Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis and Characterization of γ-CD-Citrate Oligomers
2.3. Synthesis and Characterization of MIL-100 (Al) nanoMOFs
2.4. DOX Encapsulation in nanoMOFs Coated or Not with CD-CO
2.5. Characterization of CD-CO Coated nanoMOFs
2.6. DOX Release Study
2.7. Solid-State NMR Spectroscopy
3. Results
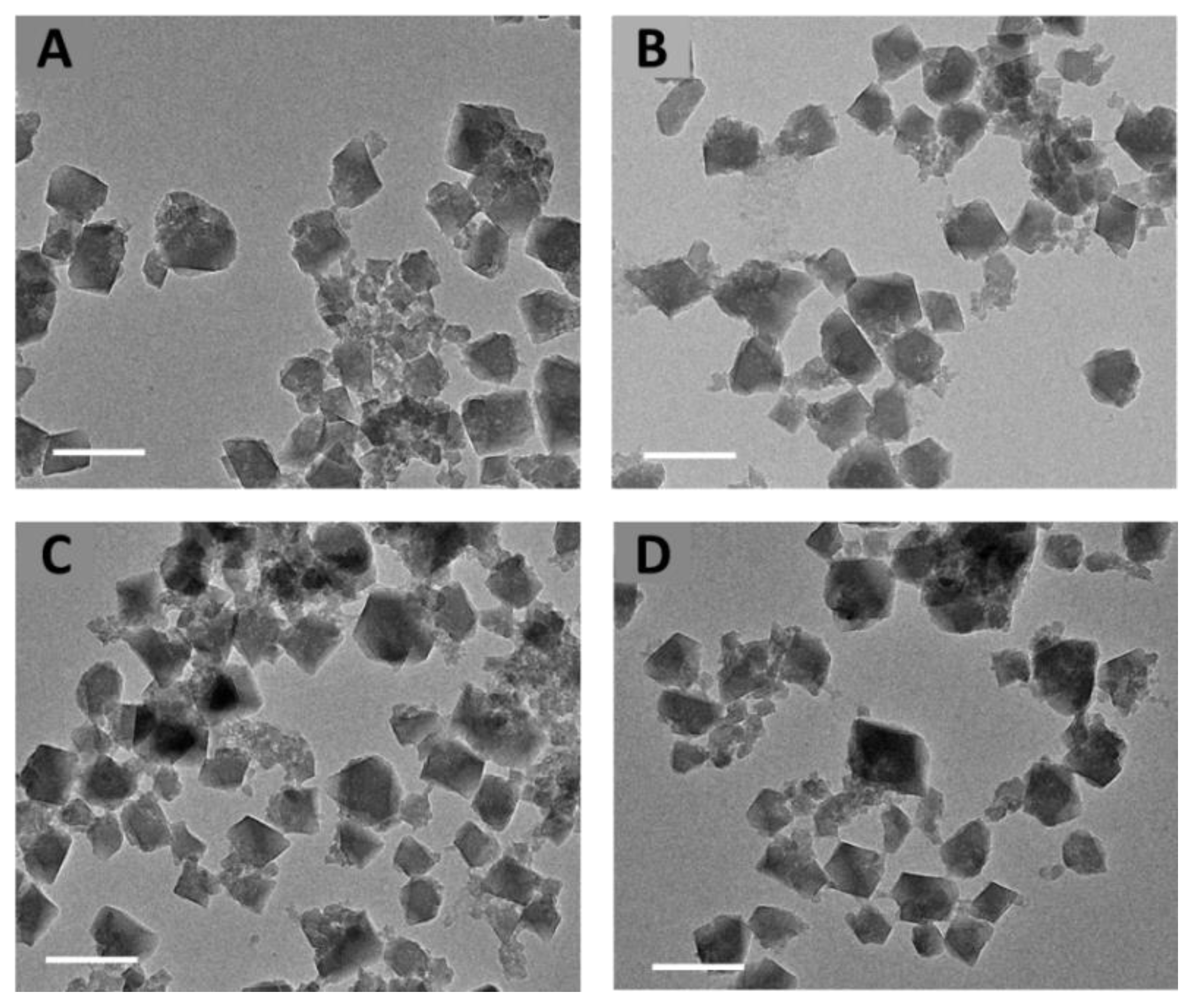
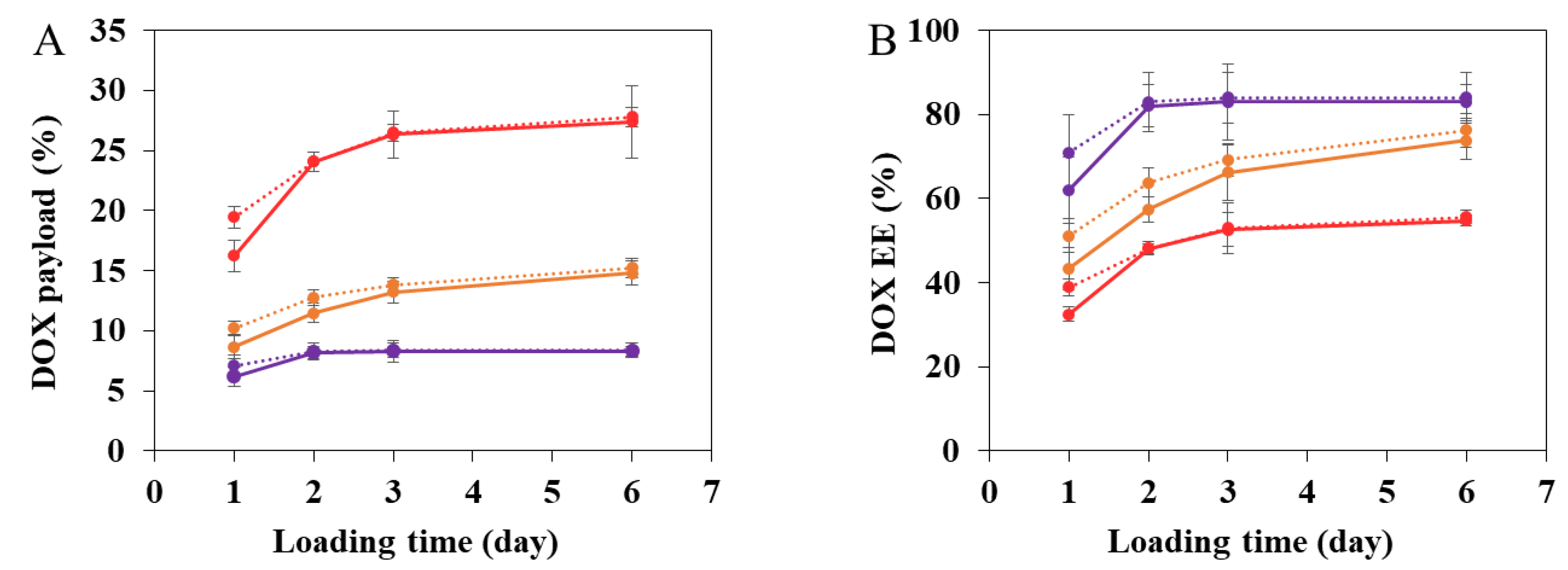
3.1. DOX Encapsulation in nanoMIL-100(Al)
3.2. DOX Encapsulation in nanoMIL-100(Al) Coated with CD-CO
3.2.1. Surface Functionalization of nanoMOFs with CD-CO
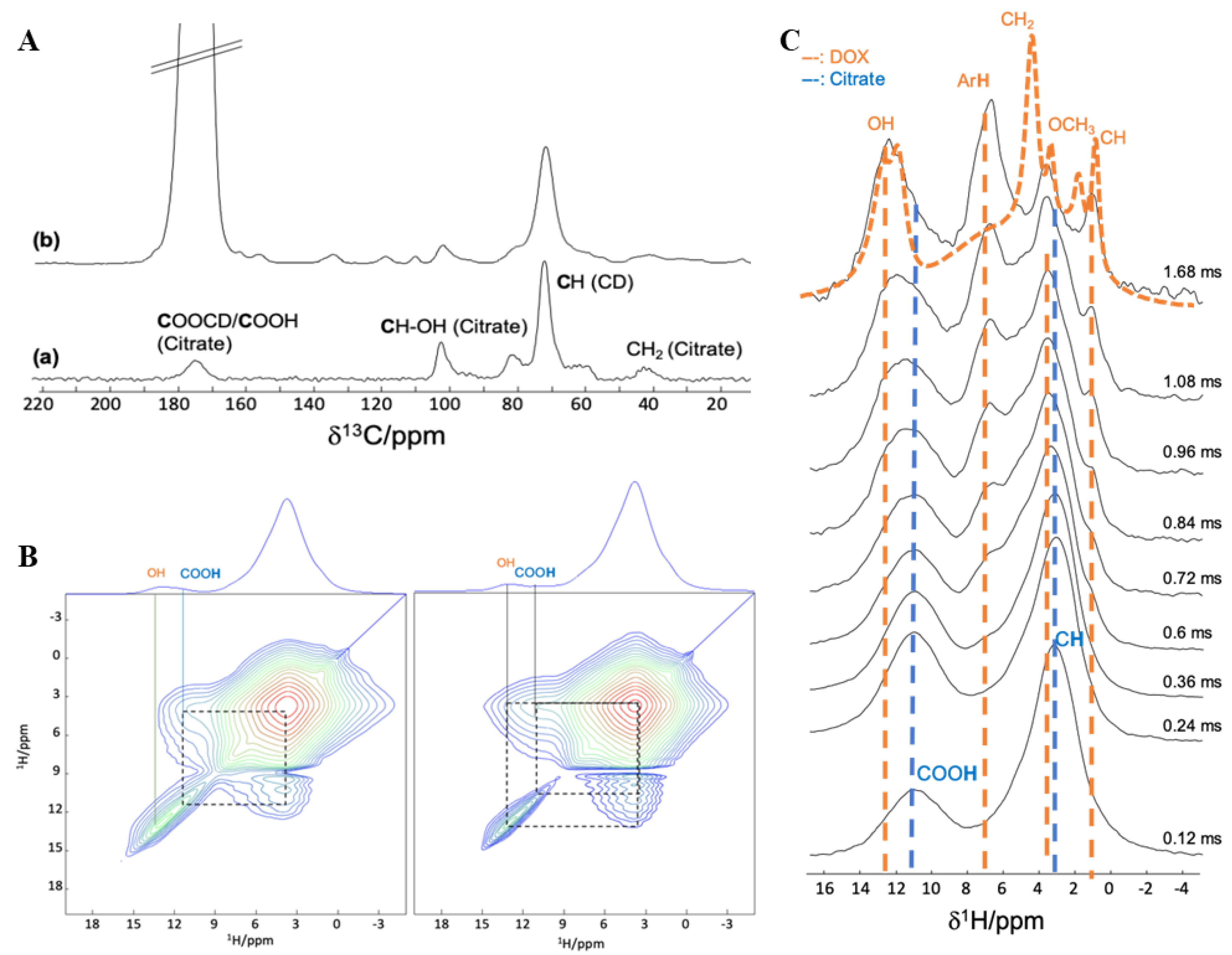
3.2.2. Interaction between DOX and CD-CO
3.2.3. Characterization of DOX Loaded MIL-100(Al) nanoMOFs Coated with CD-CO
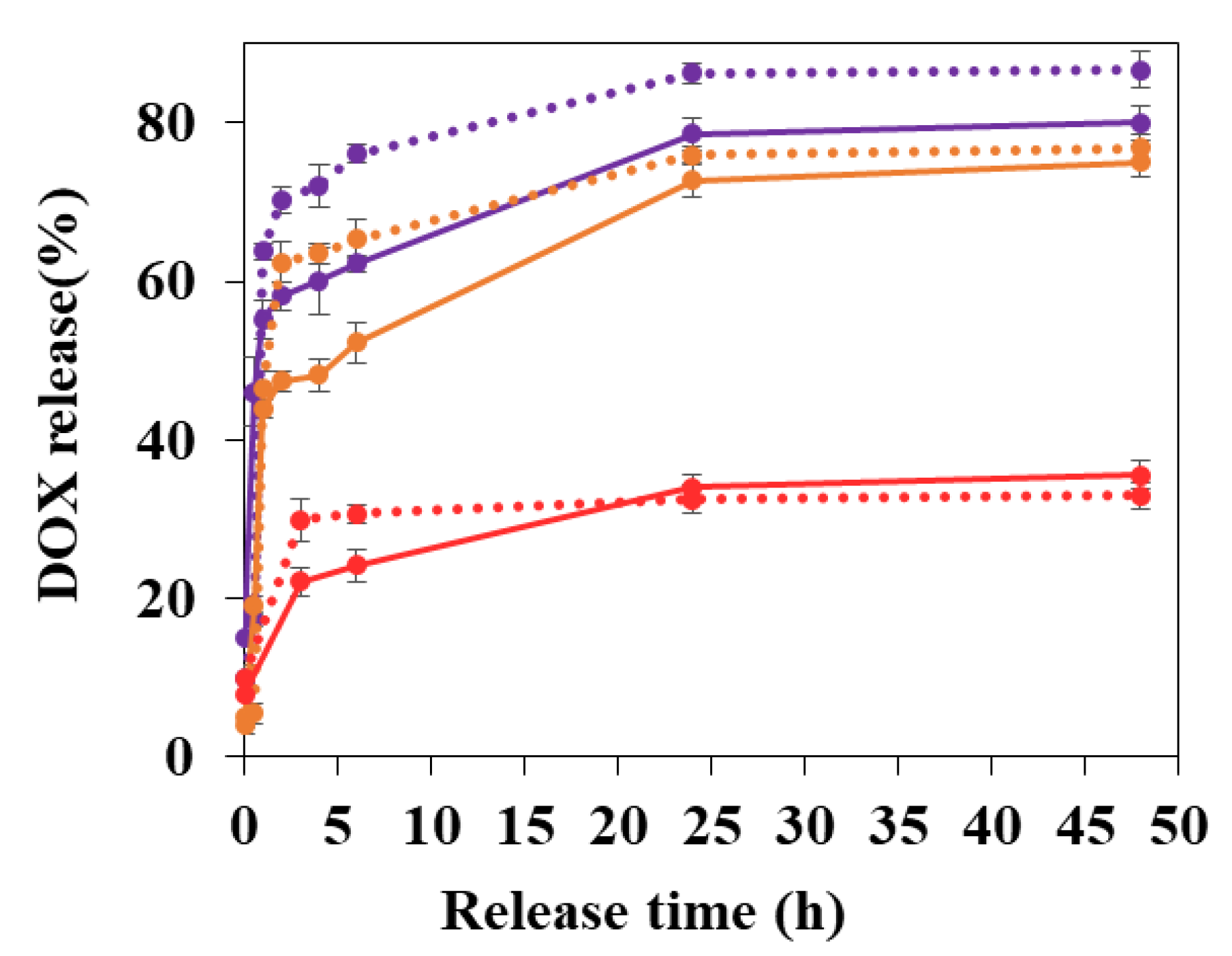
3.3. DOX Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal—Organic frameworks and coordination polymers. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Stock, N. Synthesis and structures of aluminum-based metal-organic frameworks. Metal. Org. Framew. Mater. 2014, 1–16. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Ke, F.; Zang, J.; Zhu, J. MIL-100 (Al) gels as an excellent platform loaded with doxorubicin hydrochloride for pH-triggered drug release and anticancer effect. Nanomaterials 2018, 8, 446. [Google Scholar] [CrossRef]
- Martineau-Corcos, C. NMR crystallography: A tool for the characterization of microporous hybrid solids. Curr. Opin. Colloid. Interface Sci. 2018, 33, 35–43. [Google Scholar] [CrossRef]
- Eike Brunner, M.R. Solid-state NMR spectroscopy: An advancing tool to analyse structure and properties of metal-organic frameworks. Chem Sci. 2020, 11, 4297–4304. [Google Scholar] [CrossRef]
- Hoffmann, H.C.; Debowski, M.; Müller, P.; Paasch, S.; Senkovska, I.; Kaskel, S.; Brunner, E. Solid-state NMR spectroscopy of metal–organic framework compounds (MOFs). Materials 2012, 5, 2537–2572. [Google Scholar] [CrossRef]
- Chen, S.; Mukherjee, S.; Lucier, B.; Guo, Y.; Wong, Y.T.A.; Terskikh, V.V.; Zaworotko, M.J.; Huang, Y. Cleaving carboxyls: Understanding thermally triggered hierarchical pores in the metal—Organic framework MIL-121. J. Am. Chem. Soc. 2019, 141, 14257–14271. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chen, S.; Bezrukov, A.A.; Mostrom, M.; Terskikh, V.V.; Franz, D.; Wang, S.; Kumar, A.; Chen, M.; Space, B.; et al. Ultramicropore engineering by dehydration to enable molecular sieving of H2 by calcium trimesate. Angew. Chem. Int. Ed. 2020, 59, 16188–16194. [Google Scholar] [CrossRef] [PubMed]
- Haouas, M.; Volkringer, C.; Loiseau, T. Monitoring the activation process of the giant pore MIL-100 (Al) by solid state NMR. J. Phys. Chem. C 2011, 115, 17934–17944. [Google Scholar] [CrossRef]
- Dawson, D.M.; Jamieson, L.E.; Mohideen, M.I.H.; McKinlay, A.C.; Smellie, I.A.; Cadou, R.; Keddie, N.S.; Morris, R.E.; Ashbrook, S.E. High-resolution solid-state 13 C NMR spectroscopy of the paramagnetic metal–organic frameworks, STAM-1 and HKUST-1. Phys. Chem. Chem. Phys. 2013, 15, 919–929. [Google Scholar] [CrossRef]
- Li, S.; Lafon, O.; Wang, W.; Wang, Q.; Wang, X.; Li, Y.; Xu, J.; Deng, F. Recent advances of solid-state NMR spectroscopy for microporous materials. Adv. Mater. 2020, 2002879, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mali, G. Looking into metal-organic frameworks with solid- state NMR spectroscopy. Metal. Org. Framew. 2016, 1–24. [Google Scholar] [CrossRef]
- Anand, R.; Borghi, F.; Manoli, F.; Manet, I.; Agostoni, V.; Reschiglian, P.; Gref, R.; Monti, S. Host-guest interactions in Fe(III)-trimesate MOF nanoparticles loaded with doxorubicin. J. Phys. Chem. B 2014, 118, 8532–8539. [Google Scholar] [CrossRef]
- Devautour-Vinot, S.; Martineau, C.; Diaby, S.; Ben-Yahia, M.; Miller, S.; Serre, C.; Horcajada, P.; Cunha, D.; Taulelle, F.; Maurin, G. Caffeine confinement into a series of functionalized porous zirconium MOFs: A joint experimental/modeling exploration. J. Phys. Chem. C 2013, 117, 11694–11704. [Google Scholar] [CrossRef]
- Skorupska, E.; Jeziorna, A.; Kazmierski, S.; Potrzebowski, M.J. Recent progress in solid-state NMR studies of drugs confined within drug delivery systems. Solid. State Nucl. Magn. Reson. 2014, 57–58, 2–16. [Google Scholar] [CrossRef]
- Agostoni, V.; Horcajada, P.; Noiray, M.; Malanga, M.; Aykaç, A.; Jicsinszky, L.; Vargas-Berenguel, A.; Semiramoth, N.; Daoud-Mahammed, S.; Nicolas, V.; et al. A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci. Rep. 2015, 5, 7925. [Google Scholar] [CrossRef]
- Porcino, M.; Christodoulou, I.; Dang, M.; Vuong, L.; Gref, R.; Martineau-Corcos, C. New insights on the supramolecular structure of highly porous core–shell drug nanocarriers using solid-state NMR spectroscopy. RSC Adv. 2019, 9, 32472–32475. [Google Scholar] [CrossRef]
- Cutrone, G.; Li, X.; Casas-Solvas, J.M.; Menendez-Miranda, M.; Qiu, J.; Benkovics, G.; Constantin, D.; Malanga, M.; Moreira-Alvarez, B.; Costa-Fernandez, J.M.; et al. Design of engineered cyclodextrin derivatives for spontaneous coating of highly porous metal-organic framework nanoparticles in aqueous media. Nanomaterials 2019, 9, 1103. [Google Scholar] [CrossRef]
- Qiu, J.; Li, X.; Steenkeste, K.; Barroca-Aubry, N.; Aymes-Chodur, C.; Roger, P.; Casas-Solvas, J.M.; Vargas-Berenguel, A.; Rihouey, C.; Picton, L.; et al. Self-assembled multifunctional core—Shell highly porous metal—Organic framework nanoparticles. Int. J. Pharm. 2020, 581, 119281. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Malanga, M.; Manet, I.; Tuza, K.; Aykaç, A.; Ladavière, C.; Fenyvesi, E.; Vargas-Berenguel, A.; Gref, R.; Monti, S. Citric acid–γ-cyclodextrin crosslinked oligomers as carriers for doxorubicin delivery. Photochem. Photobiol. Sci. 2013, 12, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morcellet, M. Water-soluble polymers and gels from the polycondensation between cyclodextrins and poly (carboxylic acid)s: A study of the preparation parameters. J. Appl. Polym. Sci. 2004, 97, 433–442. [Google Scholar] [CrossRef]
- Márquez, A.G.; Demessence, A.; Platero-Prats, A.E.; Heurtaux, D.; Horcajada, P.; Serre, C.; Chang, J.-S.; Férey, G.; De La Peña-O’Shea, V.A.; Boissière, C.; et al. Green microwave synthesis of MIL-100 (Al, Cr, Fe) nanoparticles for thin- film elaboration. Eur. J. Inorg. Chem. 2012, 100, 5165–5174. [Google Scholar] [CrossRef]
- Li, X.; Lachmanski, L.; Safi, S.; Serre, C.; Grenèche, J.M.; Zhang, J.; Gref, R. New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Li, X.; Semiramoth, N.; Hall, S.; Tafani, V.; Josse, J.; Laurent, F.; Salzano, G.; Foulkes, D.; Brodin, P.; Gref, R.; et al. Compartmentalized encapsulation of two antibiotics in porous nanoparticles: An efficient strategy to treat intracellular infections. Part. Part. Syst. Charact. 2019, 1800360. [Google Scholar] [CrossRef]
- Cutrone, G.; Qiu, J.; Menendez-Miranda, M.; Tafani, V.; Josse, J.; Laurent, F.; Salzano, G.; Foulkes, D.; Brodin, P.; Majlessi, L.; et al. Comb-like dextran copolymers: A versatile strategy to coat highly porous MOF nanoparticles with a PEG shell. Carbohydr. Polym. 2019, 223, 115085. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z. 13C/14N heteronuclear multiple-quantum correlation with rotary resonance and REDOR dipolar recoupling. J. Magn Reson. 2007, 184, 39–43. [Google Scholar] [CrossRef]
- Hu, B.; Trebosc, J.P.A. Comparison of several hetero-nuclear dipolar recoupling NMR methods to be used in MAS HMQC/HSQC. J. Magn. Reson. 2008, 192, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Trebosc, J.; Hu, B.; Amoureux, J.P.; Gan, Z. Through-space R3 -HETCOR experiments between spin-1/2 and half-integer quadrupolar nuclei in solid-state NMR. J. Magn. Reson. 2007, 186, 220–227. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Hu, B.; Lafon, O.; Trebosc, J. Measurement of hetero-nuclear distances using a symmetry-based pulse sequence in solid-state NMR. Phys. Chem. Chem. Phys. 2010, 12, 9395–9405. [Google Scholar] [CrossRef] [PubMed]
- Pourpoint, F.; Aany Thankamony, S.L.; Volkringer, C.; Loiseau, T.; Trébosc, J.; Aussenac, F.; Carnevale, D.; Bodenhausen, G.; Vezin, H.; Lafon, O.; et al. Probing 27Al–13C proximities in metal–organic frameworks using dynamic nuclear polarization enhanced NMR spectroscopy. Chem. Comm. 2014, 50, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.M.; Harris, R.K.E. Encyclopedia of nuclear magnetic resonance. In Advances in NMR; Wiley: Hoboken, NJ, USA, 2005; p. 861. [Google Scholar]
- Brinkmann, A.; Kentgens, A.P.M. Proton-selective 17O-1H distance measurements in fast magic-angle-spinning solid-state NMR spectroscopy for the determination of hydrogen bond lengths. J. Am. Chem Soc. 2006, 128, 14758–14759. [Google Scholar] [CrossRef] [PubMed]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Pieper, S.; Langer, K. Doxorubicin-loaded PLGA nanoparticles—A systematic evaluation of preparation techniques and parameters. Mater. Today Proc. 2017, 4, 188–192. [Google Scholar] [CrossRef]
- DOXIL® (doxorubicin HCl liposome injection) for intravenous infusion Initial U.S. Manufactured by: Ben Venue Laboratories, Inc. Bedford, OH 44146, Distributed by: Ortho Biotech Products, LP Raritan, NJ 08869-0670, Approval: 1995. 1–33
- Xue, T.; Xu, C.; Wang, Y.; Wang, Y.; Tian, H. Doxorubicin-loaded nanoscale metal-organic framework for tumor targeting combined chemotherapy and chemodynamic therapy. Biomater. Sci. 2019, 7, 4615–4623. [Google Scholar] [CrossRef]
- Munnier, E.; Tewes, F.; Cohen-Jonathan, S.; Linassier, C.; Douziech-Eyrolles, L.; Marchais, H.; Soucé, M.; Hervé, K.; Dubois, P.; Chourpa, I. On the interaction of doxorubicin with oleate ions: Fluorescence spectroscopy and liquid–liquid extraction study. Chem. Pharm. Bull. 2007, 55, 1006–1010. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, V.; Maksimenko, A.; Anand, R.; Monti, S.; Agostoni, V.; Couvreur, P.; Lampropoulou, M.; Yannakopoulou, K.; Gref, R. Efficient “green” encapsulation of a highly hydrophilic anticancer drug in metal-organic framework nanoparticles. J. Drug Target. 2015, 23, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Di-Nunzio, M.R.; Agostoni, V.; Cohen, B.; Gref, R.; Douhal, A. A “ship in a bottle” strategy to load a hydrophilic anticancer drug in porous metal organic framework nanoparticles: Efficient encapsulation, matrix stabilization, and photodelivery. J. Med. Chem. 2014, 57, 411–420. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Porcino, M.; Qiu, J.; Constantin, D.; Martineau-Corcos, C.; Gref, R. Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy. Nanomaterials 2021, 11, 945. https://doi.org/10.3390/nano11040945
Li X, Porcino M, Qiu J, Constantin D, Martineau-Corcos C, Gref R. Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy. Nanomaterials. 2021; 11(4):945. https://doi.org/10.3390/nano11040945
Chicago/Turabian StyleLi, Xue, Marianna Porcino, Jingwen Qiu, Doru Constantin, Charlotte Martineau-Corcos, and Ruxandra Gref. 2021. "Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy" Nanomaterials 11, no. 4: 945. https://doi.org/10.3390/nano11040945
APA StyleLi, X., Porcino, M., Qiu, J., Constantin, D., Martineau-Corcos, C., & Gref, R. (2021). Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy. Nanomaterials, 11(4), 945. https://doi.org/10.3390/nano11040945





