Preparation of Carbon-Covered Phosphorus-Modified Alumina with Large Pore Size and Adsorption of Rhodamine B
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Phosphorus-Modified Alumina
2.3. Synthesis of Carbon-Covered Phosphorus-Modified Alumina
2.4. Material Characterization
2.5. Adsorption of RhB
3. Results
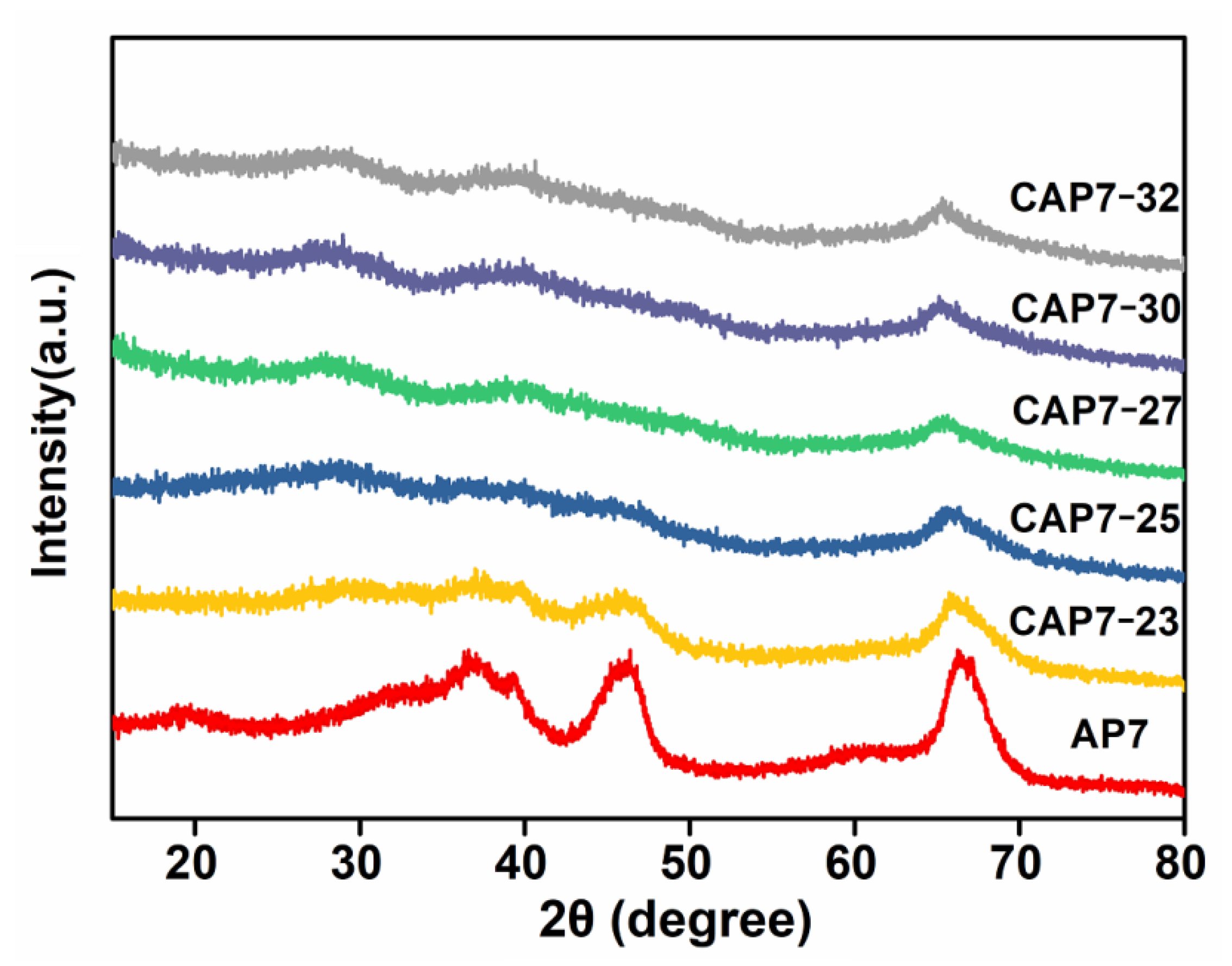
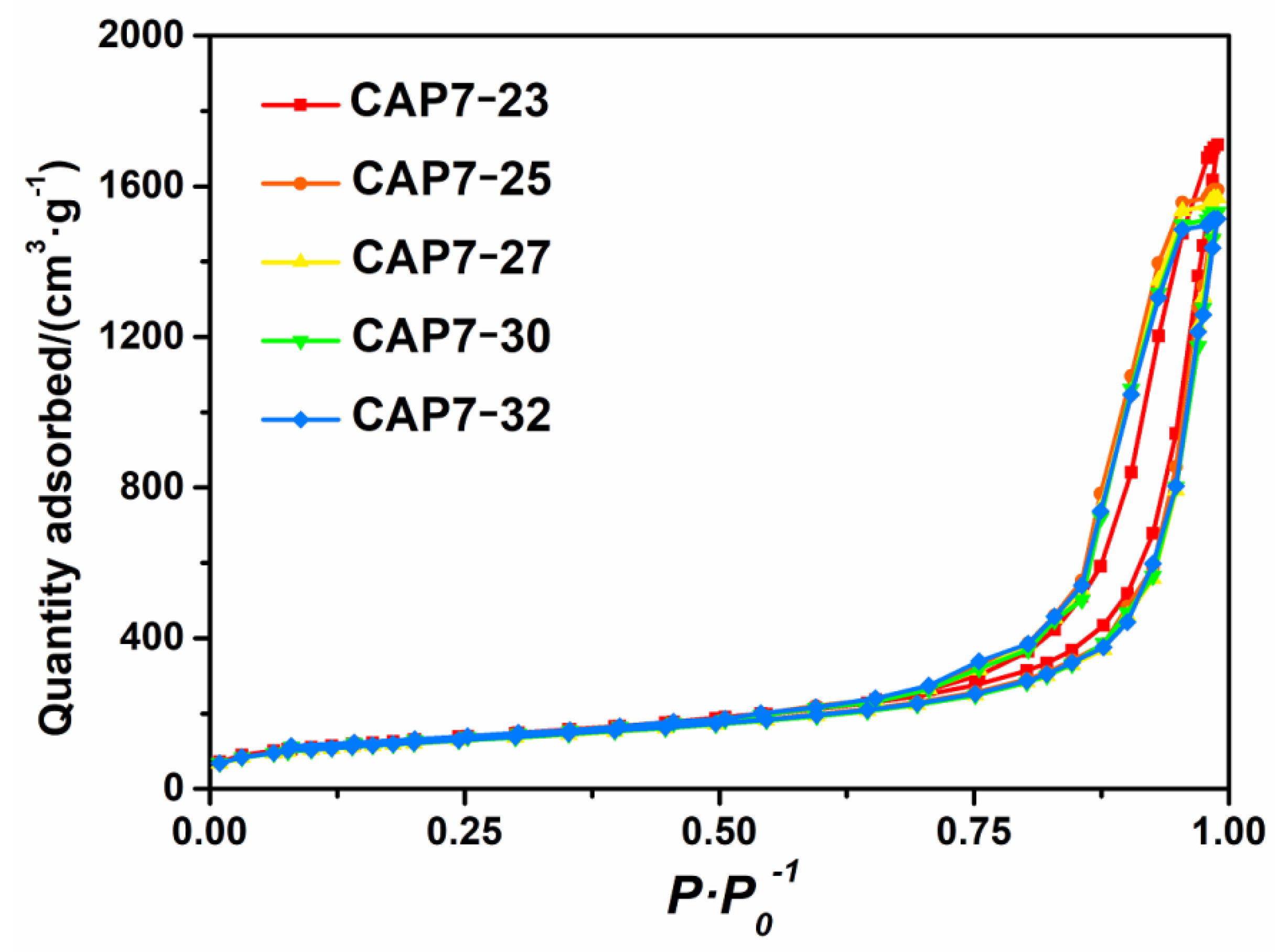
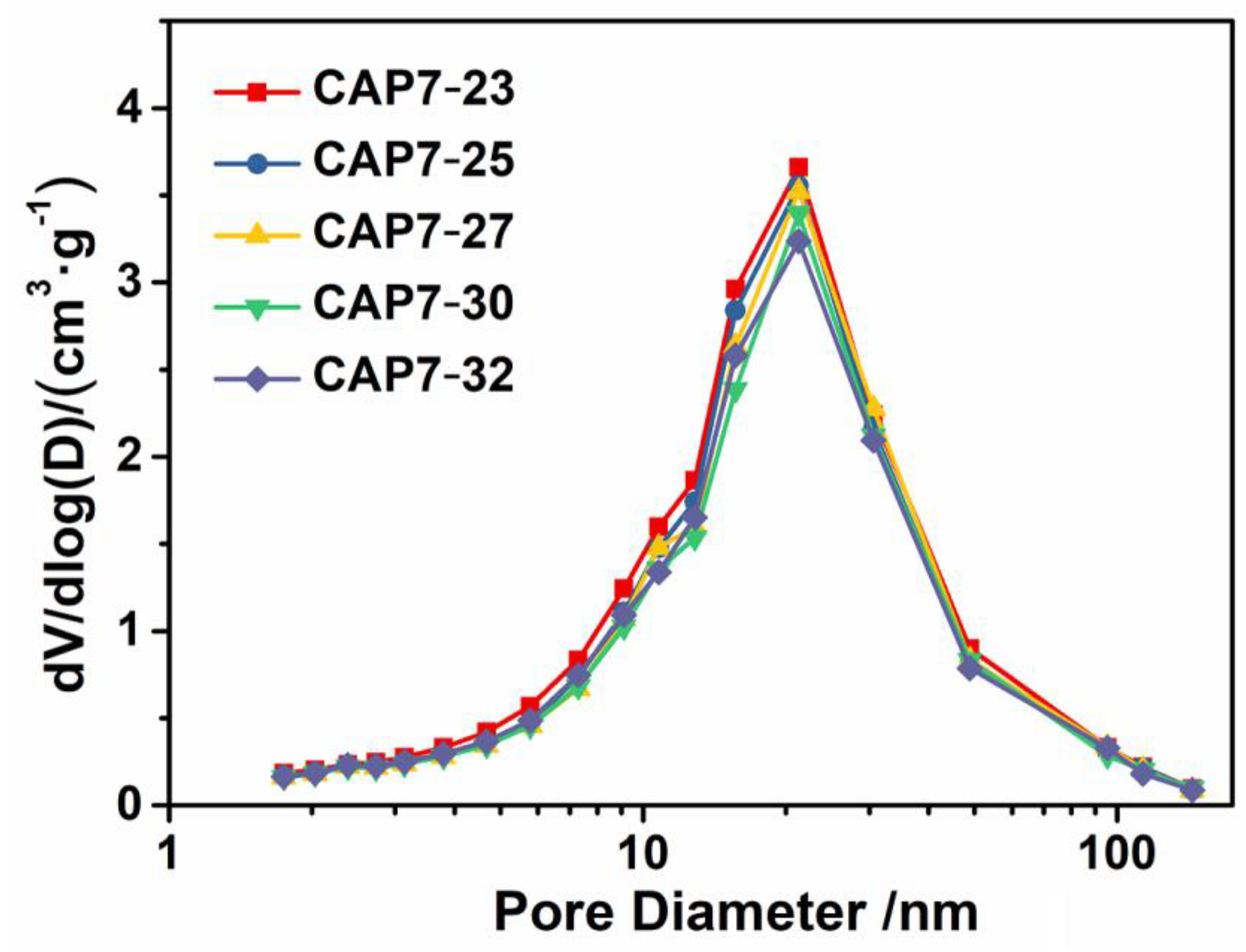
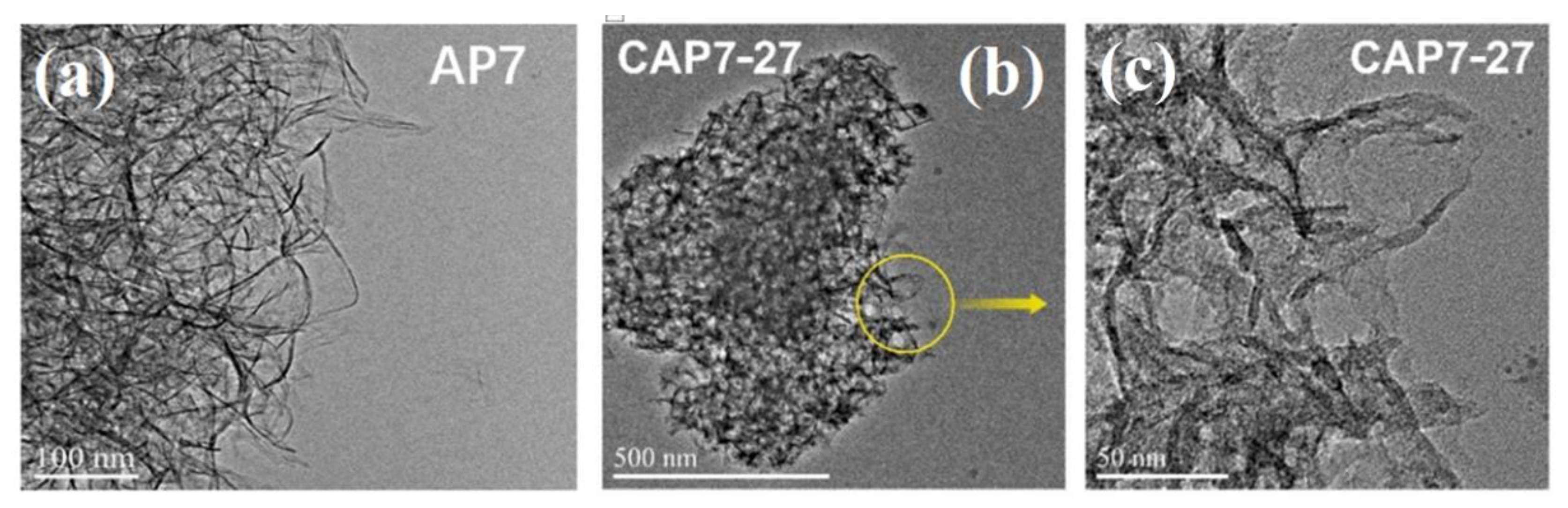
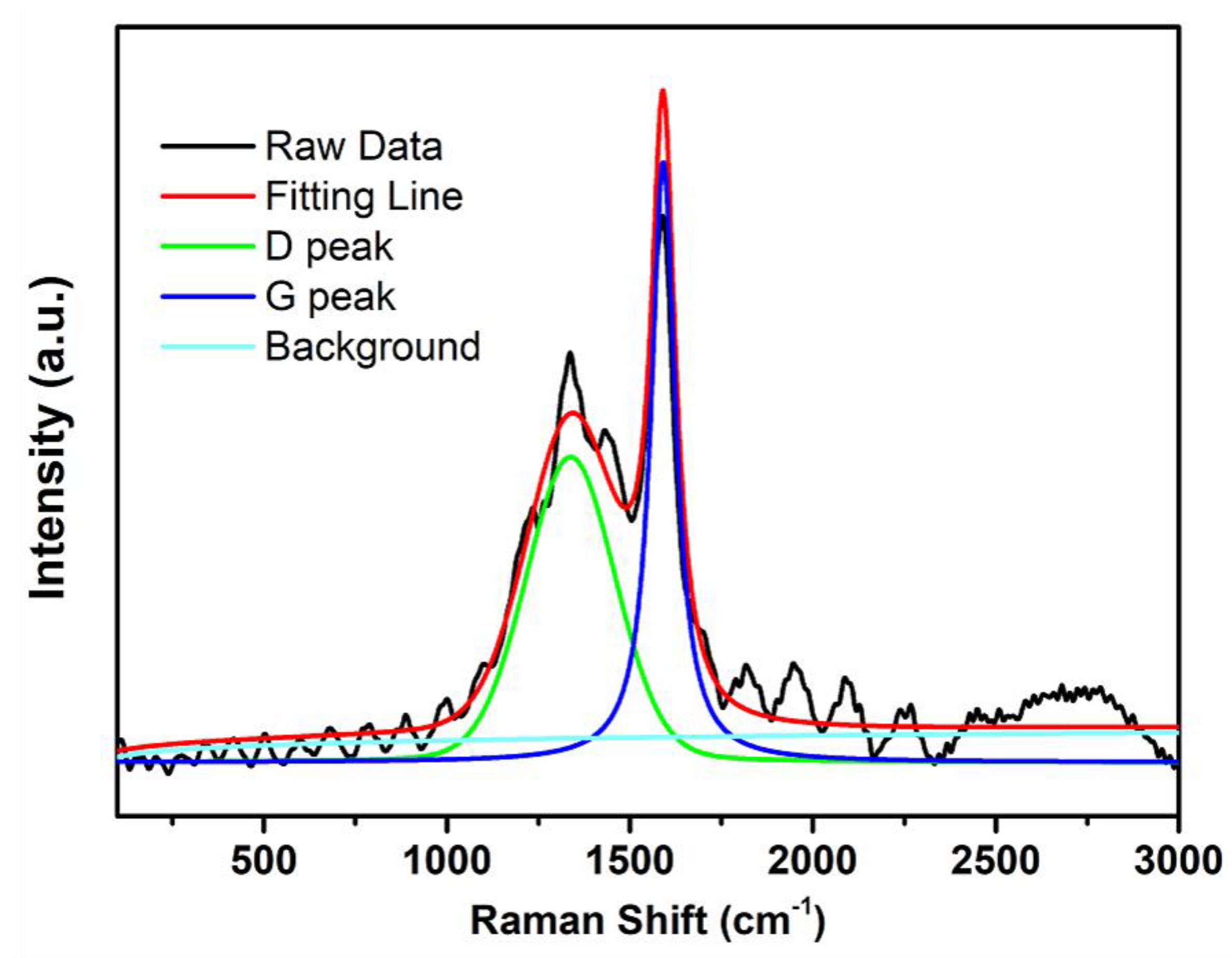
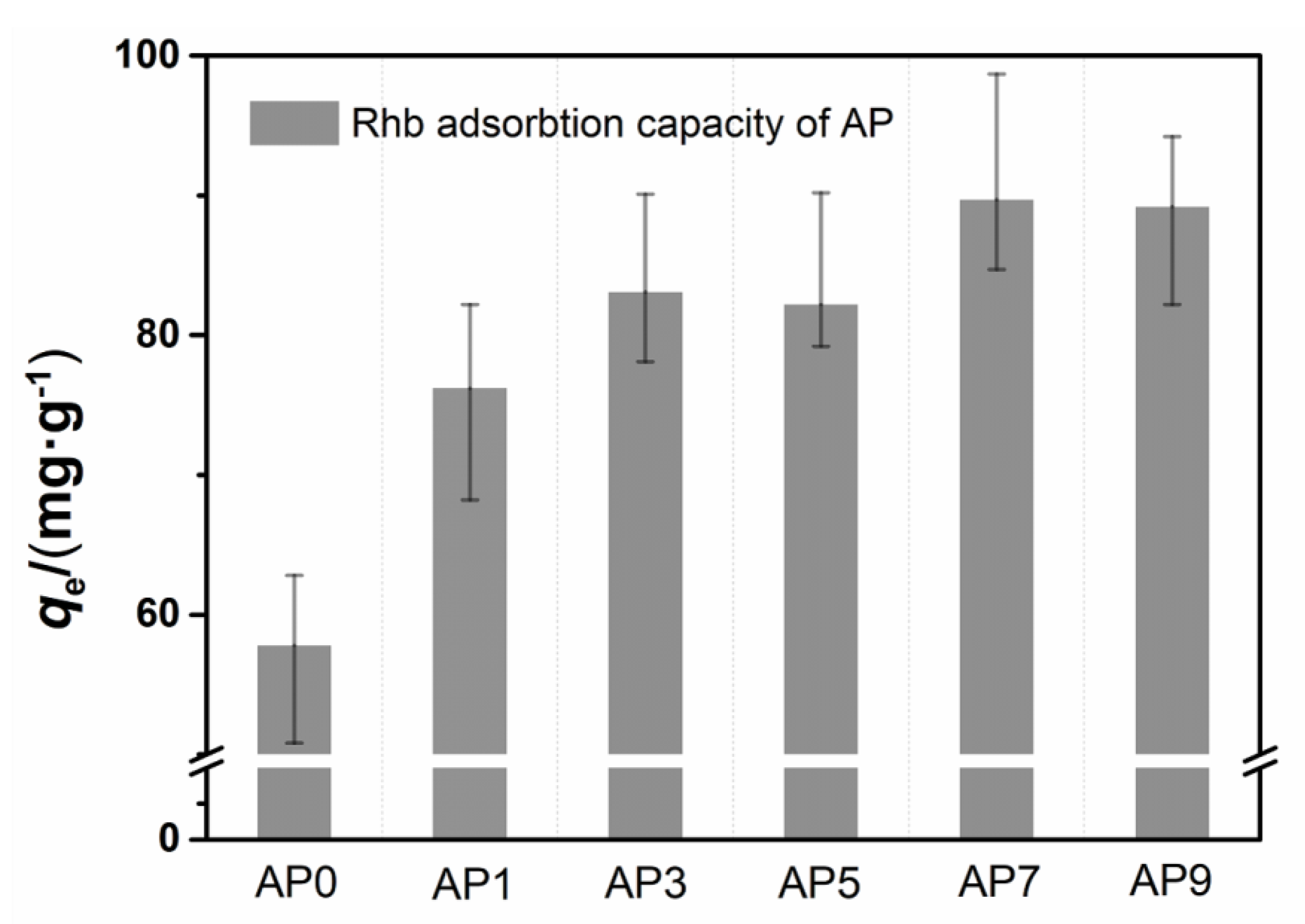
3.1. Characterizations of Carbon-Covered Phosphorus-Modified Alumina
3.2. Characterizations of Carbon-Covered Phosphorus-Modified Alumina
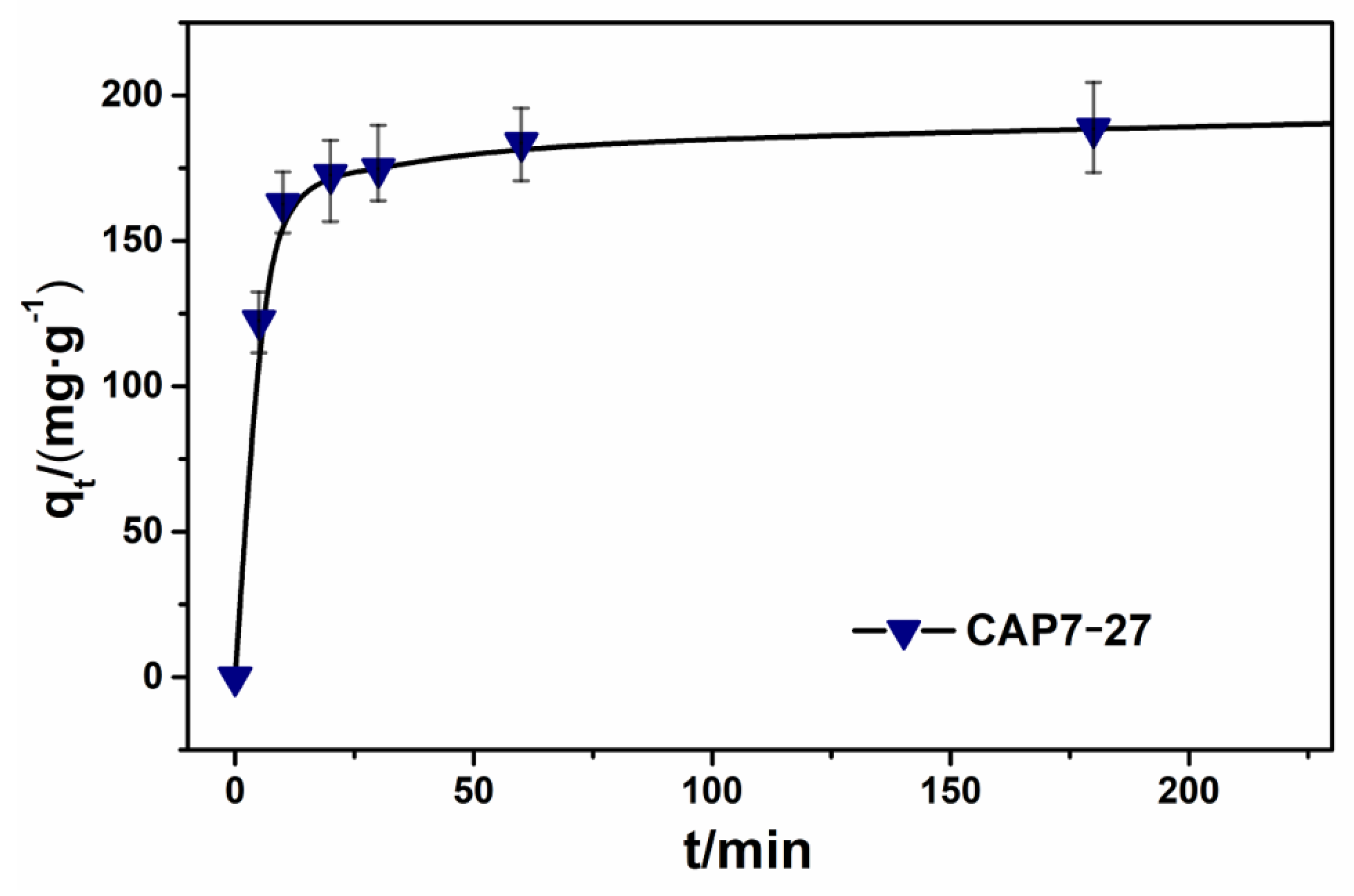
3.3. Adsorption Kinetic Studies
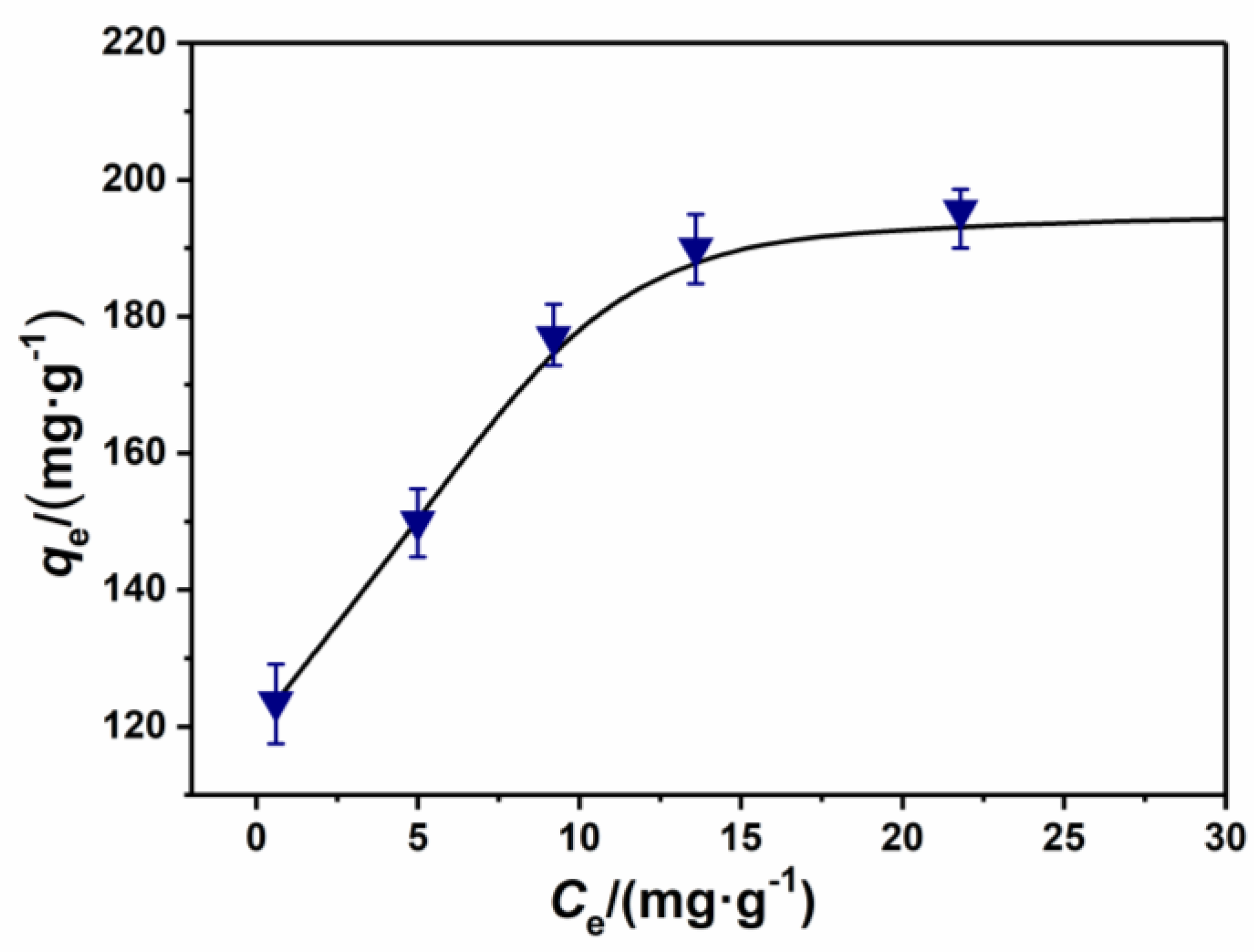
3.4. Adsorption Isotherm Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilhelm, P.; Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J. Photochem Photobiol. B 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Wu, J.M.; Zhang, T.W. Photodegradation of rhodamine B in water assisted by titania films prepared through a novel procedure. J. Photoch. Photobio. A 2004, 162, 171–177. [Google Scholar] [CrossRef]
- Yeamin, B.; Islam, M.M.; Chowdhury, A.; Awual, R. Efficient encapsulation of toxic dyes from wastewater using several biodegradable natural polymers and their composites. J. Clean. Prod. 2021, 291, 125920. [Google Scholar] [CrossRef]
- Shakir, K.; Elkafrawy, A.F.; Ghoneimy, H.F.; Beheir, S.G.E.; Refaat, M. Removal of rhodamine B (a basic dye) and thoron (an acidic dye) from dilute aqueous solutions and wastewater simulants by ion flotation. Water. Res. 2010, 44, 1449–1661. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.F.; Xie, J.M.; Jiang, D.L.; Yan, Z.X.; Jing, J.; Liu, D. Enhanced adsorption of hydroxyl contained/anionic dyes on non functionalized Ni@SiO2, core–shell nanoparticles: Kinetic and thermodynamic profile. Appl. Surf. Sci. 2014, 292, 301–310. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Robinson, T.; Mcmullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 47. [Google Scholar] [CrossRef]
- Jing, L.Q.; Sun, X.J.; Shang, J.; Cai, W.M.; Xu, Z.L.; Du, Y.G.; Fu, H.G. Review of surface photovoltage spectra of nano-sized semiconductor and its applications in heterogeneous photocatalysis. Sol. Energ. Mat. Sol. C. 2003, 79, 133–151. [Google Scholar]
- Matthews, R.W. Photooxidative degradation of coloured organics in water using supported catalysts. TiO2 on sand. Water. Res. 1991, 25, 1169–1176. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hasan, M.N.; Awual, M.R.; Islam, M.M.; Iqbal, J. Biodegradable natural carbohydrate polymeric sustainable adsorbents for efficient toxic dye removal from wastewater. J. Mol. Liq. 2020, 319, 114356. [Google Scholar]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Constapel, M.; Schellenträger, M.; Marzinkowski, J.M. Degradation of reactive dyes in wastewater from the textile industry by ozone: Analysis of the products by accurate masses. Water. Res. 2009, 43, 733–743. [Google Scholar] [CrossRef]
- Zhang, L.J.; Su, Z.X.; Jiang, F.L.; Yang, L.L.; Qian, J.J.; Zhou, Y.F.; Li, W.M.; Hong, M.C. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions. Nanoscale 2014, 6, 6590–6602. [Google Scholar] [CrossRef] [PubMed]
- Hema, M.; Arivoli, S. Rhodamine B adsorption by activated carbon: Kinetic and equilibrium studies. Indian J. Chem. Technol. 2009, 16, 8–45. [Google Scholar]
- Kaur, S.; Walia, T.P.S.; Kansal, I. Removal of Rhodamine-B by adsorption on walnut shell charcoal. J. Surface. Sci. Technol. 2008, 24, 179–193. [Google Scholar]
- Kadirvelu, K.; Karthika, C.; Vennilamani, N. Activated carbon from industrial solid waste as an adsorbent for the removal of Rhodamine-B from aqueous solution: Kinetic and equilibrium studies. Chemosphere 2005, 60, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, A.J.; Balakrishnan, V.; Arivoli, S. Kinetic and equilibrium studies of Rhodamine B adsorption by low cost activated carbon. Arch. Appl. Sci. Res. 2011, 3, 154–166. [Google Scholar]
- Ramakrishna, K.R.; Viraraghavan, T. Dye removal using low cost adsorbents. Water Sci. Technol. 1997, 36, 189–196. [Google Scholar] [CrossRef]
- Nigam, P.; Banat, I.M.; Singh, D.; Marchant, R. Microbial decolourization of textile-dye-containing effluents. Bioresour. Technol. 1996, 5, 217–227. [Google Scholar]
- Ding, L.L.; Zou, B.; Gao, W.; Liu, Q.; Wang, Z.C.; Guo, Y.P.; Gou, W.F.; Wang, X.F.; Liu, Y.H.; Marchant, R. Microbial decolorization of textile-dye containing effluents: A review. Colloid. Surface. A 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Namasivayam, C.; Radhika, R.; Suba, S. Uptake of dyes by a promising locally available agricultural solid waste: Coir pith. Waste Manag. 2001, 21, 381–387. [Google Scholar] [CrossRef]
- Rehman, R.; Mahmud, T.; Anwar, J.; Salman, M.; Ali, F. Removal of alizarin red s (dye) from aqueous media by using alumina as an adsorbent. J. Chem. Soc. Pakistan 2011, 33, 228–232. [Google Scholar]
- Banerjee, S.; Gautam, R.K.; Jaiswal, A.; Chattopadhyaya, M.C.; Chandra Sharma, Y. Rapid scavenging of methylene blue dye from aqueous solutions by adsorption on nanoalumina. Rsc. Adv. 2015, 5, 14425–14440. [Google Scholar] [CrossRef]
- Lin, L. Studies on Novel Carbon/Oxide Composites. Ph. D. Thesis, Peking University, Beijing, China, 2007. [Google Scholar]
- Hasan, M.M.; Shenashen, M.A.; Hasan, M.N.; Znad, H.; Salman, M.S.; Awual, M.R. Natural biodegradable polymeric bioadsorbents for efficient cationic dye encapsulation from wastewater. J. Mol. Liq. 2021, 323, 114587. [Google Scholar] [CrossRef]
- Song, J.Q.; Li, Z.H.; Xu, X.Y.; He, M.Y.; Li, Z.F.; Wang, Q.; Yan, L.J. Organic-free Synthesis of Boehmite Nanofibers by Al2(SO4)3·18H2O with High Pore Volume. Ind. Eng. Chem. Res. 2013, 52, 7752–7757. [Google Scholar] [CrossRef]
- Nascimento, R.C.S.; Silva, A.O.S.; Meili, L. Carbon-covered mesoporous silica and its application in Rhodamine B adsorption. Environ. Technol. 2017, 39, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.L.; Li, Y.X.; Liu, Z. Fabrication of graphene oxide/silicalite-1 composites with hierarchical porous structure and investigation on their adsorption performance for rhodamine B. J. Ind. Eng. Chem. 2017, 55, 234–243. [Google Scholar] [CrossRef]
- Wang, J.; Tsuzuki, T.; Tang, B.; Hou, X.; Sun, L.; Wang, X. Reduced graphene oxide/ZnO composite: Reusable adsorbent for pollutant management. ACS Appl. Mater Interfaces 2012, 4, 3084–3090. [Google Scholar] [CrossRef]
- Mishra, A.K.; Arockiadoss, T.; Ramaprabhu, S. Study of removal of azo dye by functionalized multi walled carbon nanotubes. Chem. Eng. J. 2010, 162, 1026–1034. [Google Scholar] [CrossRef]
- Qin, Y.L.; Long, M.C.; Tan, B.H.; Zhou, B.X. RhB Adsorption performance of magnetic adsorbent Fe3O4/RGO composite and its regeneration through a Fenton-like reaction. Nano-Micro. Lett. 2014, 6, 125–135. [Google Scholar] [CrossRef]
- Sun, J.C.; Gao, A.W.; Wang, X.H.; Xu, X.Y.; Song, J.Q. Removal of phosphorus from wastewater by different morphological alumina. Molecules 2020, 25, 3092. [Google Scholar] [CrossRef]
- Naoufal, B.; Tadahiko, W. New sol–gel route for the preparation of pure α-alumina at 950 °C. J. Am. Ceram. Soc. 2010, 83, 2324–2326. [Google Scholar]
- Macêdo, M.I.F.; Osawa, C.C.; Bertran, C.A. Sol-gel synthesis of transparent alumina gel and pure gamma alumina by urea hydrolysis of alumina nitrate. J. Sol-gel Sci. Tech. 2004, 30, 135–140. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.C.; Zhu, Y.X.; Xie, Y.C. Applications of monolayer-dispersed organic compounds in the preparation of related materials. Acta Physico-Chimica Sin. 2012, 28, 2327–2335. [Google Scholar]
- Li, L.; Liu, S.X.; Zhu, T. Application of actived carbon derived from scrap tires for adsorption of Rhodamine B. J. Environ. Sci. 2007, 85, 956–964. [Google Scholar]
- Mittal, H.; Mishra, S.B. Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohyd. Polym. 2014, 101, 1255–1264. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, J.H.; Chen, Y.N.; Hu, Y.Y. Enhanced adsorption performance of MoS2 nanosheet-coated MIL-101 hybrids for the removal of aqueous rhodamine B. J. Colloid Interface Sci. 2017, 504, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Zeinali, N.; Ghaedi, A.M.; Teimuori, M.; Tashkhourian, J. Artificial neural network-genetic algorithm based optimization for the adsorption of methylene blue and brilliant green from aqueous solution by graphite oxide nanoparticle. Spectrochim. Acta Part A 2014, 125, 264–277. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.G.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Lin, Z.J.; Wang, Z.G.; Lin, L.Y. Formation, Structure and Fluorescence of CdS Clusters In A Mesoporous Zeolite. Sol. Stat. Commun. 1998, 105, 129–134. [Google Scholar] [CrossRef]
- Chen, W.; Lin, Z.J.; Wang, Z.G.; Qian, J.J.; Lin, L.Y. Some New Observation On The Formation and Optical Properties of CdS Clusters In Zeolite-Y. Sol. Stat. Commun. 1996, 100, 101–104. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.H.; Huang, Y.N. Luminescence Enhancement of EuS Nanoclusters In USY-Zeolite. Appl. Phys. Lett. 2000, 76, 2328–2330. [Google Scholar] [CrossRef]
- Wang, W.; Guo, R.; Xiong, X.H.; Liu, H.; Chen, W.; Hu, S.M.; Amador, E.; Chen, B.J.; Zhang, X.H.; Wang, L. Improved Stability and Efficiency of Perovskite Via a Simple Solid Diffusion Method, Mater. Today Phys. 2021, in press. [Google Scholar]

| Adsorbent | Adsorption Capacity (mg·g−1) | References |
|---|---|---|
| Alumina | 3.6 | [24] |
| Carbon-Covered alumina | 47.9 | [24] |
| Adsorbent of wheat flour | 142.3 | [25] |
| Magnesium silicate/carbon composite | 244 | [27] |
| Graphene oxide/silicalite-1 composite | 57.0 | [28] |
| Carbon nanotubes | 69.0 | [29] |
| Zn/Co ZIFs-derived carbon | 116.2 | [30] |
| Fe3O4/rGO | 142.9 | [31] |
| Carbon-Covered phosphorus-modified alumina | 198.0 | This work |
| Sample | Specific Surface Area (m²·g−1) | Pore Volume (cm3·g−1) | Most Probable Aperture (nm) |
|---|---|---|---|
| AP0 | 307.4 | 0.96 | 11.8 |
| AP1 | 356.1 | 1.17 | 16.9 |
| AP3 | 453.6 | 2.88 | 20.1 |
| AP5 | 467.8 | 3.01 | 21.2 |
| AP7 | 496.2 | 3.03 | 21.9 |
| AP9 | 517.9 | 2.99 | 21.0 |
| Sample | Specific Surface Area (m²·g−1) | Pore Volume (cm3·g−1) | Most Probable Aperture (nm) |
|---|---|---|---|
| CAP7–23 | 470.1 | 2.65 | 21.3 |
| CAP7–25 | 440.0 | 2.46 | 21.2 |
| CAP7–27 | 435.3 | 2.43 | 21.2 |
| CAP7–30 | 436.3 | 2.37 | 21.3 |
| CAP7–32 | 441.9 | 2.34 | 21.0 |
| Adsorbent | qe (mg·g−1) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||
|---|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g·mg−1·min−1) | R2 | ||
| CAP7–27 | 197.3 | 0.0075 | 0.9574 | 0.0017 | 0.9997 |
| Adsorbent | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| qm (mg·g−1) | KL (L·mg−1) | R2 | KF (mg·g−1)(L·mg−1)1/n | 1/n | R2 | |
| CAP7–27 | 195.3 | 1.28 | 0.9983 | 8.298 | 0.1308 | 0.9513 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, X.; Tong, W.; Sun, J.; Xu, X.; Song, J.; Gong, J.; Chen, W. Preparation of Carbon-Covered Phosphorus-Modified Alumina with Large Pore Size and Adsorption of Rhodamine B. Nanomaterials 2021, 11, 799. https://doi.org/10.3390/nano11030799
Chen S, Wang X, Tong W, Sun J, Xu X, Song J, Gong J, Chen W. Preparation of Carbon-Covered Phosphorus-Modified Alumina with Large Pore Size and Adsorption of Rhodamine B. Nanomaterials. 2021; 11(3):799. https://doi.org/10.3390/nano11030799
Chicago/Turabian StyleChen, Shuaiqi, Xuhui Wang, Weiyi Tong, Jianchuan Sun, Xiangyu Xu, Jiaqing Song, Jianyi Gong, and Wei Chen. 2021. "Preparation of Carbon-Covered Phosphorus-Modified Alumina with Large Pore Size and Adsorption of Rhodamine B" Nanomaterials 11, no. 3: 799. https://doi.org/10.3390/nano11030799
APA StyleChen, S., Wang, X., Tong, W., Sun, J., Xu, X., Song, J., Gong, J., & Chen, W. (2021). Preparation of Carbon-Covered Phosphorus-Modified Alumina with Large Pore Size and Adsorption of Rhodamine B. Nanomaterials, 11(3), 799. https://doi.org/10.3390/nano11030799






