Synthesis of Highly Active Pd@Cu–Pt/C Methanol Oxidation Electrocatalysts via Continuous, Co-Electroless Deposition
Abstract
1. Introduction
2. Materials and Methods
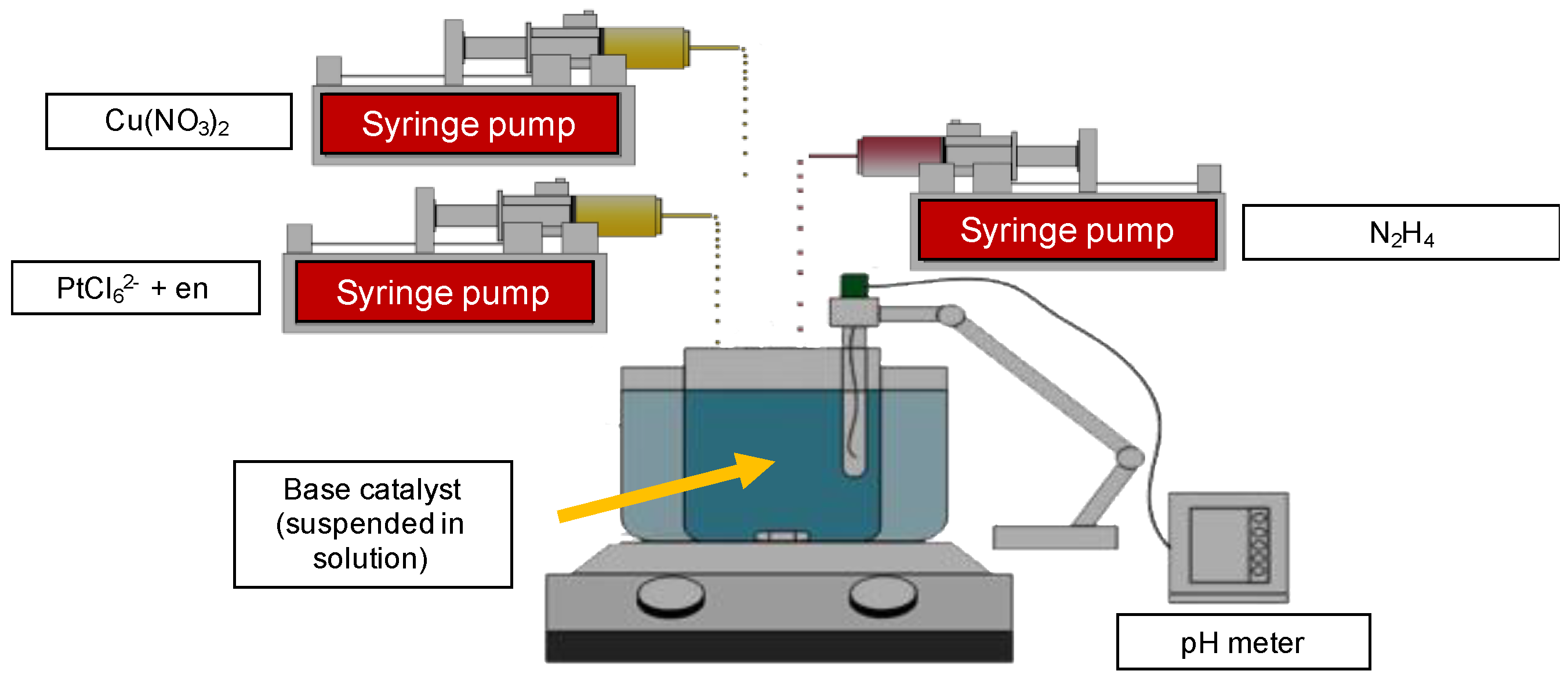
2.1. Catalyst Preparation
2.2. Characterization
2.3. Cyclic Voltammetry
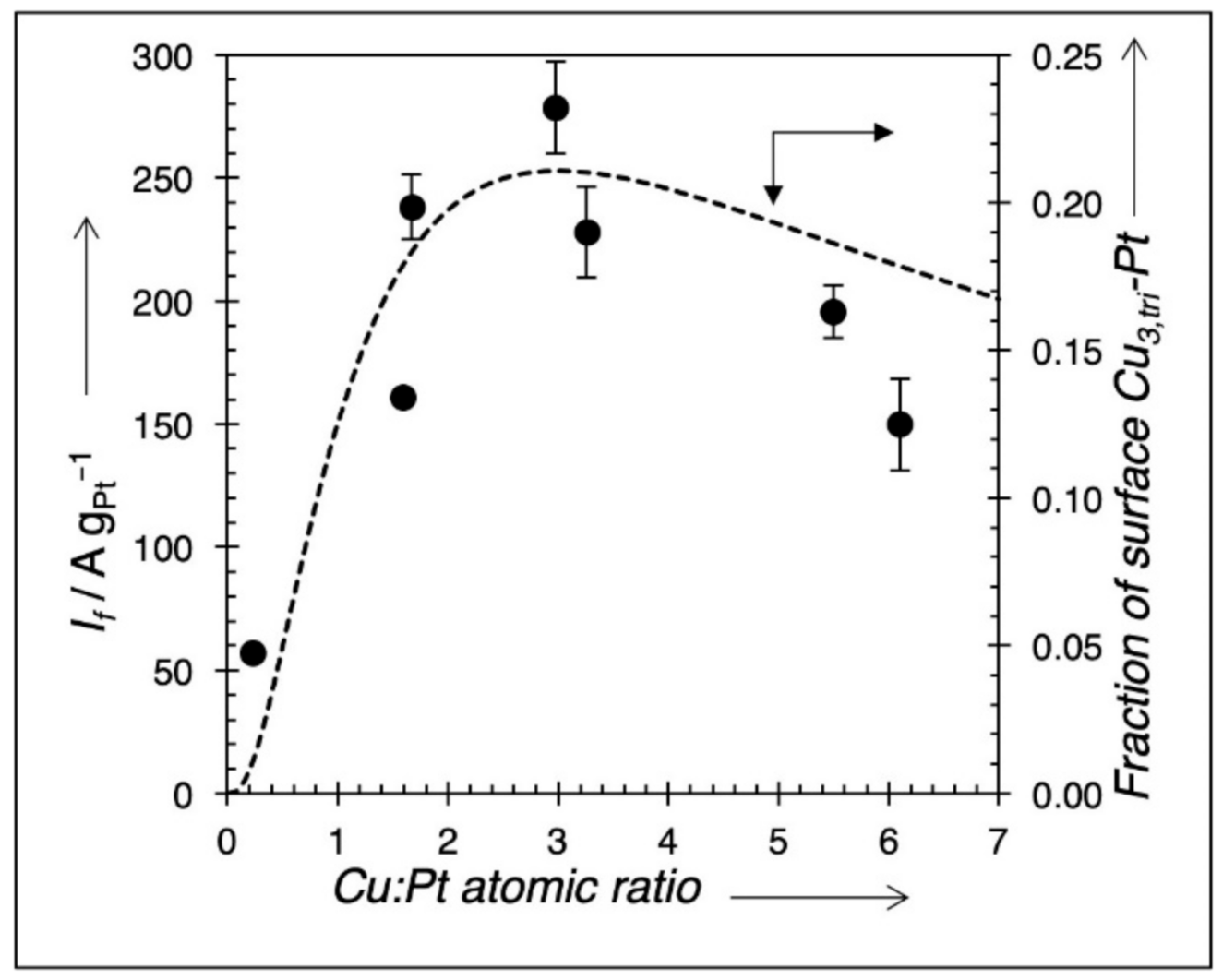
3. Results
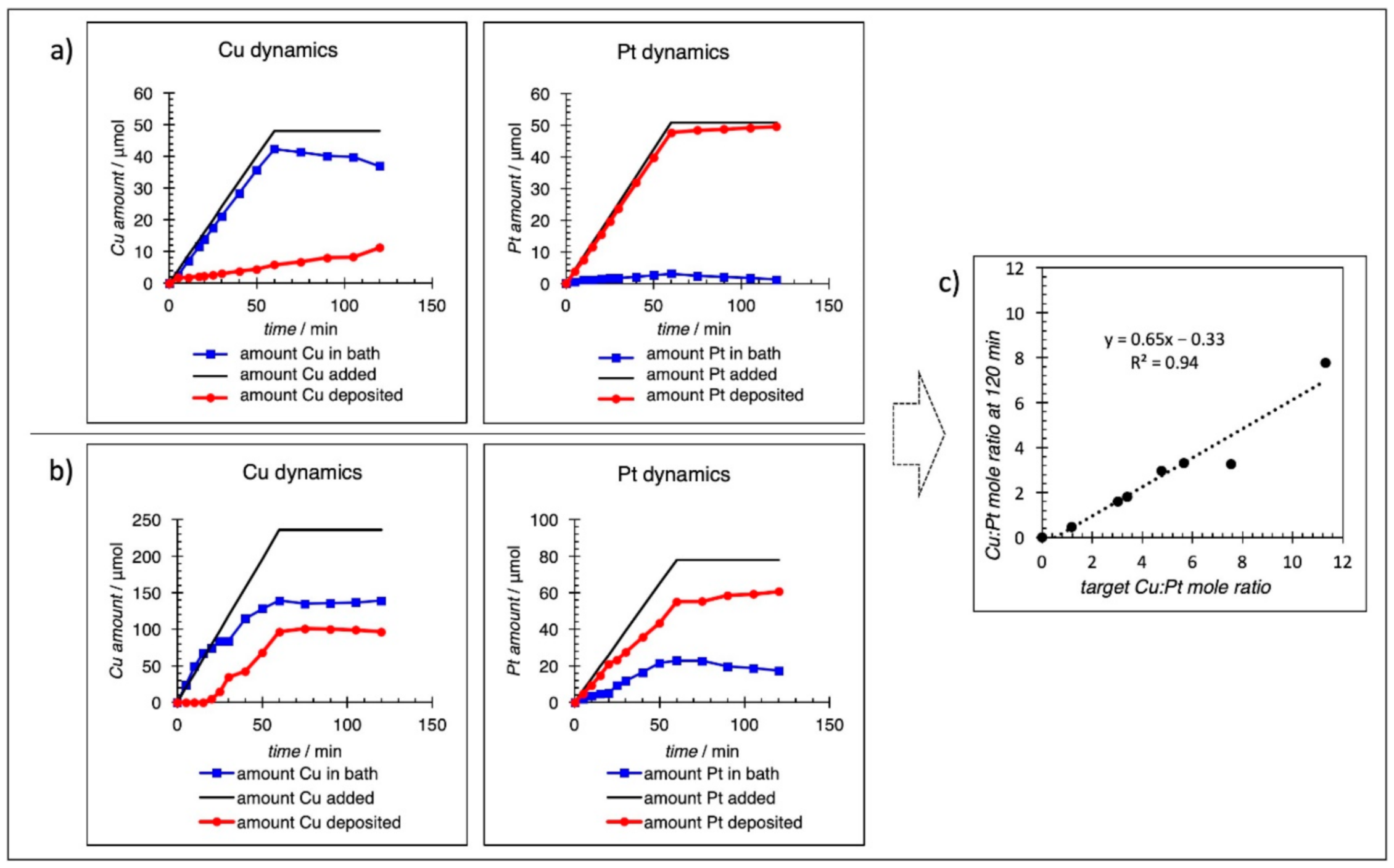
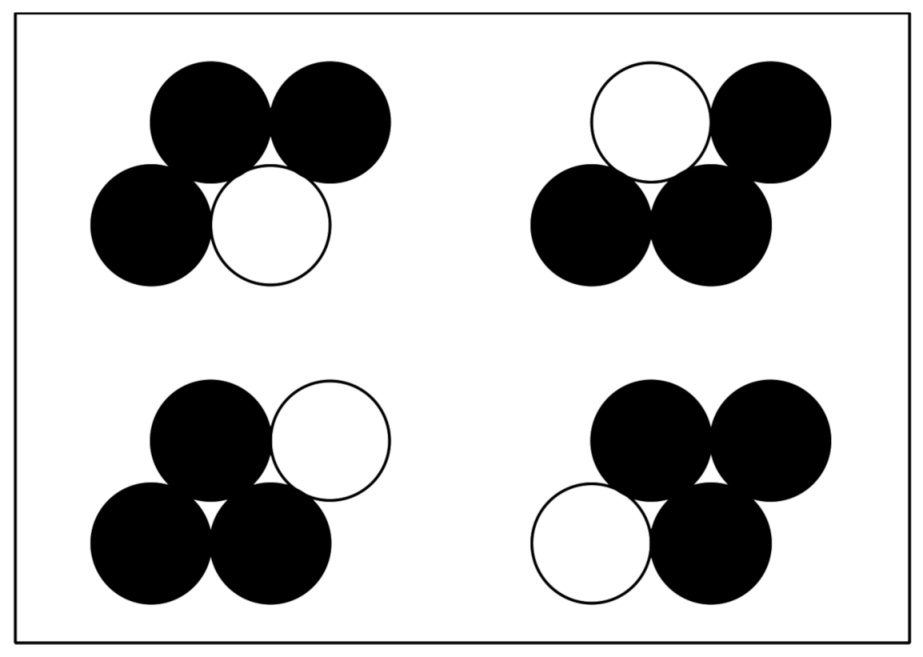
3.1. Preparation/Synthesis
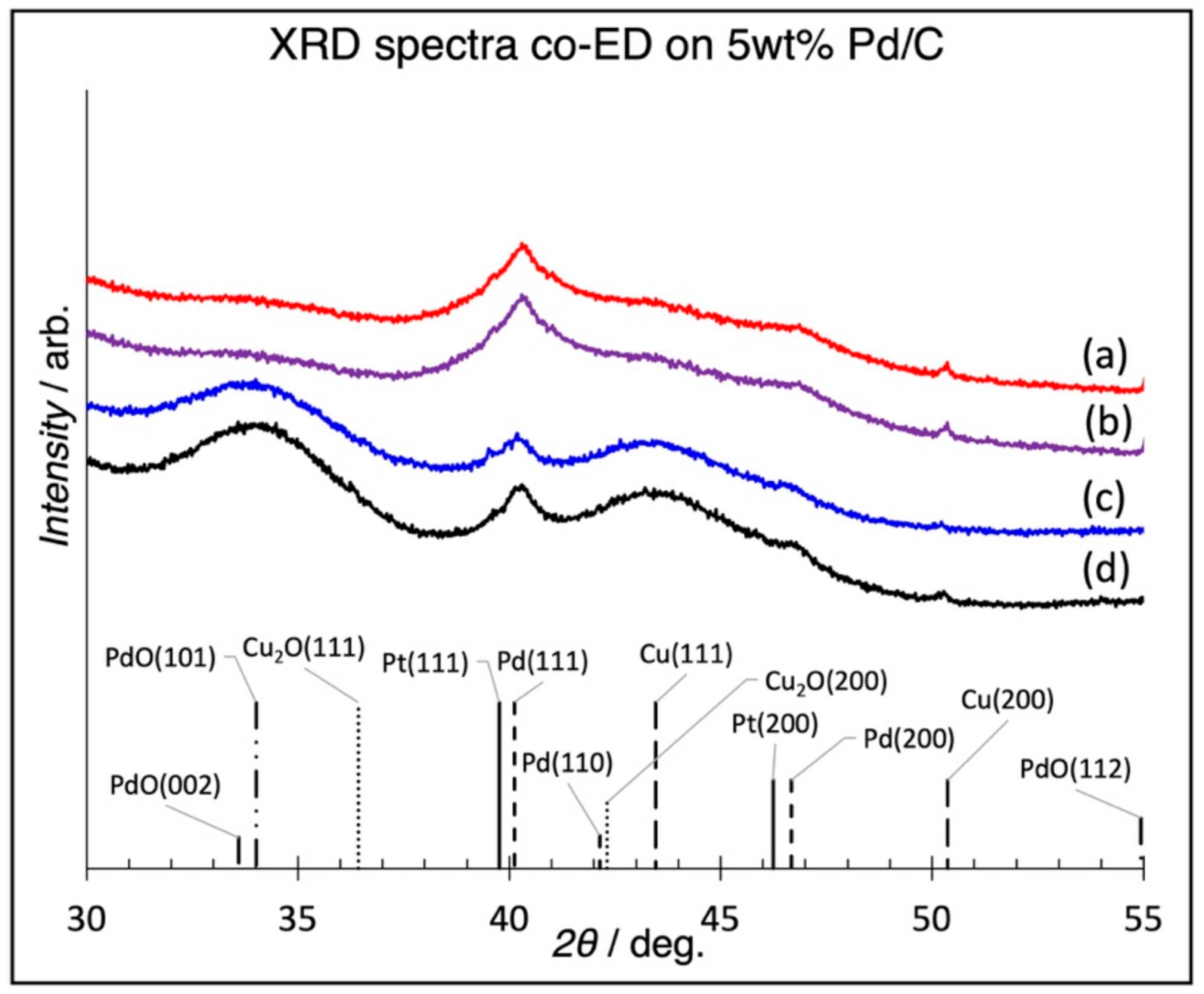
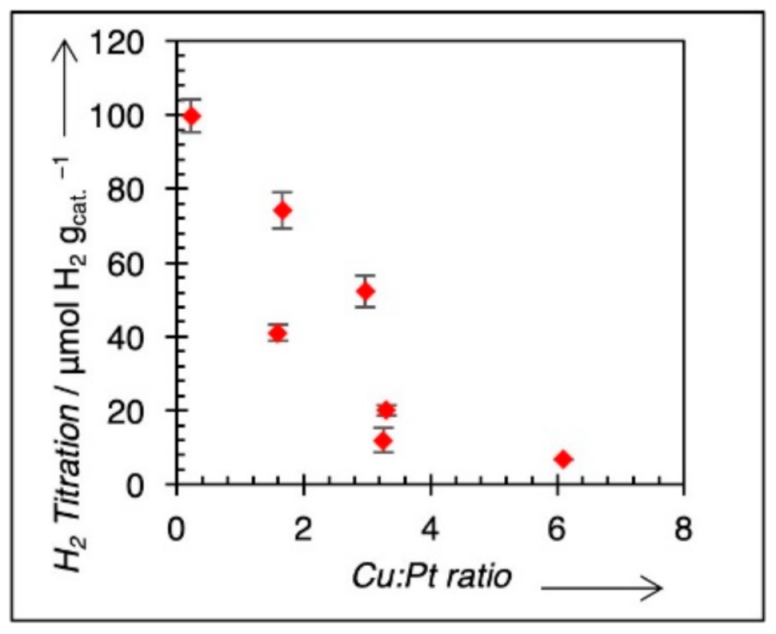
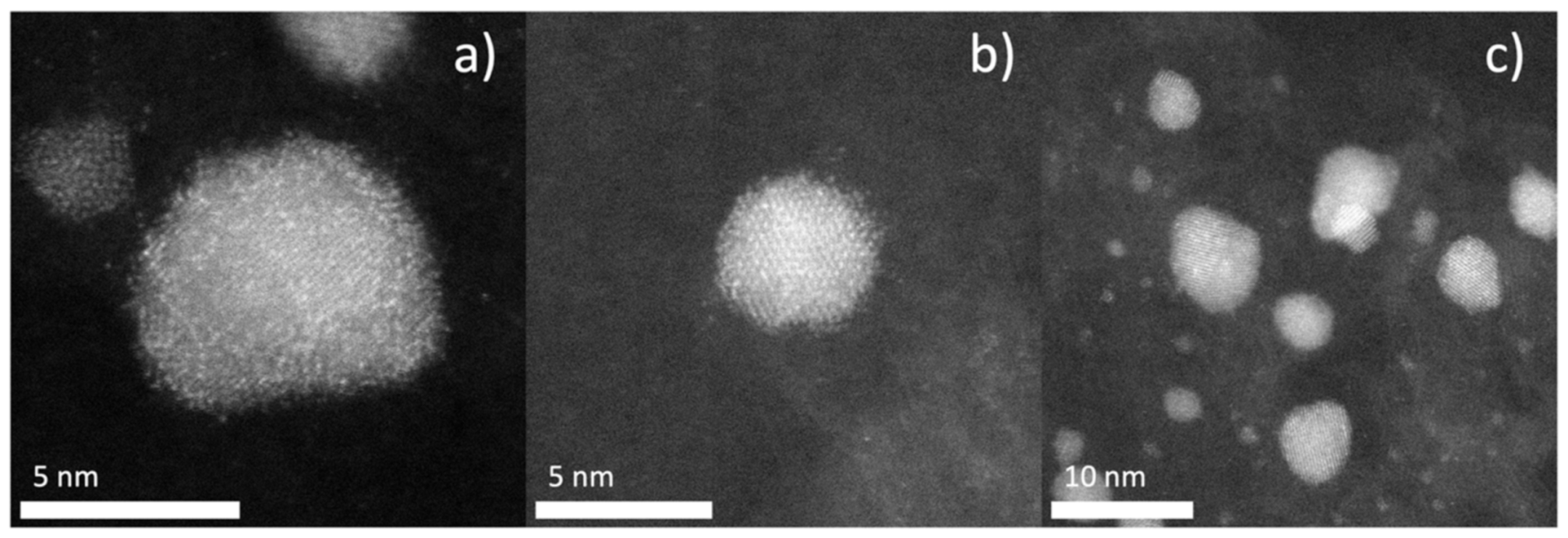
3.2. Physicochemical Characterization
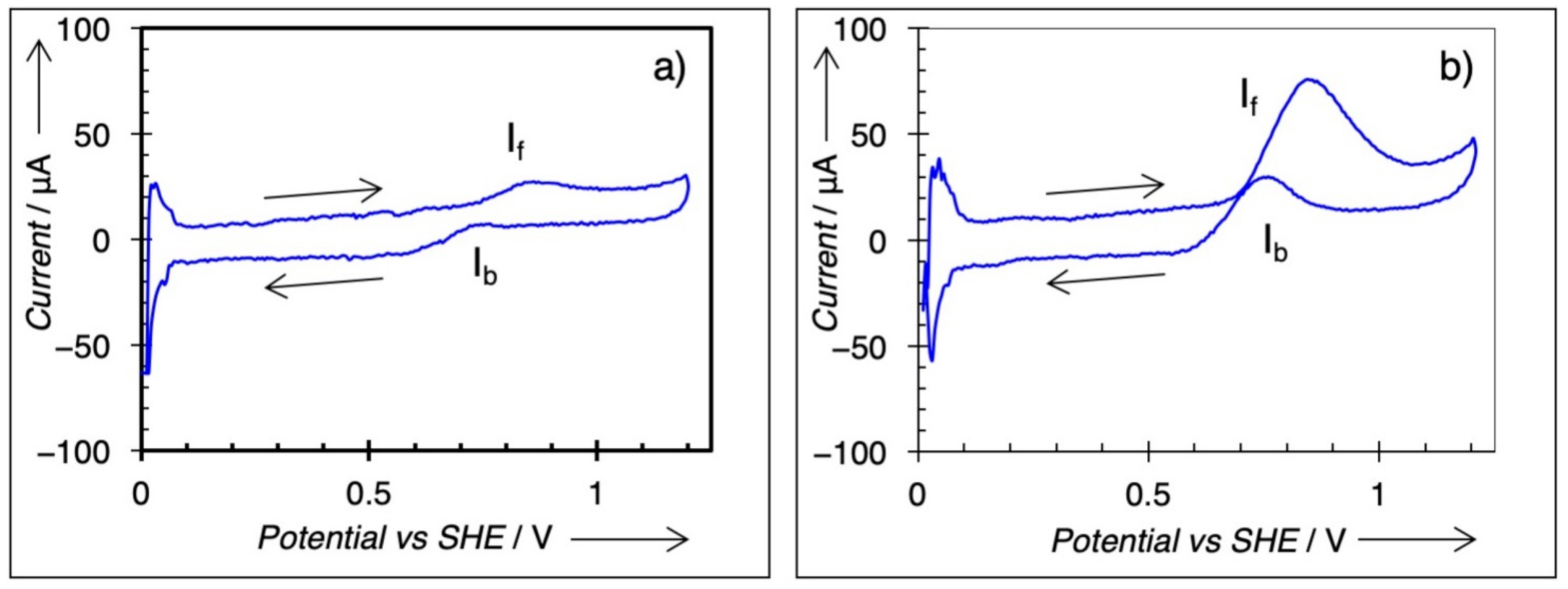
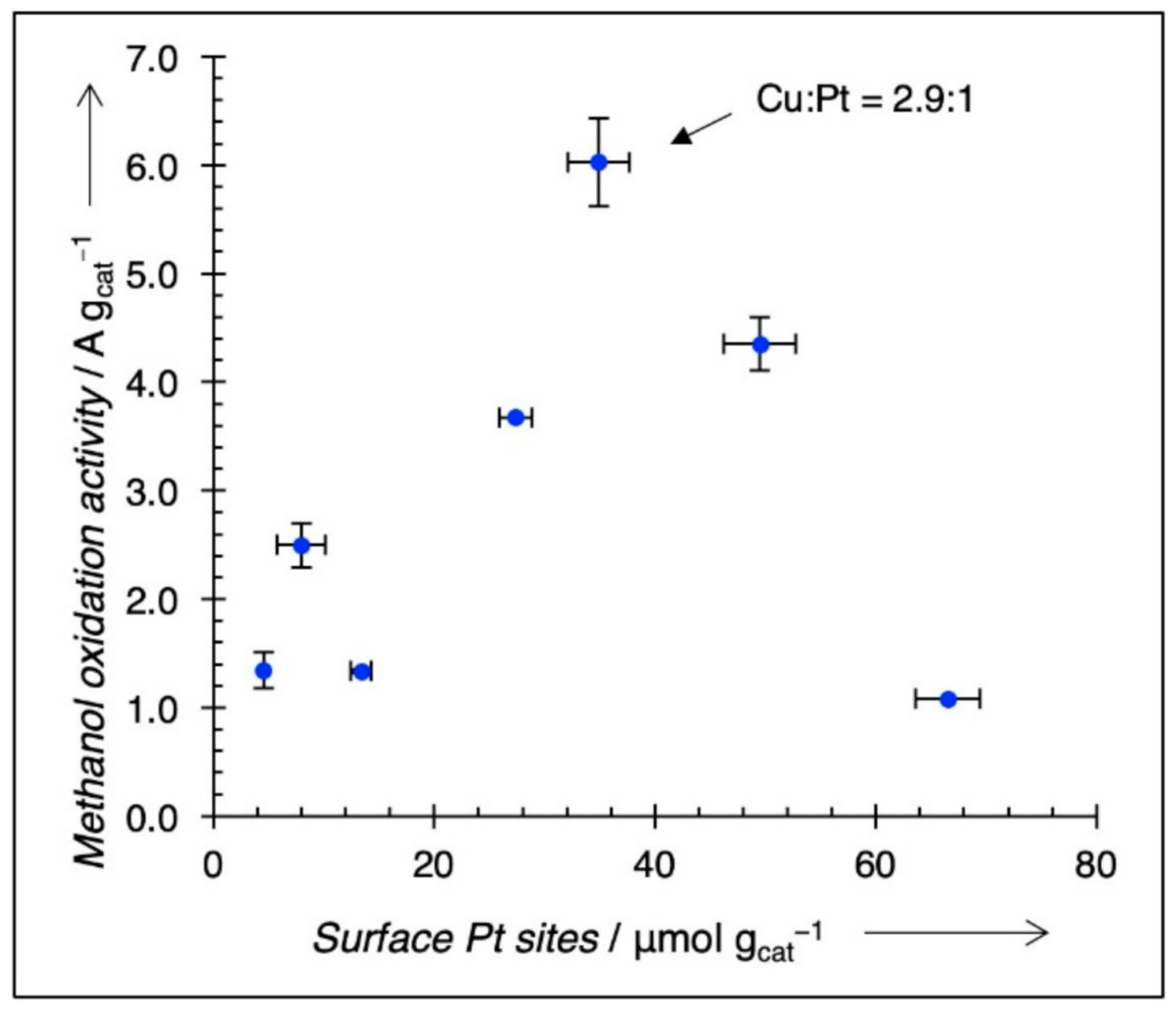
3.3. Electrochemical Characterization
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koppel, T. Powering the Future: The Ballard Fuel Cell and the Race to Change the World; John and Wiley and Sons Canada Ltd: Mississauga, ON, Canada, 1999. [Google Scholar]
- Poling, B.E.; Thomson, G.H.; Friend, D.G.; Rowley, R.L.; Wilding, W.V. Perry’s Chemical Engineers’ Handbook, 8th ed.; Green, D.W., Perry, R.H., Eds.; McGraw-Hill: New York, NY, USA, 2008; pp. 2–198. [Google Scholar]
- Chung, D.Y.; Lee, K.-J.; Sung, Y.-E. Methanol Electro-oxidation on Pt Surface: Revisiting the Cyclic Volatammetry Intrepretation. J. Phys. Chem. C 2010, 120, 9028–9035. [Google Scholar] [CrossRef]
- Day, J.B.; Vuissoz, P.-A.; Oldfield, E.; Wieckowski, A.; Ansermet, J.-P. Nuclear Magnetic Resonance Spectroscopic Study of the Electrochemical Oxidation Product of Methanol on Platinum Black. J. Am. Chem. Soc. 1996, 118, 13046–13050. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Ferrin, P.; Tritsaris, G.A.; Nilekar, A.U.; Koh, S.; Bae, S.E.; Brankovic, S.R.; Strasser, P.; Mavrikakis, M. Bifunctional anode catalysts for direct methanol fuel cells. Energy Environ. Sci. 2012, 5, 8335. [Google Scholar] [CrossRef]
- Tritsaris, G.A.; Rossmeisl, J. Methanol Oxidation on Model Elemental and Bimetallic Transition Metal Surfaces. J. Phys. Chem. C 2012, 116, 11980–11986. [Google Scholar] [CrossRef]
- Lai, S.C.S.; Lebedeva, N.P.; Housmans, T.H.M.; Koper, M.T.M. Mechanisms of Carbon Monoxide and Methanol Oxidation at Single-crystal Electrodes. Top. Catal. 2007, 46, 320–333. [Google Scholar] [CrossRef]
- Schlapka, A.; Lischka, M.; Groß, A.; Käsberger, U.; Jakob, P. Surface Strain versus Substrate Interaction in Heteroepitaxial Metal Layers: Pt on Ru(0001). Phys. Rev. Lett. 2003, 91, 016101. [Google Scholar] [CrossRef]
- Grabow, L.; Xu, Y.; Mavrikakis, M. Lattice strain effects on CO oxidation on Pt(111). Phys. Chem. Chem. Phys. 2006, 8, 3369–3374. [Google Scholar] [CrossRef]
- Garrick, T.R.; Diao, W.; Tengco, J.M.; Stach, E.A.; Senanayake, S.D.; Chen, D.A.; Monnier, J.R.; Weidner, J.W. The Effect of the Surface Composition of Ru-Pt Bimetallic Catalysts for Methanol Oxidation. Electrochim. Acta 2016, 195, 106–111. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by ad-atoms: Part II. Enhancement of the oxidation of methanol on platinum by ruthenium ad-atoms. J. Electroanal. Chem. 1975, 60, 267–273. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; White, R.; Shakouri, A.; Regalbuto, J.R.; Weidner, J.W.; Monnier, J.R. Ruthenium-platinum bimetallic catalysts with controlled surface compositions and enhanced performance for methanol oxidation. Catal. Today 2019, 334, 156–161. [Google Scholar] [CrossRef]
- Diao, W.; Tengco, J.M.M.; Regalbuto, J.R.; Monnier, J.R. Preparation and Characterization of Pt–Ru Bimetallic Catalysts Synthesized by Electroless Deposition Methods. ACS Catal. 2015, 5, 5123–5134. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. Chapter One—A Review of Preparation Methods for Suported Metal Catalysts. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 61, pp. 1–35. [Google Scholar]
- Diao, W.; Tengco, J.M.M.; Gaffney, A.M.; Regalbuto, J.R.; Monnier, J.R. Rational synthesis of bimetallic catalysts using electroless deposition methods. In Catalysis; Spivey, J., Han, Y.F., Shekhawat, D., Eds.; Royal Society of Chemistry: London, UK, 2020; Volume 32, pp. 116–150. [Google Scholar]
- Beard, K.; Schaal, M.; van Zee, J.; Monnier, J.R. Preparation of highly dispersed PEM fuel cell catalysts using electroless deposition methods. Appl. Catal. B 2007, 72, 262–271. [Google Scholar] [CrossRef]
- Tengco, J.; Mehrabadi, B.T.; Zhang, Y.; Wongkaew, A.; Regalbuto, J.R.; Weidner, J.; Monnier, J.R. Synthesis and Electrochemical Evaluation of Carbon Supported Pt-Co Bimetallic Catalysts Prepared by Electroless Deposition and Modified Charge Enhanced Dry Impregnation. Catalysts 2016, 6, 83. [Google Scholar] [CrossRef]
- Wongkaew, A.; Zhang, Y.; Tengco, J.M.M.; Blom, D.A.; Sivasubramanian, P.; Fanson, P.T.; Regalbuto, J.R.; Monnier, J.R. Characterization and evaluation of Pt-Pd electrocatalysts prepared by electroless deposition. Appl. Catal. B 2016, 188, 367–375. [Google Scholar] [CrossRef]
- Ohashi, M.; Beard, K.D.; Ma, S.; Blom, D.A.; St-Pierre, J.; van Zee, J.W.; Monnier, J.R. Electrochemical and structural characterization of carbon-supported Pt–Pd bimetallic electrocatalysts prepared by electroless deposition. Electrochim. Acta 2010, 55, 7376–7384. [Google Scholar] [CrossRef]
- Galhenage, R.P.; Xie, K.; Diao, W.; Tengco, J.M.M.; Seuser, G.S.; Monnier, J.R.; Chen, D.A. Platinum–ruthenium bimetallic clusters on graphite: A comparison of vapor deposition and electroless deposition methods. Phys. Chem. Chem. Phys. 2015, 17, 28354–28363. [Google Scholar] [CrossRef]
- Xu, L.; Lin, J.; Bai, Y.; Mavrikakis, M. Atomic and Molecular Adsorption on Cu(111). Top. Catal. 2018, 61, 736–750. [Google Scholar] [CrossRef]
- Potekaev, A.I.; Klopotov, A.A.; Starostenkov, M.D.; Klopotov, V.D.; Markova, T.N.; Morozov, M.M. Structure-phase states in the Cu-Pd-Pt system. Steel Transl. 2013, 43, 184–187. [Google Scholar] [CrossRef]
- Nakahigashi, K. L11-Type Ordered Phase in Cu-Pt-Pd Ternary Alloys. Jpn. J. Appl. Phys. 1986, 25, 1284–1287. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, B.-J. Durability screening of Pt ternary alloy (111) surfaces for oxygen reduction reaction using Density Functional Theory. Surf. Interfaces 2020, 18, 100440. [Google Scholar] [CrossRef]
- Mitsui, K.; Takahashi, M. Effect of ternary addition on the formation of Cu3Pt and CuPt order phases in the Cu-Pt system. Philos. Mag. 1998, 77, 49–57. [Google Scholar] [CrossRef]
- Wang, B.; Tao, L.; Cheng, Y.; Yang, F.; Jin, Y.; Zhou, C.; Yu, H.; Yang, Y. Electrocatalytic Oxidation of Small Molecule Alcohols over Pt, Pd, and Au Catalysts: The Effect of Alcohol’s Hydrogen Bond Donation Ability and Molecular Structure Properties. Catalysts 2019, 9, 387. [Google Scholar] [CrossRef]
- Capon, A.; Parsons, R. The oxidation of formic acid on noble metal electrodes: II. A comparison of the behaviour of pure electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1973, 44, 239–254. [Google Scholar] [CrossRef]
- Kadirgan, F.; Beden, B.; Leger, J.M.; Lamy, C. Synergistic effect in the electrocatalytic oxidation of methanol on platinum+palladium alloy electrodes. J. Electroanal. Chem. 1981, 125, 89–103. [Google Scholar] [CrossRef]
- Vecchio, C.L.; Alegre, C.; Sabastián, D.; Stassi, A.; Aricò, A.S.; Baglio, V. Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance. Materials 2015, 8, 7997–8008. [Google Scholar] [CrossRef]
- Tate, G.L.; Kenvin, A.; Diao, W.; Monnier, J.R. Preparation of Pt-containing bimetallic and trimetallic catalysts using continuous electroless deposition methods. Catal. Today 2019, 334, 113–121. [Google Scholar] [CrossRef]
- Ohno, I.; Wakabayashi, O.; Haruyama, S. Anodic Oxidation of Reductants in Electroless Plating. J. Electrochem. Soc. 1985, 132, 2323–2330. [Google Scholar] [CrossRef]
- Djokić, S.S. Modern Aspects of Electrochemistry; Conway, B.E., White, R.E., Eds.; Springer: Boston, MA, USA, 2002; Volume 35, pp. 51–133. [Google Scholar]
- Benson, J.E.; Boudart, M. Hydrogen-oxygen titration method for the measurement of supported platinum surface areas. J. Catal. 1965, 4, 704–710. [Google Scholar] [CrossRef]
- Vannice, M.A.; Benson, J.E.; Boudart, M. Determination of surface area by chemisorption: Unsupported platinum. J. Catal. 1970, 16, 348–356. [Google Scholar] [CrossRef]
- Meites, L. Handbook of Analytical Chemistry; Meites, L., Ed.; McGraw-Hill: New York, NY, USA, 1963; p. 13. [Google Scholar]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- O’Connell, K.; Regalbuto, J.R. High Sensitivity Silicon Slit Detectors for 1 nm Powder XRD Size Detection Limit. Catal. Lett. 2015, 145, 777–783. [Google Scholar] [CrossRef]
- King, H.W. Quantitative size-factors for metallic solid solutions. J. Mater. Sci. 1966, 1, 79–90. [Google Scholar] [CrossRef]
- Banerjee, R.; Liu, Q.; Tengco, J.M.M.; Regalbuto, J.R. Detection of Ambient Oxidation of Ultrasmall Supported Platinum Nanoparticles with Benchtop Powder X-Ray Diffraction. Catal. Lett. 2017, 147, 1754–1764. [Google Scholar] [CrossRef]
- Vanýsek, P. Handbook of Chemistry and Physics, 92nd ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 5–80. [Google Scholar]
- The Platinum Metals and Their Alloys; Vines, R.F., Wise, E.M., Eds.; The International Nickel Company: New York, NY, USA, 1941; p. 84. [Google Scholar]
- Hansen, M. Constitution of Binary Alloys; McGraw-Hill: New York, NY, USA, 1958; p. 1121. [Google Scholar]
- Punyawudho, K.; Blom, D.A.; van Zee, J.W.; Monnier, J.R. Comparison of different methods for determination of Pt surface site concentrations for supported Pt electrocatalysts. Electrochim. Acta 2010, 55, 5349–5356. [Google Scholar] [CrossRef]
- Punyawudho, K.; Vorayos, N.; Zhang, Y.; Shimpalee, S.; Monnier, J.R. Identification and quantification of performance losses for PEM fuel cells as determined by selective chemisorption and ESA measurements. Int. J. Hydrog. Energy 2014, 39, 11110. [Google Scholar] [CrossRef]
- Bett, J.; Kinoshita, K.; Routsis, K.; Stonehart, P. A comparison of gas-phase and electrochemical measurements for chemisorbed carbon monoxide and hydrogen on platinum crystallites. J. Catal. 1973, 29, 160–168. [Google Scholar] [CrossRef]
- Larsen, M.J.; Morales, I.J.; Cavaliere, S.; Zajac, J.; Jones, D.J.; Rozière, J.; Kaluža, L.; Gulková, D.; Odgaard, M. Development of tailored high-performance and durable electrocatalysts for advanced PEM fuel cells. Int. J. Hydrog. Energy 2017, 42, 7166–7176. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley-Interscience: New York, NY, USA, 2001; p. 92. [Google Scholar]
- Catalyzing Commercialization: Creating Novel Bimetallic Catalysts for Methanol Fuel Cells. Chem. Eng. Progr. 2019, 4, 14.

| Empirical Formula | Pt (wt %) | Cu (wt %) | ML (Cu + Pt) | Number of Pts (1018 Sites × g−1 Cat) |
|---|---|---|---|---|
| Cu0.2Pt1 | 1.9 | 0.14 | 1.2 | 40 |
| Cu1.6Pt1 | 2.4 | 1.2 | 3.1 | 17 |
| Cu1.7Pt1 | 1.9 | 1.0 | 2.5 | 30 |
| Cu3.0Pt1 | 2.3 | 2.2 | 4.5 | 21 |
| Cu3.3Pt1 | 1.1 | 1.2 | 2.4 | 8.1 |
| Cu5.5Pt1 | 0.90 | 1.8 | 3.3 | 4.8 |
| Cu6.1Pt1 | 1.1 | 2.0 | 3.7 | 2.7 |
| Empirical Formula | Peak Current (A × g−1 Pt) |
|---|---|
| Pt (commercial) | 146 |
| Cu0.2Pt1 | 57 |
| Cu1.6Pt1 | 161 |
| Cu1.7Pt1 | 238 |
| Cu3.0Pt1 | 278 |
| Cu3.3Pt1 | 228 |
| Cu5.5Pt1 | 296 |
| Cu6.1Pt1 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tate, G.L.; Mehrabadi, B.A.T.; Xiong, W.; Kenvin, A.; Monnier, J.R. Synthesis of Highly Active Pd@Cu–Pt/C Methanol Oxidation Electrocatalysts via Continuous, Co-Electroless Deposition. Nanomaterials 2021, 11, 793. https://doi.org/10.3390/nano11030793
Tate GL, Mehrabadi BAT, Xiong W, Kenvin A, Monnier JR. Synthesis of Highly Active Pd@Cu–Pt/C Methanol Oxidation Electrocatalysts via Continuous, Co-Electroless Deposition. Nanomaterials. 2021; 11(3):793. https://doi.org/10.3390/nano11030793
Chicago/Turabian StyleTate, Gregory L., Bahareh Alsadat Tavakoli Mehrabadi, Wen Xiong, Adam Kenvin, and John R. Monnier. 2021. "Synthesis of Highly Active Pd@Cu–Pt/C Methanol Oxidation Electrocatalysts via Continuous, Co-Electroless Deposition" Nanomaterials 11, no. 3: 793. https://doi.org/10.3390/nano11030793
APA StyleTate, G. L., Mehrabadi, B. A. T., Xiong, W., Kenvin, A., & Monnier, J. R. (2021). Synthesis of Highly Active Pd@Cu–Pt/C Methanol Oxidation Electrocatalysts via Continuous, Co-Electroless Deposition. Nanomaterials, 11(3), 793. https://doi.org/10.3390/nano11030793






