Fluorescent Polyelectrolyte System to Track Anthocyanins Delivery inside Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Anthocyanins Extraction
2.3. Polyelectrolyte System Fabrication
2.4. Polyelectrolyte System Characterization
2.5. HPLC-DAD Analysis
2.6. Cell Culture
2.7. Proliferation Assay
2.8. Lifetime Fluorescence Imaging Microscopy
3. Results
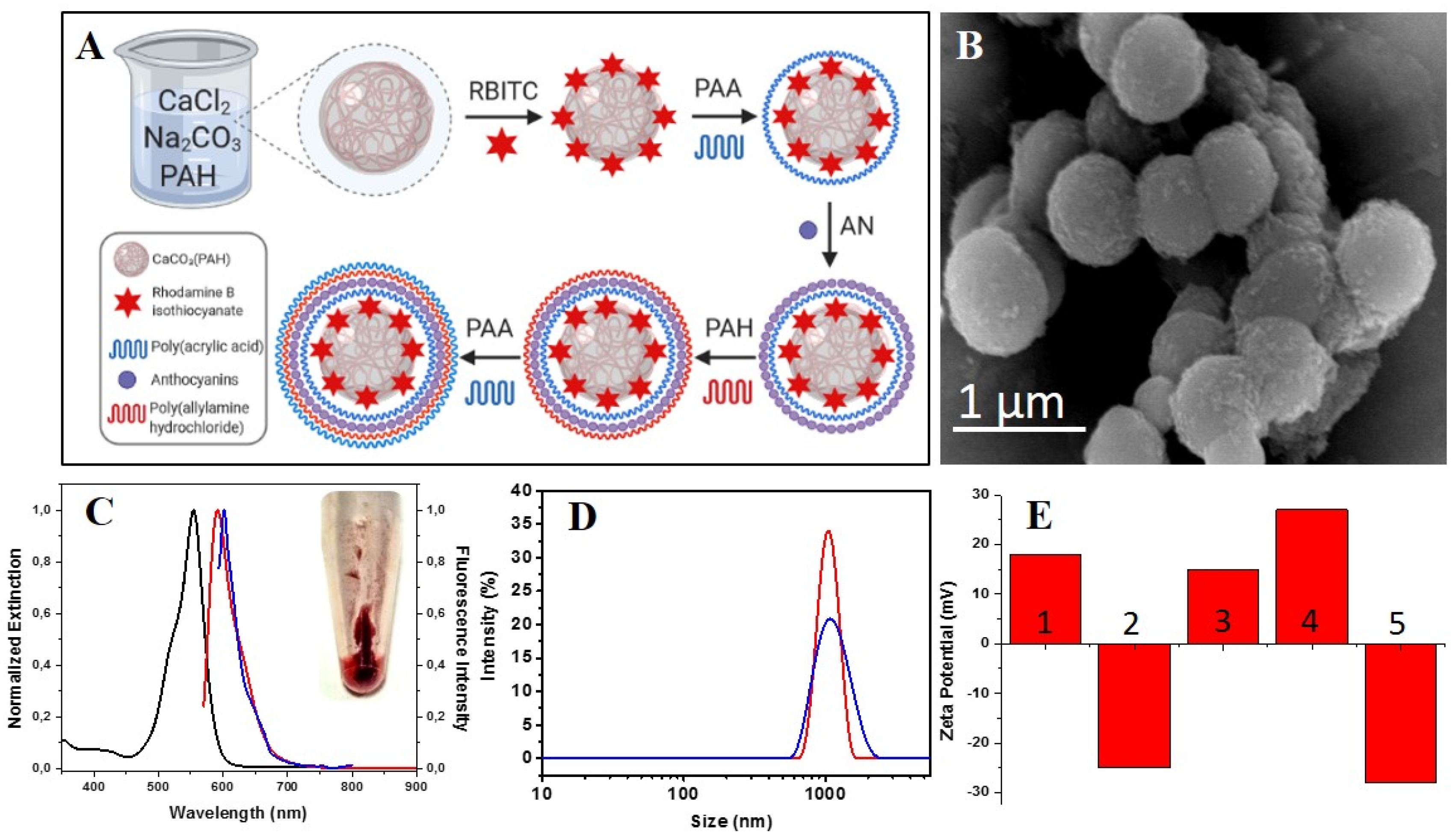
3.1. Fabrication and Characterization of Polyelectrolyte Microcapsules
3.2. Anthocyanins Entrapment Efficiency
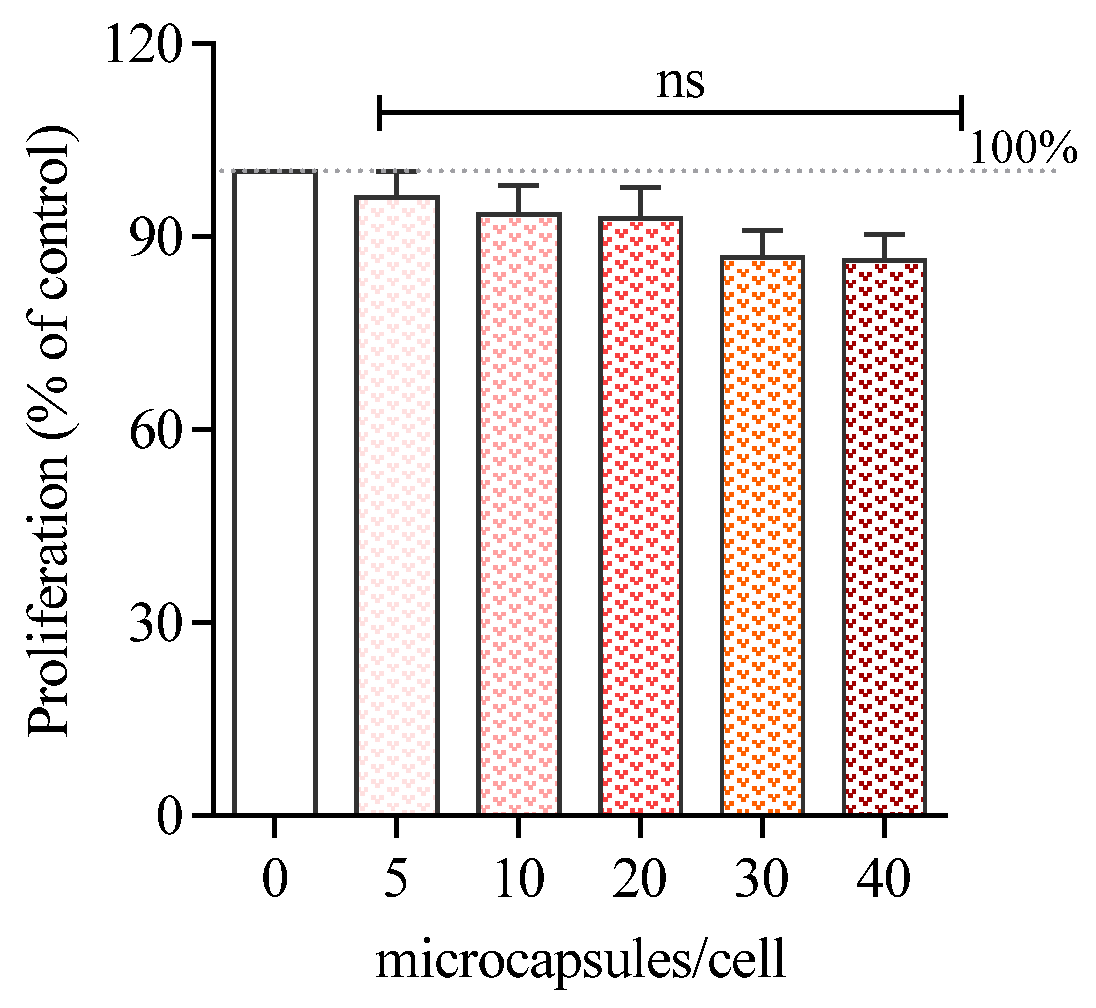
3.3. Cell Proliferation
3.4. FLIM Imaging of Microcapsules
3.5. FLIM Imaging of Internalized Microcapsules in Melanoma Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bunea, A.; Rugina, D.; Sconta, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tabaran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kwon, J.Y.; Seo, S.K.; Son, J.E.; Jung, S.K.; Min, S.Y.; Hwang, M.K.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Cyanidin suppresses ultraviolet B-induced COX-2 expression in epidermal cells by targeting MKK4, MEK1, and Raf-1. Biochem. Pharmacol. 2010, 79, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-drying microencapsulation of anthocyanins by natural biopolymers: A review. Drying Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Tan, C.; Selig, M.J.; Lee, M.C.; Abbaspourrad, A. Polyelectrolyte microcapsules built on CaCO3 scaffolds for the integration, encapsulation, and controlled release of copigmented anthocyanins. Food Chem. 2018, 246, 305–312. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kong, F. Anthocyanin extraction, microencapsulation, and release properties during in vitro digestion. Food Rev. Int. 2016, 32, 46–67. [Google Scholar] [CrossRef]
- Cai, X.R.; Du, X.F.; Cui, D.M.; Wang, X.N.; Yang, Z.K.; Zhu, G.L. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S. Development and evaluation of floating alginate microspheres for oral delivery of anthocyanins—A preliminary investigation. Food Sci. Nutr. 2017, 5, 713–721. [Google Scholar] [CrossRef]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Impact of liposomal encapsulation on degradation of anthocyanins of black carrot extract by adding ascorbic acid. Food Funct. 2017, 8, 1085–1093. [Google Scholar] [CrossRef]
- Svanberg, L.; Wassen, S.; Gustinelli, G.; Ohgren, C. Design of microcapsules with bilberry seed oil, cold-set whey protein hydrogels and anthocyanins: Effect of pH and formulation on structure formation kinetics and resulting microstructure during purification processing and storage. Food Chem. 2019, 280, 146–153. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Velasco, J.I.; de Oliveira, R.A. Active edible films based on arrowroot starch with microparticles of blackberry pulp obtained by freeze-drying for food packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Garapaty, A.; Champion, J.A. Engineering Polyelectrolyte capsules with independently controlled size and shape. Langmuir 2015, 31, 7601–7608. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-cellulose nanocrystal microencapsulation to improve encapsulation efficiency and stability of entrapped fruit anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef]
- Xiao, F.X.; Pagliaro, M.; Xu, Y.J.; Liu, B. Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, H.; Tong, W.; Gao, C. Fabrication of triple-labeled polyelectrolyte microcapsules for localized ratiometric pH sensing. J. Colloid Interface Sci. 2014, 416, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Nifontova, G.; Efimov, A.; Agapova, O.; Agapov, I.; Nabiev, I. Bioimaging tools based on polyelectrolyte microcapsules encoded with fluorescent semiconductor nanoparticles: Design and characterization of the fluorescent properties. Nanoscale Res. Lett. 2019, 14, 29. [Google Scholar] [CrossRef]
- Ahmad, M.; Ashraf, B.; Gani, A.; Gani, A. Microencapsulation of saffron anthocyanins using beta glucan and beta cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion. Int. J. Biol. Macromol. 2018, 109, 435–442. [Google Scholar]
- Diaconeasa, Z.; Ayvaz, H.; Rugina, D.; Leopold, L.; Stanila, A.; Socaciu, C.; Tabaran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma Inhibition by Anthocyanins is associated with the reduction of oxidative stress biomarkers and changes in mitochondrial membrane potential. Plant. Foods Hum. Nutr. 2017, 72, 404–410. [Google Scholar] [CrossRef]
- König, K. Clinical multiphoton tomography. J. Biophotonics 2008, 1, 13–23. [Google Scholar] [CrossRef]
- Boens, N.; Qin, W.; Basarić, N.; Hofkens, J.; Ameloot, M.; Pouget, J.; Lefèvre, J.-P.; Valeur, B.; Gratton, E.; van de Ven, M.; et al. Fluorescence lifetime standards for time and frequency domain fluorescence spectroscopy. Anal. Chem. 2007, 79, 2137–2149. [Google Scholar] [CrossRef]
- Datta, R.; Heaster, T.M.; Sharick, J.T.; Gillette, A.A.; Skala, C.M. Fluorescence lifetime imaging microscopy: Fundamentals and advances in instrumentation, analysis, and applications. J. Biomed. Opt. 2020, 25, 071203. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.-O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, G.B.; Rogach, A.L.; Zebli, B.; Liedl, T.; Skirtach, A.G.; Köhler, K.; Antipov, A.A.; Gaponik, N.; Susha, A.S.; Winterhalter, M.; et al. Nanoengineered polymer capsules: Tools for detection, controlled delivery, and site-specific manipulation. Small 2005, 1, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef]



| Synthesis Steps | Non-Entrapped AN (µg/mL) | Non-Entrapped AN (%) | EE (%) |
|---|---|---|---|
| ANI | 48.02 ± 0.008 | 3.87 ± 0.006 | 96.12 ± 0.004 |
| ANII | 18.09 ± 0.001 | 1.46 ± 0.001 | 98.53 ± 0.009 |
| ANIII | 0.77 ± 0.006 | 0.06 ± 0.002 | 99.93 ± 0.008 |
| TOTAL | 66.89 ± 0.003 | 5.397 ± 0.002 | 94.60 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiman, R.; Nistor, M.; Focșan, M.; Pintea, A.; Aștilean, S.; Rugina, D. Fluorescent Polyelectrolyte System to Track Anthocyanins Delivery inside Melanoma Cells. Nanomaterials 2021, 11, 782. https://doi.org/10.3390/nano11030782
Ghiman R, Nistor M, Focșan M, Pintea A, Aștilean S, Rugina D. Fluorescent Polyelectrolyte System to Track Anthocyanins Delivery inside Melanoma Cells. Nanomaterials. 2021; 11(3):782. https://doi.org/10.3390/nano11030782
Chicago/Turabian StyleGhiman, Raluca, Madalina Nistor, Monica Focșan, Adela Pintea, Simion Aștilean, and Dumitrita Rugina. 2021. "Fluorescent Polyelectrolyte System to Track Anthocyanins Delivery inside Melanoma Cells" Nanomaterials 11, no. 3: 782. https://doi.org/10.3390/nano11030782
APA StyleGhiman, R., Nistor, M., Focșan, M., Pintea, A., Aștilean, S., & Rugina, D. (2021). Fluorescent Polyelectrolyte System to Track Anthocyanins Delivery inside Melanoma Cells. Nanomaterials, 11(3), 782. https://doi.org/10.3390/nano11030782










