Nanonutraceuticals: The New Frontier of Supplementary Food
Abstract
1. Introduction
2. Methods of Bibliographic Search
3. Positive Effects of Nutraceuticals on Human Health
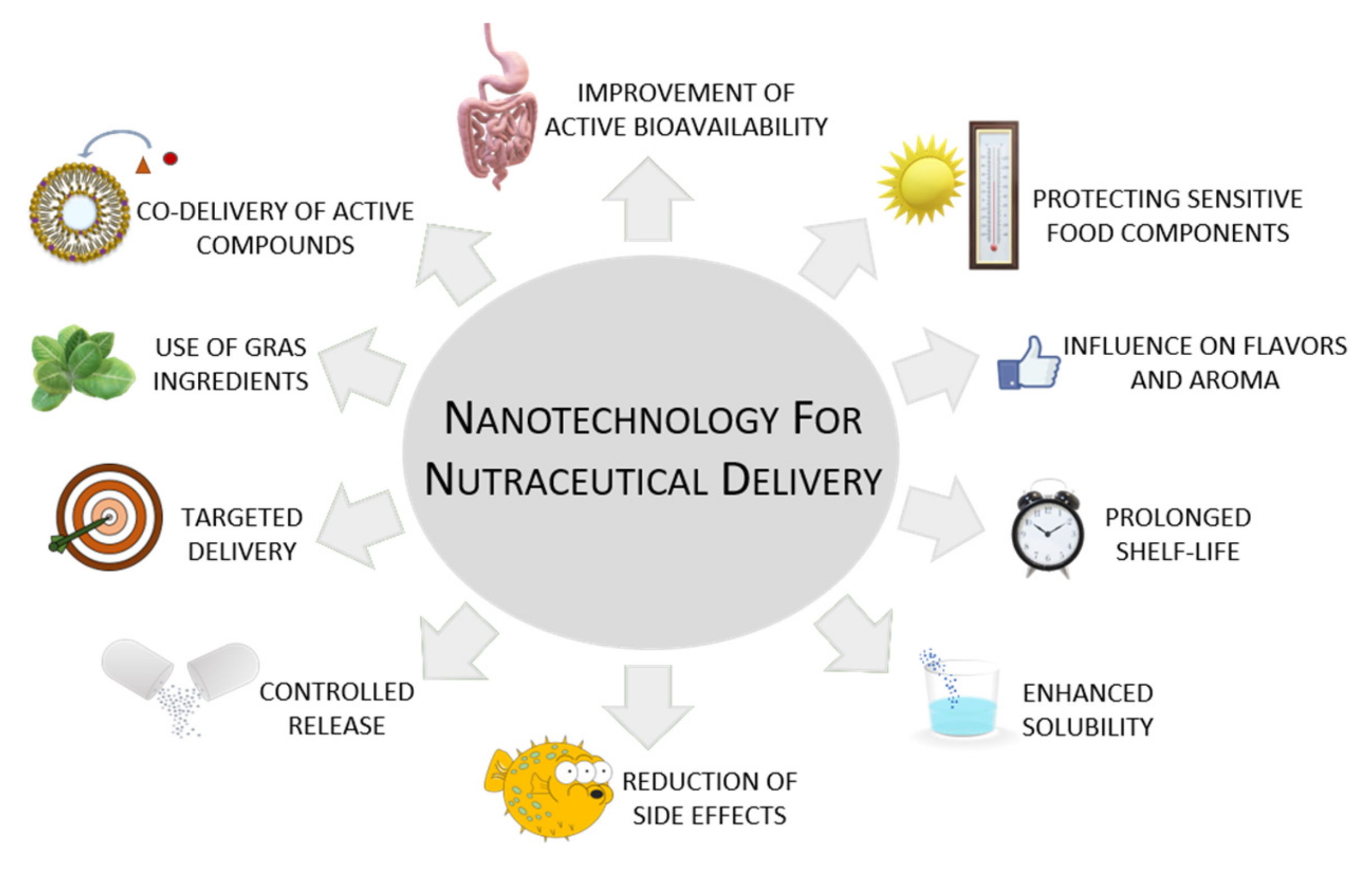
4. Nanotechnology as a Nutraceutical Properties Enhancement Strategy
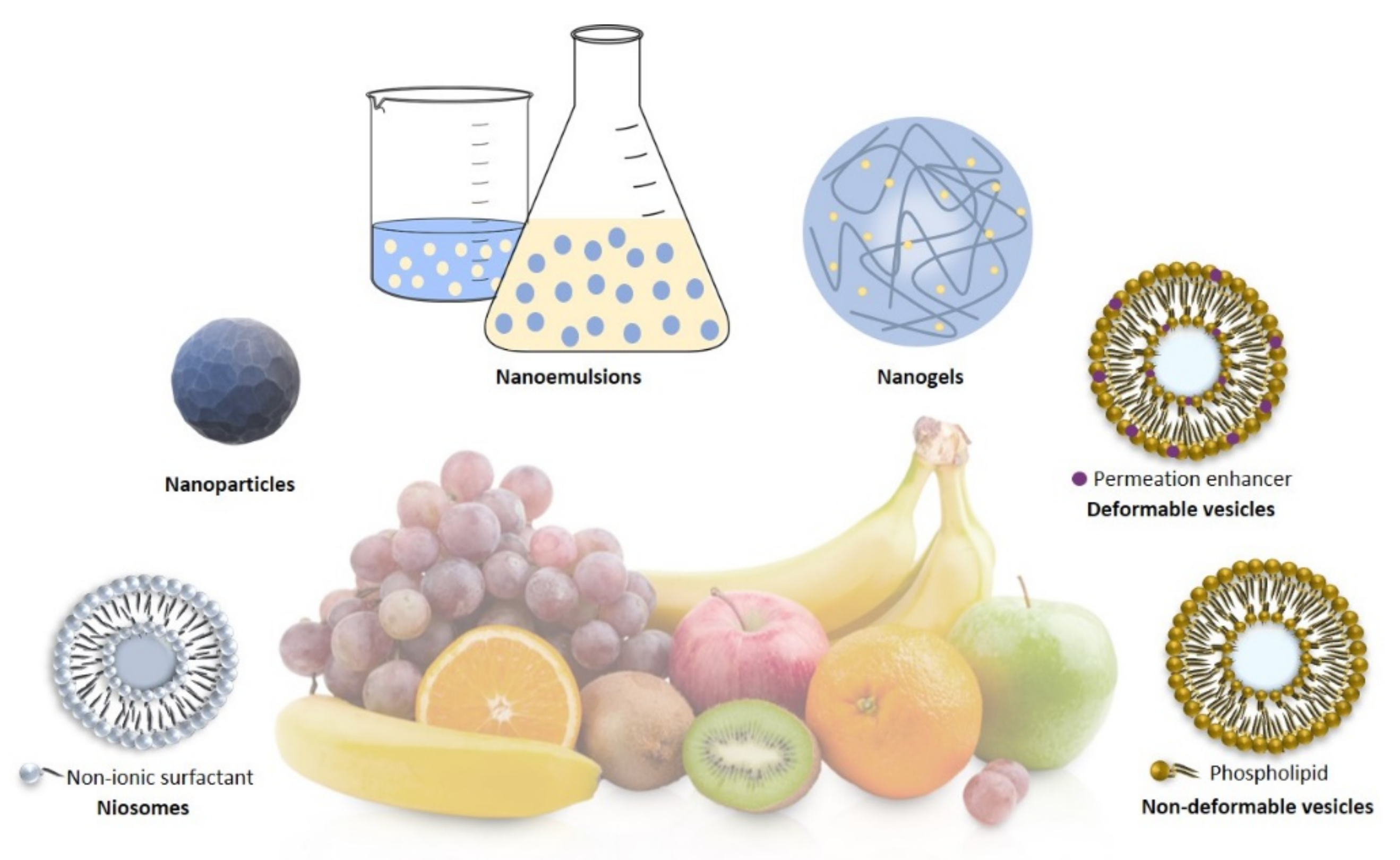
4.1. Nanoparticles
Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
4.2. Liposomes
4.3. Nanoemulsions
4.4. Nanogels
5. The Other Face of Nanosystems Focuses on Their Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Song, E.J.; Do Nam, Y.; Lee, S.Y. Probiotics in human health and disease: From nutribiotics to pharmabiotics. J. Microbiol. 2018, 56, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Shinde, N.; Bangar, B.; Deshmukh, S.; Kumbhar, P. Nutraceuticals: A review on current status. RJPT 2014, 7, 110–113. [Google Scholar]
- Gruss, S.M.; Nhim, K.; Gregg, E.; Bell, M.; Luman, E.; Albright, A. Public health approaches to type 2 diabetes prevention: The US National Diabetes Prevention Program and beyond. Curr. Diabetes Rep. 2019, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diab. Rep. 2019, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef]
- Natarajan, T.D.; Ramasamy, J.R.; Palanisamy, K. Nutraceutical potentials of synergic foods: A systematic review. J. Ethn. Foods. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease revention and management. Expert Rev. Clin. Pharm. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Leonard, N.B. Stability testing of nutraceuticals and functional foods. In Handbook of Nutraceuticals and Functional Foods; CRC Press: Boca Raton, Fl, USA, 2000. [Google Scholar]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. F 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Sandhu, K.S.; Kaur, M.; Siroha, A.K. Nanotechnology: A Successful Approach to Improve Nutraceutical Bioavailability. In Nanobiotechnology Bioformulation; Springer: Cham, Switzerland, 2019; pp. 119–133. [Google Scholar]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, 50–57. [Google Scholar] [CrossRef]
- Pandey, M.; Verma, R.K.; Saraf, S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin. Res. 2010, 3, 11–15. [Google Scholar]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Joshi, K.; Sharma, V.; Ranjan, R.; Bhattacharya, K.; Varshney, A. Cytokines driven anti-inflammatory and anti-psoriasis like efficacies of nutraceutical sea buckthorn (hippophae rhamnoides) oil. Front. Pharm. 2019, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a nutraceutical approach for inflammatory related diseases. Front. Pharm. 2019, 10, 486. [Google Scholar] [CrossRef]
- Derosa, G.; Limas, C.P.; Maciás, P.C.; Estrella, A.; Maffioli, P. Dietary and nutraceutical approach to type 2 diabetes. Arch. Med. Sci. 2014, 10, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phyther. Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Hidalgo, J.M.; Romero, M.; Duarte, J.; López-huertas, E. Antihypertensive effects of virgin olive oil (Unfiltered) low molecular weight peptides with ace inhibitory activity in spontaneously hypertensive rats. Nutrients 2020, 12, 271. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.C.; Gluba-Brzózka, A.; Mikhailidis, D.P.; Cicero, A.F.; Rysz, J.; Banach, M. Lipid-modifying effects of nutraceuticals: An evidence-based approach. Nutrition 2016, 32, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Mannarino, M.R.; Sahebkar, A.; Cosentino, T.; Pirro, M. Cholesterol-Lowering Nutraceuticals Affecting Vascular Function and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2018, 20, 53. [Google Scholar] [CrossRef]
- Cimaglia, P.; Dalla Sega, F.V.; Vitali, F.; Lodolini, V.; Bernucci, D.; Passarini, G.; Fortini, F.; Marracino, L.; Aquila, G.; Rizzo, P.; et al. Effectiveness of a novel nutraceutical compound containing red yeast rice, polymethoxyflavones and antioxidants in the modulation of cholesterol levels in subjects with hypercholesterolemia and low-moderate cardiovascular risk: The NIRVANA study. Front. Physiol. 2019, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Attanzio, A.; Tesoriere, L.; Poojary, M.M.; Cilla, A. Fruit and Vegetable Derived Waste as a Sustainable Alternative Source of Nutraceutical Compounds. J. Food Qual. 2018, 2018, 8136190. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects–A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-haq, I.; Ur-rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.; Singh, R.; Buttar, H.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef]
- Kaur, S.; Dhiman, M.; Mantha, A.K. Ferulic Acid: A Natural Antioxidant with Application towards Neuroprotection against Alzheimer’s Disease. In Functional Food and Human Health; Springer: Singapore, 2018; pp. 575–586. [Google Scholar]
- Oboh, G.; Ademosun, A.O. Shaddock peels (Citrus maxima) phenolic extracts inhibit α-amylase, α-glucosidase and angiotensin I-converting enzyme activities approach to diab: A nutraceutical etes management. Diabetes Metab. Syndr. Clin. Res. Rev. 2011, 5, 148–152. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L.; variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; D’avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A pilot screening of agro-food waste products as sources of nutraceutical formulations to improve simulated postprandial glycaemia and insulinaemia in healthy subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef]
- Aklakur, M.; Asharf Rather, M.; Kumar, N. Nanodelivery: An Emerging Avenue for Nutraceuticals and Drug Delivery. Crit. Rev. Food Sci. Nutr. 2016, 56, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Colloid Interface Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Aldalaen, S.; Nasr, M.; El-Gogary, R.I. Angiogenesis and collagen promoting nutraceutical-loaded nanovesicles for wound healing. J. Drug Deliv. Sci. Technol. 2020, 56, 101548. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-Loaded Ufasomes Improved the Nutraceutical Efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Rasti, B.; Erfanian, A.; Salamat, J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017, 230, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Meenambal, R.; Bharath, M.M.S. Nanocarriers for effective nutraceutical delivery to the brain. Neurochem. Int. 2020, 140, 104851. [Google Scholar] [CrossRef]
- Fresta, M.; Mancuso, A.; Cristiano, M.C.; Urbanek, K.; Cilurzo, F.; Cosco, D.; Iannone, M.; Paolino, D. Targeting of the Pilosebaceous Follicle by Liquid Crystal Nanocarriers: In Vitro and In Vivo Effects of the Entrapped Minoxidil. Pharmaceutics 2020, 12, 1127. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Fox, C.B.; Sivananthan, S.J.; Duthie, M.S.; Vergara, J.; Guderian, J.A.; Moon, E.; Coblentz, D.; Reed, S.G.; Carter, D. A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7. J. Nanobiotechnol. 2014, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Tripathi, D.K.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Gumezescu, A.; Oprea, A.E. Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety, 1st ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Aditya, N.P.; Espinosa, Y.G.; Norton, I.T. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kailasapathy, K. Encapsulation technologies for functional foods and nutraceutical product development. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2009, 4, 1–19. [Google Scholar] [CrossRef]
- Ali, A.; Ahmad, U.; Akhtar, J.; Badruddeen; Khan, M.M. Engineered nano scale formulation strategies to augment efficiency of nutraceuticals. J. Funct. Foods. 2019, 62, 103554. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef] [PubMed]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, S.; Zhang, L.; Dai, L.; Tai, K.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y.; Mackie, A. Fabrication, characterization and in vitro digestion of food grade complex nanoparticles for co-delivery of resveratrol and coenzyme Q10. Food Hydrocoll. 2020, 105, 105791. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Zhu, J.; Liu, C.; Sun, X.; Wang, D.; Xu, Y. Fabrication and characterization of zein nanoparticles by dextran sulfate coating as vehicles for delivery of curcumin. Int. J. Biol. Macromol. 2020, 151, 1074–1083. [Google Scholar] [CrossRef]
- Xiong, K.; Zhou, L.; Wang, J.; Ma, A.; Fang, D.; Xiong, L.; Sun, Q. Construction of food-grade pH-sensitive nanoparticles for delivering functional food ingredients. Trends Food Sci. Technol. 2020, 96, 102–113. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y.; Yu, Y.; Zhou, H.; Guo, T.; Zhu, H.; Wang, H.; Zhang, Y. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Colloids Surf. B Biointerfaces 2020, 196, 111294. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 285854. [Google Scholar] [CrossRef] [PubMed]
- Defrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Luo, Y.; Wang, Q. Nanoparticles Synthesized from Soy Protein: Preparation, Characterization, and Application for Nutraceutical Encapsulation. J. Agric. Food Chem. 2012, 60, 2712–2720. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, P.; Li, X.; Zhang, N.; Tang, C. Novel Soy β-Conglycinin Core–Shell Nanoparticles As Outstanding Ecofriendly Nanocarriers for Curcumin. J. Agric. Food Chem. 2019, 67, 6292–6301. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Antimicrobial activities of green synthesized gums-stabilized nanoparticles loaded with flavonoids. Sci. Rep. 2019, 9, 3122. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Jaglan, S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci. Technol. 2016, 54, 114–126. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Ichwan, S.J.A.; Soundharrajan, I.; Govindan, N. Nutraceuticals as potential therapeutic agents for colon cancer: A review. Acta Pharm. Sin. B 2014, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Encapsulation of Nutraceutical Ingredients in Liposomes and Their Potential for Cancer Treatment. Nutr. Cancer. 2018, 70, 1184–1198. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Mare, R.; Paolino, D.; Salvatici, M.C.; Cilurzo, F.; Fresta, M. Sclareol-loaded hyaluronan-coated PLGA nanoparticles: Physico-chemical properties and in vitro anticancer features. Int. J. Biol. Macromol. 2019, 132, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Dimas, K.; Kokkinopoulos, D.; Demetzos, C.; Vaos, B.; Marselos, M.; Malamas, M.; Tzavaras, T. The effect of sclareol on growth and cell cycle progression of human leukemic cell lines. Leuk. Res. 1999, 23, 217–234. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. Npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Wark, P.A.B.; Garg, M.L. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid. Redox Signal. 2010, 13, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Venuti, V.; Cannavà, C.; Cristiano, M.C.; Fresta, M.; Majolino, D.; Paolino, D.; Stancanelli, R.; Tommasini, S.; Ventura, C.A. A characterization study of resveratrol/sulfobutyl ether-β-cyclodextrin inclusion complex and in vitro anticancer activity. Colloids Surf. B Biointerfaces 2014, 115, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cheng, B.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Poonia, N.; Lather, V.; Narang, J.K.; Beg, S.; Pandita, D. Resveratrol-loaded folate targeted lipoprotein-mimetic nanoparticles with improved cytotoxicity, antioxidant activity and pharmacokinetic profile. Mater. Sci. Eng. C 2020, 114, 111016. [Google Scholar] [CrossRef]
- Brotons-Canto, A.; Gonzalez-Navarro, C.J.; Gurrea, J.; González-Ferrero, C.; Irache, J.M. Zein nanoparticles improve the oral bioavailability of resveratrol in humans. J. Drug Deliv. Sci. Technol. 2020, 57, 101704. [Google Scholar] [CrossRef]
- Chen, S.; McClements, D.J.; Jian, L.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Core-Shell Biopolymer Nanoparticles for Co-Delivery of Curcumin and Piperine: Sequential Electrostatic Deposition of Hyaluronic Acid and Chitosan Shells on the Zein Core. ACS Appl. Mater. Interfaces 2019, 11, 38103–38115. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; Julian, D.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Food Hydrocolloids Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal diges. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Sanna, V.; Lubinu, G.; Madau, P.; Pala, N.; Nurra, S.; Mariani, A.; Sechi, M. Polymeric nanoparticles encapsulating white tea extract for nutraceutical application. J. Agric. Food Chem. 2015, 63, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Cilurzo, F.; Fresta, M. Innovative drug delivery systems for the administration of natural compounds. Curr. Bioact. Compd. 2007, 3, 262–277. [Google Scholar] [CrossRef]
- Paolino, D.; Stancampiano, A.H.S.; Cilurzo, F.; Cosco, D.; Puglisi, G.; Pignatello, R. Nanostructured lipid carriers (NLC) for the topical delivery of lutein. Drug Deliv. Lett. 2011, 1, 32–39. [Google Scholar]
- Da Silva Santos, V.; Badan Ribeiro, A.P.; Andrade Santana, M.H. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: Overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 1863–1873. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci. Technol. 2015, 46, 114–157. [Google Scholar] [CrossRef]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physico-chemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Ghanbarzadeh, B.; Mohammadi, M.; Fathollahi, I.; Hamishehkar, H. Encapsulation of vitamin A palmitate in nanostructured lipid carrier (NLC)-effect of surfactant concentration on the formulation properties. Adv. Pharm. Bull. 2014, 4, 563–568. [Google Scholar] [PubMed]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, Antimicrobial and Physicochemical Properties of Turmeric Extract-Loaded Nanostructured Lipid Carrier (NLC). Colloids Interface Sci. Commun. 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Neves, A.R.; Martins, S.; Segundo, M.A.; Reis, S. Nanoscale delivery of resveratrol towards enhancement of supplements and nutraceuticals. Nutrients 2016, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2019, 30, 336–365. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Ahmad, R.R.H.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Hewavitharana, A.K.; Shaw, P.N.; Smyth, H.D.C.; Samaranayake, L.P. A novel, quorum sensor-infused liposomal drug delivery system suppresses Candida albicans biofilms. Int. J. Pharm. 2020, 578, 119096. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Gawne, P.J.; de Rosales, R.T.M. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 14, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Ferrati, S.; Bansal, S.; van de Ven, A.L.; Ruozi, B.; Zabre, E.; Hosali, S.; Paolino, D.; Sarpietro, M.G.; Fine, D.; et al. Drug Delivery: Sustained Zero-Order Release of Intact Ultra-Stable Drug-Loaded Liposomes from an Implantable Nanochannel Delivery System. Adv. Healthc. Mater. 2014, 3, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Subramani, T.; Ganapathyswamy, H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Angka, L.; Spagnuolo, P.A. From food to clinical medicine-nutraceuticals as clinical therapeutics for hematological malignancies. Curr. Opin. Food Sci. 2015, 4, 7–12. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, Y.; Wang, L.; Liu, H.; Cui, G. Enhanced anticancer activity of glutamate prodrugs of all-trans retinoic acid. J. Pharm. Pharm. 2009, 61, 1353–1358. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B Biointerfaces 2017, 150, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phyther. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Bakheit, A.H.; Zargar, S.; Alanazi, Z.S.; Al-Majed, A.A. Influence of antioxidant flavonoids quercetin and rutin on the in-vitro binding of neratinib to human serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118977. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Maduni, I.V.; Gajski, G.; Popi, J.; Garaj-vrhovac, V. Apigenin: A dietary fl avonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; M’hiri, N.; Chaaban, H.; Boudhrioua, N.M.; Ghoul, M. Effect of the process, temperature, light and oxygen on naringin extraction and the evolution of its antioxidant activity. Int. J. Food Sci. Technol. 2018, 53, 2754–2760. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Marongiu, F.; Castangia, I.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G.; D’hallewin, G.; Bacchetta, G.; Fadda, A.M. Polymer-associated liposomes for the oral delivery of grape pomace extract. Colloids Surf. B Biointerfaces 2016, 146, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; De Gaetano, F.; Ventura, C.A.; Fresta, M.; Paolino, D. The Rheolaser Master™ and Kinexus Rotational Rheometer® to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel. Molecules 2020, 25, 1979. [Google Scholar] [CrossRef] [PubMed]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid. Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cristiano, M.C.; Fresta, M.; Paolino, D. The Challenge of Nanovesicles for Selective Topical Delivery for Acne Treatment: Enhancing Absorption Whilst Avoiding Toxicity. Int. J. Nanomed. 2020, 15, 9197–9210. [Google Scholar] [CrossRef] [PubMed]
- Dima, Ş.; Dima, C.; Iordăchescu, G. Encapsulation of functional lipophilic food and drug biocomponents. Food Eng. Rev. 2015, 7, 417–438. [Google Scholar] [CrossRef]
- Basiri, L.; Rajabzadeh, G.; Bostan, A. Physicochemical properties and release behavior of Span 60/Tween 60 niosomes as vehicle for α-Tocopherol delivery. LWT Food Sci. Technol. 2017, 84, 471–478. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; De Cindio, B. Co-encapsulation of antioxidants into niosomal carriers: Gastrointestinal release studies for nutraceutical applications. Colloids Surf. B Biointerfaces 2014, 114, 82–88. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech. 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qi, J.; Wu, W. Absorption, Disposition and Pharmacokinetics of Nanoemulsions. Curr. Drug Metab. 2012, 13, 396–417. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Z.; McClements, D.J. Nanoemulsions: An emerging platform for increasing the efficacy of nutraceuticals in foods. Colloids Surf. B Biointerfaces 2020, 194, 111202. [Google Scholar] [CrossRef]
- Quagliariello, V.; Vecchione, R.; De Capua, A.; Lagreca, E.; Iaffaioli, R.V.; Botti, G.; Netti, P.A.; Maurea, N. Nano-encapsulation of coenzyme Q10 in secondary and tertiary nano-emulsions for enhanced cardioprotection and hepatoprotection in human cardiomyocytes and hepatocytes during exposure to anthracyclines and trastuzumab. Int. J. Nanomed. 2020, 15, 4859–4876. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; McClements, D.J.; Li, F.; Liu, H.; Cao, Y.; Xiao, H. Nanoemulsion-Based Delivery Systems for Nutraceuticals: Influence of Long-Chain Triglyceride (LCT) Type on In Vitro Digestion and Astaxanthin Bioaccessibility. Food Biophys. 2018, 13, 412–421. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chemie Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Manirujjaman, M.; Imran-Ul-Haque, M.A.; Sharmin, S. An overview of nanogel drug delivery system. J. Appl. Pharm. Sci. 2013, 3, 95–105. [Google Scholar]
- Theune, L.E.; Charbaji, R.; Kar, M.; Wedepohl, S.; Hedtrich, S.; Calderón, M. Critical parameters for the controlled synthesis of nanogels suitable for temperature-triggered protein delivery. Mater. Sci. Eng. C 2019, 100, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Farazi, S.; Chen, F.; Foster, H.; Boquiren, R.; McAlpine, S.R.; Chapman, R. Real time monitoring of peptide delivery: In vitro using high payload pH responsive nanogels. Polym. Chem. 2020, 11, 425–432. [Google Scholar] [CrossRef]
- Novikau, I.S.; Minina, E.S.; Sánchez, P.A.; Kantorovich, S.S. Suspensions of magnetic nanogels at zero field: Equilibrium structural properties. J. Magn. Magn. Mater. 2020, 498, 166152. [Google Scholar] [CrossRef]
- Feng, J.; Wu, S.; Wang, H.; Liu, S. Improved bioavailability of curcumin in ovalbumin-dextran nanogels prepared by Maillard reaction. J. Funct. Foods 2016, 27, 55–68. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.X.; Zhang, C.; Dai, C.; Ju, X.; He, R. Fabrication of Stable and Self-Assembling Rapeseed Protein Nanogel for Hydrophobic Curcumin Delivery. J. Agric. Food Chem. 2019, 67, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Baba, W.N.; Shah, U.; Gani, A.; Gani, A.; Masoodi, F.A. Nutraceutical properties of the green tea polyphenols. J. Food Process. Technol. 2014, 5, 11. [Google Scholar]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Z.; Kong, Q.; Zhang, R.; Yang, X. Enhancing the antitumor activity of tea polyphenols encapsulated in biodegradable nanogels by macromolecular self-assembly. RSC Adv. 2019, 9, 10004–10016. [Google Scholar] [CrossRef]
- Podila, R.; Brown, J.M. Toxicity of engineered nanomaterials: A physico-chemical perspective. J. Biochem. Mol. Toxicol. 2013, 27, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Rajwade, J.M.; Paknikar, K.M. Nanotoxycology and in vitro studies: The need of the hour. Toxicol. Appl. Pharm. 2012, 258, 151–165. [Google Scholar] [CrossRef]
- Ciappellano, S.G.; Tedesco, E.; Venturini, M.; Benedetti, F. In vitro toxicity assessment of oral nanocarriers. Adv. Drug Deliv. Rev. 2016, 106, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Potential of Nanonutraceuticals in Increasing Immunity. Nanomaterials 2020, 10, 2224. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mrsny, R.J.; Brayden, D.J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Iannone, M.; Fresta, M.; Fiorito, S.; Celia, C.; Paolino, D. In vitro and in vivo trans-epidermal water loss evaluation following topical drug delivery systems application for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2020, 186, 113295. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed]

| Nanocarrier Features | Advantages | Limits |
|---|---|---|
| - Nature of nanosystems (polymeric, lipidic, metal based, miscellaneous) | - Increased efficiency | - Possibility of conformational modification |
| - Size distribution | - Improved stability | - Possibility of immunotoxicity |
| - Biocompatibility and biodegradability | - Enhanced bioavailability | - Absence of guidelines and standardized protocols |
| - Encapsulation efficiency | - Toxic effects depending on carrier features | |
| - Drug release profile | - Absence of guidelines and standardized protocols | |
| - Targeting on the surface of system |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, D.; Mancuso, A.; Cristiano, M.C.; Froiio, F.; Lammari, N.; Celia, C.; Fresta, M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials 2021, 11, 792. https://doi.org/10.3390/nano11030792
Paolino D, Mancuso A, Cristiano MC, Froiio F, Lammari N, Celia C, Fresta M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials. 2021; 11(3):792. https://doi.org/10.3390/nano11030792
Chicago/Turabian StylePaolino, Donatella, Antonia Mancuso, Maria Chiara Cristiano, Francesca Froiio, Narimane Lammari, Christian Celia, and Massimo Fresta. 2021. "Nanonutraceuticals: The New Frontier of Supplementary Food" Nanomaterials 11, no. 3: 792. https://doi.org/10.3390/nano11030792
APA StylePaolino, D., Mancuso, A., Cristiano, M. C., Froiio, F., Lammari, N., Celia, C., & Fresta, M. (2021). Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials, 11(3), 792. https://doi.org/10.3390/nano11030792