Li2(BH4)(NH2) Nanoconfined in SBA-15 as Solid-State Electrolyte for Lithium Batteries
Abstract
1. Introduction
2. Materials and Methods
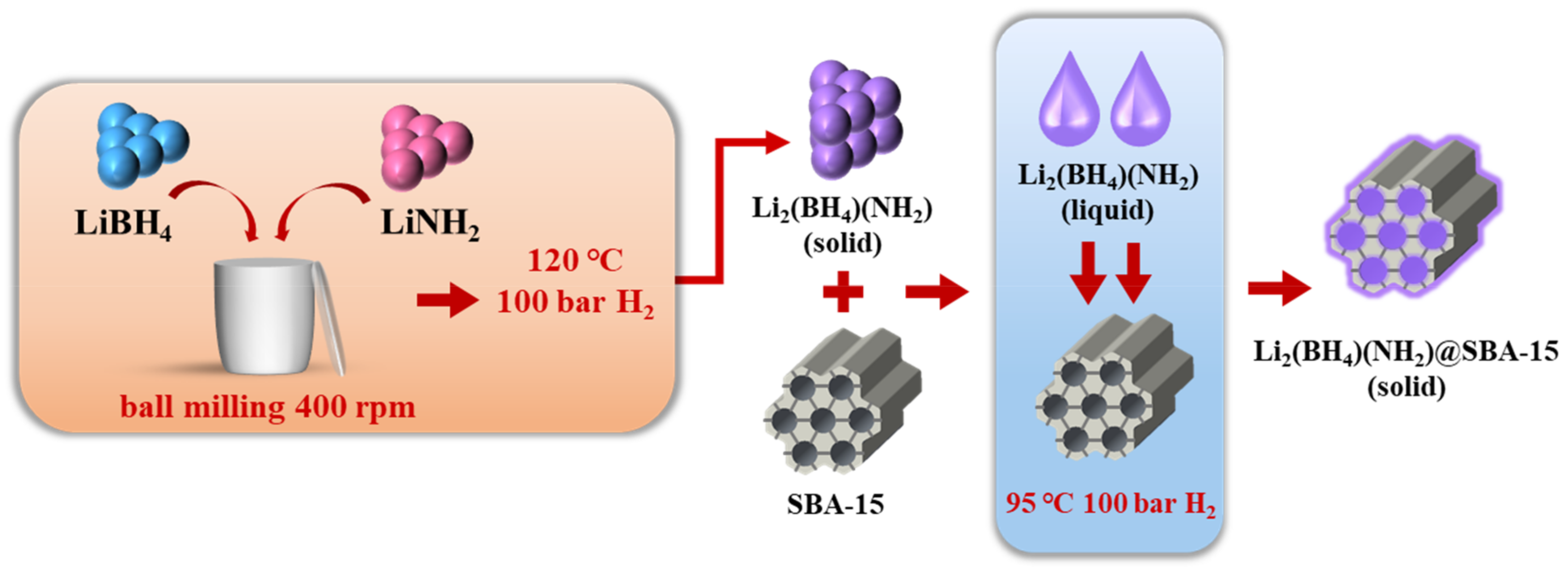
2.1. Materials Synthesis
2.2. Structural Characterization
2.3. Cell Assemblies and Electrochemical Measurements
3. Results and Discussion
3.1. Preparation of Li2(BH4)(NH2)@SBA-15
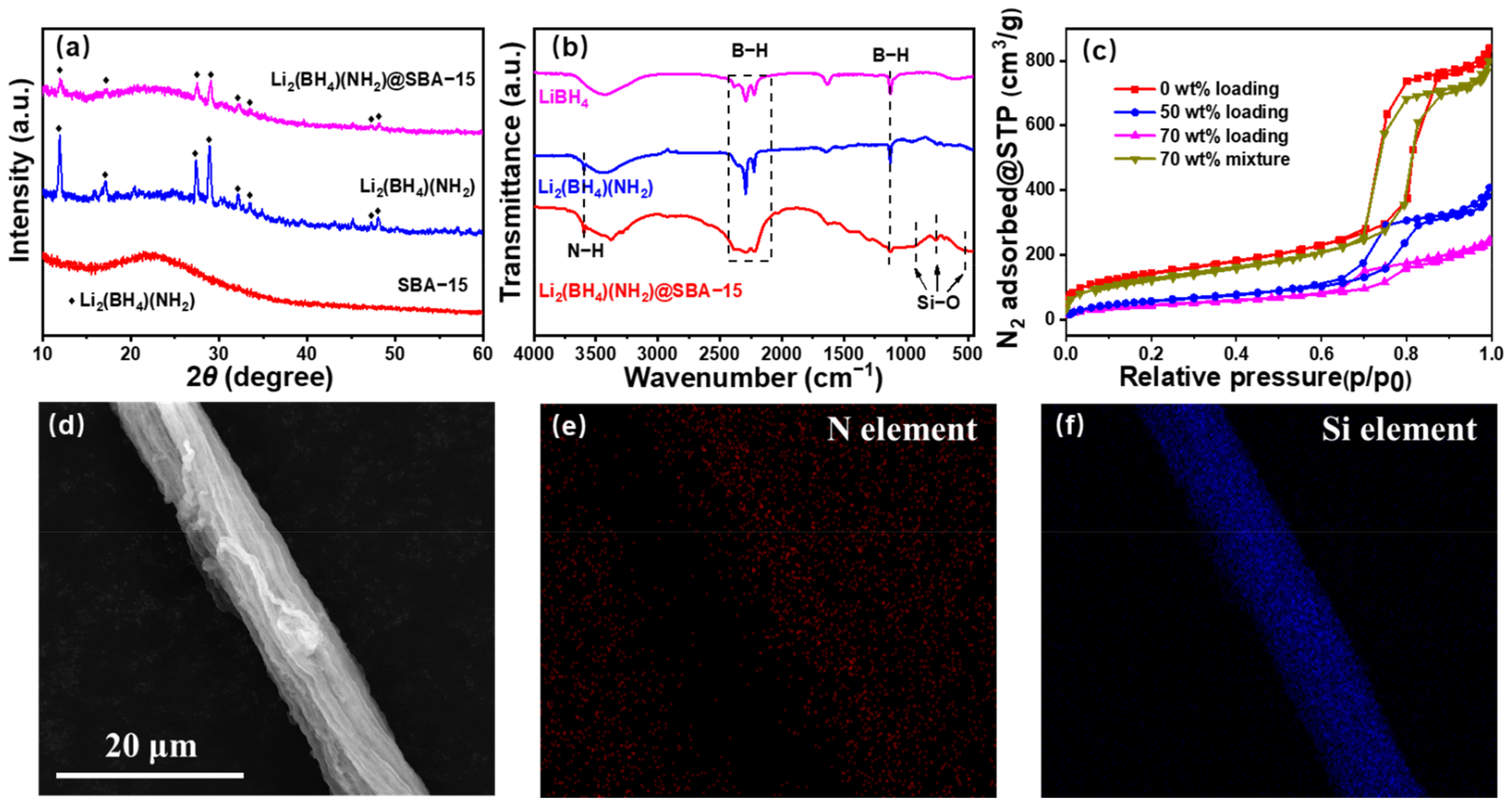
3.2. Structures of Li2(BH4)(NH2)@SBA-15
3.3. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, S.C.N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nat. Cell Biol. 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W.-H. Development of Electrolytes towards Achieving Safe and High-Performance Energy-Storage Devices: A Review. ChemElectroChem 2014, 2, 22–36. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Sun, Y.-K. Promising All-Solid-State Batteries for Future Electric Vehicles. ACS Energy Lett. 2020, 5, 3221–3223. [Google Scholar] [CrossRef]
- Khurana, R.; Schaefer, J.L.; Archer, L.A.; Coates, G.W. Suppression of Lithium Dendrite Growth Using Cross-Linked Polyethylene/Poly(ethylene oxide) Electrolytes: A New Approach for Practical Lithium-Metal Polymer Batteries. J. Am. Chem. Soc. 2014, 136, 7395–7402. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Mangal, R.; Agrawal, A.; Archer, L.A. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 2015, 6, 10101. [Google Scholar] [CrossRef] [PubMed]
- Martinez, U.; Babu, S.K.; Holby, E.F.; Zelenay, P. Durability challenges and perspective in the development of PGM-free electrocatalysts for the oxygen reduction reaction. Curr. Opin. Electrochem. 2018, 9, 224–232. [Google Scholar] [CrossRef]
- Inaguma, Y.; Itoh, M. Influences of carrier concentration and site percolation on lithium ion conductivity in perovskite-type oxides. Solid State Ion. 1996, 86-88, 257–260. [Google Scholar] [CrossRef]
- Amores, M.; El-Shinawi, H.; McClelland, I.; Yeandel, S.R.; Baker, P.J.; Smith, R.I.; Playford, H.Y.; Goddard, P.; Corr, S.A.; Cussen, E.J. Li1.5La1.5MO6 (M = W6+, Te6+) as a new series of lithium-rich double perovskites for all-solid-state lithium-ion batteries. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Tolganbek, N.; Yerkinbekova, Y.; Khairullin, A.; Bakenov, Z.; Kanamura, K.; Mentbayeva, A. Enhancing purity and ionic conductivity of NASICON-typed Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Bruce, P.G.; West, A.R. The A-C Conductivity of Polycrystalline LISICON, Li2 + 2x Zn1 − x GeO4, and a Model for Intergranular Constriction Resistances. J. Electrochem. Soc. 1983, 130, 662–669. [Google Scholar] [CrossRef]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef]
- Kotobuki, M.; Kanamura, K.; Sato, Y.; Yoshida, T. Fabrication of all-solid-state lithium battery with lithium metal anode using Al2O3-added Li7La3Zr2O12 solid electrolyte. Lancet 2011, 196, 7750–7754. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, S.; Zhang, X.; Liu, Y.; Hu, J.; Huang, Z.; Gao, M.; Pan, H. Amorphous Dual-Layer Coating: Enabling High Li-Ion Conductivity of Non-Sintered Garnet-Type Solid Electrolyte. Adv. Funct. Mater. 2021, 2009692. [Google Scholar] [CrossRef]
- Mo, Y.; Ong, S.P.; Ceder, G. First Principles Study of the Li10GeP2S12 Lithium Super Ionic Conductor Material. Chem. Mater. 2012, 24, 15–17. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Minami, T.; Tatsumisago, M. Formation of superionic crystals from mechanically milled Li2S–P2S5 glasses. Electrochem. Commun. 2003, 5, 111–114. [Google Scholar] [CrossRef]
- Muramatsu, H.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Structural change of Li2S–P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ion. 2011, 182, 116–119. [Google Scholar] [CrossRef]
- Kato, A.; Yamamoto, M.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Mechanical Properties of Li2S–P2S5 Glasses with Lithium Halides and Application in All-Solid-State Batteries. ACS Appl. Energy Mater. 2018, 1, 1002–1007. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Mackanic, D.; Burke, W.; Bao, Z.; Cui, Y. Status, promises, and challenges of nanocomposite solid-state electrolytes for safe and high performance lithium batteries. Mater. Today Nano 2018, 4, 1–16. [Google Scholar] [CrossRef]
- Lim, H.-D.; Park, J.-H.; Shin, H.-J.; Jeong, J.; Kim, J.T.; Nam, K.-W.; Jung, H.-G.; Chung, K.Y. A review of challenges and issues concerning interfaces for all-solid-state batteries. Energy Storage Mater. 2019, 25, 224–250. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Meng, X.; Ju, X. Improvement of the LiBH4 hydrogen desorption by confinement in modified carbon nanotubes. J. Alloys Compd. 2015, 645, S112–S116. [Google Scholar] [CrossRef]
- Chen, K.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Zhang, Y.; Zhu, M. Converting H+ from coordinated water into H− enables super facile synthesis of LiBH4. Green Chem. 2019, 21, 4380–4387. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, Y.; Gao, M.; Ouyang, L.; Liu, J.; Wang, H.; Zhu, M.; Pan, H. A mechanical-force-driven physical vapour deposition approach to fabricating complex hydride nanostructures. Nat. Commun. 2014, 5, 3519. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Orimo, S.-I. Lithium Fast-Ionic Conduction in Complex Hydrides: Review and Prospects. Adv. Energy Mater. 2011, 1, 161–172. [Google Scholar] [CrossRef]
- Campanella, D.; Belanger, D.; Paolella, A. Beyond garnets, phosphates and phosphosulfides solid electrolytes: New ceramic perspectives for all solid lithium metal batteries. J. Power Sources 2021, 482, 228949. [Google Scholar] [CrossRef]
- Matsuo, M.; Nakamori, Y.; Orimo, S.-I.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Motoaki, M. Complex hydrides with (BH4)− and (NH2)− anions as new lithium fast-ion conductors. J. Am. Chem. Soc. 2009, 131, 16389–16391. [Google Scholar]
- Yan, Y.; Kühnel, R.-S.; Remhof, A.; Duchêne, L.; Reyes, E.C.; Rentsch, D.; Łodziana, Z.; Battaglia, C. A Lithium Amide-Borohydride Solid-State Electrolyte with Lithium-Ion Conductivities Comparable to Liquid Electrolytes. Adv. Energy Mater. 2017, 7, 7. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Song, T.; Miyaoka, H.; Shinzato, K.; Miyaoka, H.; Ichikawa, T.; Shi, S.; Zhang, X.; Isobe, S.; et al. Ammonia, a Switch for Controlling High Ionic Conductivity in Lithium Borohydride Ammoniates. Joule 2018, 2, 1522–1533. [Google Scholar] [CrossRef]
- Zhu, M.; Pang, Y.; Lu, F.; Shi, X.; Yang, J.; Zheng, S. In Situ Formed Li–B–H Complex with High Li-Ion Conductivity as a Potential Solid Electrolyte for Li Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14136–14141. [Google Scholar] [CrossRef]
- Gulino, V.; Brighi, M.; Murgia, F.; Ngene, P.; de Jongh, P.; Černý, R.; Baricco, M. Room-Temperature Solid-State Lithium-Ion Battery Using a LiBH4–MgO Composite Electrolyte. ACS Appl. Energy Mater. 2021, 4, 1228–1236. [Google Scholar] [CrossRef]
- Blanchard, D.; Nale, A.; Sveinbjörnsson, D.; Eggenhuisen, T.M.; Verkuijlen, M.H.W.; Suwarno; Vegge, T.; Kentgens, A.P.M.; de Jongh, P.E. Nanoconfined LiBH4 as a Fast Lithium Ion Conductor. Adv. Funct. Mater. 2015, 25, 184–192. [Google Scholar] [CrossRef]
- De Kort, L.M.; Harmel, J.; de Jongh, P.E.; Ngene, P. The effect of nanoscaffold porosity and surface chemistry on the Li-ion conductivity of LiBH4-LiNH2/metal oxide nanocomposites. J. Mater. Chem. A 2020, 8, 20687–20697. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Dehydriding and rehydriding reactions of LiBH4. J. Alloys Compd. 2005, 404, 427–430. [Google Scholar] [CrossRef]
- Lu, F.; Pang, Y.; Zhu, M.; Han, F.; Yang, J.; Fang, F.; Sun, D.; Zheng, S.; Wang, C. A High-Performance Li–B–H Electrolyte for All-Solid-State Li Batteries. Adv. Funct. Mater. 2019, 29, 1809219. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 2017, 8, 15893. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, Y.-S.; Oh, K.H.; Cho, Y.W. Interface-enhanced Li ion conduction in a LiBH4–SiO2 solid electrolyte. Phys. Chem. Chem. Phys. 2016, 18, 22540–22547. [Google Scholar] [CrossRef] [PubMed]
- Lefevr, J.; Cervini, L.; Griffin, J.M.; Blanchard, D. Lithium Conductivity and Ions Dynamics in LiBH4/SiO2 Solid Electrolytes Studied by Solid-State NMR and Quasi-Elastic Neutron Scattering and Applied in Lithium–Sulfur Batteries. J. Phys. Chem. C 2018, 122, 15264–15275. [Google Scholar] [CrossRef]
- Unemoto, A.; Ikeshoji, T.; Yasaku, S.; Matsuo, M.; Stavila, V.; Udovic, T.J.; Orimo, S.-I. Stable Interface Formation between TiS2 and LiBH4 in Bulk-Type All-Solid-State Lithium Batteries. Chem. Mater. 2015, 27, 5407–5416. [Google Scholar] [CrossRef]
- Unemoto, A.; Nogami, G.; Tazawa, M.; Taniguchi, M.; Orimo, S.-I. Development of 4V-Class Bulk-Type All-Solid-State Lithium Rechargeable Batteries by a Combined Use of Complex Hydride and Sulfide Electrolytes for Room Temperature Operation. Mater. Trans. 2017, 58, 1063–1068. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; De Jongh, P.E.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4 Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef]


| LiBH4-Based Materials | Ea2 (eV) | Electrochemical Window (V vs. Li/Li+) | Applications in ASSLBs | Ref. | |
|---|---|---|---|---|---|
| LiBH4 (P63mc) | 10−3 (120 °C) | 0.53 | 5 | Li||TiS2 | [34,48] |
| LiBH4 (Pnma) | 10−8 | 0.69 | 5 | - | [34] |
| LiBH4@SBA-15 | 9 × 10−4 | 0.43 | 3.5 | Li||S | [40,50] |
| Li2(BH4)(NH2) | 9 × 10−4 | 0.66 | - | - | [32,35] |
| Li(NH3)nBH4 (0.5 ≤ n ≤ 1) | 10−3–10−2 (40 °C) | - | 4 | - | [37] |
| LiBH4–LiX (X = Cl, Br and I) | 10−6−10−4 | 0.39–0.64 | - | LiNbO3-coated LiCoO2/KB/80Li2S 20P2S5||Li | [32,49] |
| LiBH4−MgO composites | 9 × 10−3 | 0.29 | 2.2 | Li||TiS2 | [39] |
| LiBH4–LiNH2/metal oxide nanocomposites | 10−3 | 0.86–0.90 | - | - | [41] |
| Li4(BH4)3I@SBA-15 | 8 × 10−3 | 0.46 | 5 | Li||Li4Ti5O12, Li||S, Li||LiCoO2 | [44] |
| Li2(BH4)(NH2)@SBA-15 | 5 × 10−3 | 0.49 | 3.2 | Li||TiS2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Lu, F.; Liu, Y.; Zhang, Y.; Wang, X.; Pang, Y.; Zheng, S. Li2(BH4)(NH2) Nanoconfined in SBA-15 as Solid-State Electrolyte for Lithium Batteries. Nanomaterials 2021, 11, 946. https://doi.org/10.3390/nano11040946
Yang Q, Lu F, Liu Y, Zhang Y, Wang X, Pang Y, Zheng S. Li2(BH4)(NH2) Nanoconfined in SBA-15 as Solid-State Electrolyte for Lithium Batteries. Nanomaterials. 2021; 11(4):946. https://doi.org/10.3390/nano11040946
Chicago/Turabian StyleYang, Qianyi, Fuqiang Lu, Yulin Liu, Yijie Zhang, Xiujuan Wang, Yuepeng Pang, and Shiyou Zheng. 2021. "Li2(BH4)(NH2) Nanoconfined in SBA-15 as Solid-State Electrolyte for Lithium Batteries" Nanomaterials 11, no. 4: 946. https://doi.org/10.3390/nano11040946
APA StyleYang, Q., Lu, F., Liu, Y., Zhang, Y., Wang, X., Pang, Y., & Zheng, S. (2021). Li2(BH4)(NH2) Nanoconfined in SBA-15 as Solid-State Electrolyte for Lithium Batteries. Nanomaterials, 11(4), 946. https://doi.org/10.3390/nano11040946





