route. Res. J. Phys. Sci. 2013, 1, 11–14. [Google Scholar]
- Park, S.Y.; Ryu, S.; Kwak, S. Antibacterial Metal-Fiber Hybrid with Covalent Assembly of Silver and Palladium Nanoparticles on Cellulose Fibers. Environ. Chem. 2011, 1, 183–186. [Google Scholar]
- Kvítek, O.; Siegel, J.; Hnatowicz, V.; Švorčík, V. Noble Metal Nanostructures Influence of Structure and Environment on Their Optical Properties. J. Nanomater. 2013, 2013, 743684. [Google Scholar] [CrossRef]
- Noritomi, H.; Miyagawa, S.; Igari, N.; Saito, H.; Kato, S. Application of Reverse Micelles of Alkyl Glucosides to Synthesis of Silver Nanoparticles. Adv. Nanopart. 2013, 2013, 344–349. [Google Scholar] [CrossRef][Green Version]
- Gakiya-Teruya, M.; Palomino-Marcelo, L.; Rodriguez-Reyes, J. Synthesis of Highly Concentrated Suspensions of Silver Nanoparticles by Two Versions of the Chemical Reduction Method. Methods Protoc. 2018, 2, 3. [Google Scholar] [CrossRef]
- Anderson, R.; Buscall, R.; Eldridge, R.; Mulvaney, P.; Scales, P.J. Ostwald ripening of comb polymer stabilised Ag salt nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 58–64. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Anderson, R.; Buscall, R.; Eldridge, R.; Mulvaney, P.; Scales, P. Concentrated synthesis of metal nanoparticles in water. RSC Adv. 2014, 4, 31914–31925. [Google Scholar] [CrossRef]
- Patil, R.S.; Kokate, M.R.; Jambhale, C.L.; Pawar, S.M.; Han, S.H.; Kolekar, S.S. One-pot synthesis of PVA-capped silver nanoparticles their characterization and biomedical application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 015013. [Google Scholar] [CrossRef]
- Kyrychenko, A.; Pasko, D.A.; Kalugin, O.N. Poly (vinyl alcohol) as a water protecting agent for silver nanoparticles: The role of polymer size and structure. Phys. Chem. Chem. Phys. 2017, 19, 8742–8756. [Google Scholar] [CrossRef]
- Kyrychenko, A.; Korsun, O.M.; Gubin, I.I.; Kovalenko, S.M.; Kalugin, O.N. Atomistic simulations of coating of silver nanoparticles with poly (vinylpyrrolidone) oligomers: Effect of oligomer chain length. J. Phys. Chem. C 2015, 119, 7888–7899. [Google Scholar] [CrossRef]
- Ledwith, D.M.; Whelan, A.M.; Kelly, J.M. A rapid, straight-forward method for controlling the morphology of stable silver nanoparticles. J. Mater. Chem. 2007, 17, 2459–2464. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Ghader, S. Experimental study on effect of different parameters on size and shape of triangular silver nanoparticles prepared by a simple and rapid method in aqueous solution. Arab. J. Chem. 2009, 2, 47–53. [Google Scholar] [CrossRef]
- Porel, S.; Ramakrishna, D.; Hariprasad, E.; Dutta Gupta, A.; Radhakrishnan, T.P. Polymer thin film with in situ synthesized silver nanoparticles as a potent reusable bactericide. Curr. Sci. 2011, 101, 927–934. [Google Scholar]
- Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J.; Ratoi, M.; Spikes, H.A. The effect of emulsifier concentration on the lubricating properties of oil-in-water emulsions. Tribol. Lett. 2006, 22, 53–65. [Google Scholar] [CrossRef]
- Olietti, A.; Pargoletti, E.; Diona, A.; Cappelletti, G. A novel optimized mold release oil-in-water emulsion for polyurethane foams production. J. Mol. Liq. 2018, 261, 199–207. [Google Scholar] [CrossRef]
- Lotierzo, A.; Pifferi, V.; Ardizzone, S.; Pasqualin, P.; Cappelletti, G. Insight into the role of amines in Metal Working Fluids. Corros. Sci. 2016, 110, 192–199. [Google Scholar] [CrossRef]
- Sabatini, V.; Pargoletti, E.; Longoni, M.; Farina, H.; Ortenzi, M.A.; Cappelletti, G. Stearyl methacrylate co-polymers: Towards new polymer coatings for mortars protection. Appl. Surf. Sci. 2019, 488, 213–220. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 coatings for Cultural Heritage. Prog. Org. Coatings 2012, 74, 186–191. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.; Yuan, H.; Zhou, Z.; Xiao, D. Preparation of Silver Nanoparticle and Its Application to the Determination of ct-DNA. Sensors 2007, 7, 708–718. [Google Scholar] [CrossRef]
- Pino, F.; Fermo, P.; La Russa, M.; Ruffolo, S.; Comite, V.; Baghdachi, J.; Pecchioni, E.; Fratini, F.; Cappelletti, G. Advanced mortar coatings for cultural heritage protection. Durability towards prolonged UV and outdoor exposure. Environ. Sci. Pollut. Res. 2017, 24, 12608–12617. [Google Scholar] [CrossRef] [PubMed]
- Pargoletti, E.; Motta, L.; Comite, V.; Fermo, P.; Cappelletti, G. The hydrophobicity modulation of glass and marble materials by different Si-based coatings. Prog. Org. Coatings 2019, 136, 105260. [Google Scholar] [CrossRef]
- Louie, S.M.; Gorham, J.M.; Tan, J.; Hackley, V.A. Ultraviolet photo-oxidation of polyvinylpyrrolidone (PVP) coatings on gold nanoparticles. Environ. Sci. Nano 2017, 4, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Wypych, G. Handbook of Material Weathering, 6th ed.; ChemTec Publishing: Toronto, ON, Canada, 2018. [Google Scholar]
- Sharma, A.; Jain, C.P. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res. Pharm. Sci. 2010, 5, 49–56. [Google Scholar]
- Saroj, A.L.; Singh, R.K.; Chandra, S. Studies on polymer electrolyte poly (vinyl) pyrrolidone (PVP) complexed with ionic liquid: Effect of complexation on thermal stability, conductivity and relaxation behaviour. Mater. Sci. Eng. B 2013, 178, 231–238. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Huglin, M.R. Hydrogels in medicine and pharmacy Edited by N. A.; Peppas, CRC Press Inc., Boca Raton, Florida, 1986 (Vol. l), 1987 (Vols 2 and 3). Vol. 1 Fundamentals, pp. vii + 180, £72.00, ISBN 0-8493-5546-X.; Vol. 2 Polymers, pp. vii + 171, £72.00, ISBN 0-8493-5547-8. Br. Polym. J. 1989, 21, 184. [Google Scholar] [CrossRef]
| Sample | H2O (mL) | PVP (%wt) | PVA | TSC (mM) | AgNO3 (mM) | NaBH4 (mM) | Seeds (μM) | AA (mM) | Ag0 (μM) | |
|---|---|---|---|---|---|---|---|---|---|---|
| One-step | Yellow | 18.7 | − | − | 2.5 | 2.5 | 3.0 | − | − | 63 |
| Two-step | Red_PVP | 15.0 | 20.0 | − | 6.1 | 4.1 | − | 6.83 | 4.3 | 190 |
| Red_PVA | 15.0 | − | 10.0 | 6.9 | 4.1 | − | 6.83 | 4.3 | 190 | |
| Blue_PVP | 15.0 | 10.0 | − | 6.4 | 5.7 | − | 3.60 | 4.3 | 330 | |
| Blue_PVA | 15.0 | − | 10.0 | 6.5 | 5.7 | − | 3.60 | 4.3 | 330 | |
| Green_PVP | 15.0 | 20.0 | − | 6.7 | 4.9 | − | 1.84 | 4.5 | 225 | |
| Green_PVA | 15.0 | − | 10.0 | 6.7 | 4.9 | − | 1.84 | 4.5 | 225 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pargoletti, E.; Ortenzi, M.A.; Cappelletti, G. Stable Coloured Micrometric Films from Highly Concentrated Nano-Silver Sols: The Role of the Stabilizing Agents. Nanomaterials 2021, 11, 980. https://doi.org/10.3390/nano11040980
Pargoletti E, Ortenzi MA, Cappelletti G. Stable Coloured Micrometric Films from Highly Concentrated Nano-Silver Sols: The Role of the Stabilizing Agents. Nanomaterials. 2021; 11(4):980. https://doi.org/10.3390/nano11040980
Chicago/Turabian StylePargoletti, Eleonora, Marco Aldo Ortenzi, and Giuseppe Cappelletti. 2021. "Stable Coloured Micrometric Films from Highly Concentrated Nano-Silver Sols: The Role of the Stabilizing Agents" Nanomaterials 11, no. 4: 980. https://doi.org/10.3390/nano11040980
APA StylePargoletti, E., Ortenzi, M. A., & Cappelletti, G. (2021). Stable Coloured Micrometric Films from Highly Concentrated Nano-Silver Sols: The Role of the Stabilizing Agents. Nanomaterials, 11(4), 980. https://doi.org/10.3390/nano11040980