Synthesis and Characterization of Layered Double Hydroxides as Materials for Electrocatalytic Applications
Abstract
1. Introduction
2. Preparation of the Devices
- (i)
- the chemical bulk synthesis of the LDH and subsequent electrode coating;
- (ii)
- the direct synthesis of LDH on the electrode surface.
2.1. LDH Bulk Synthesis
2.2. In Situ Growth Methods
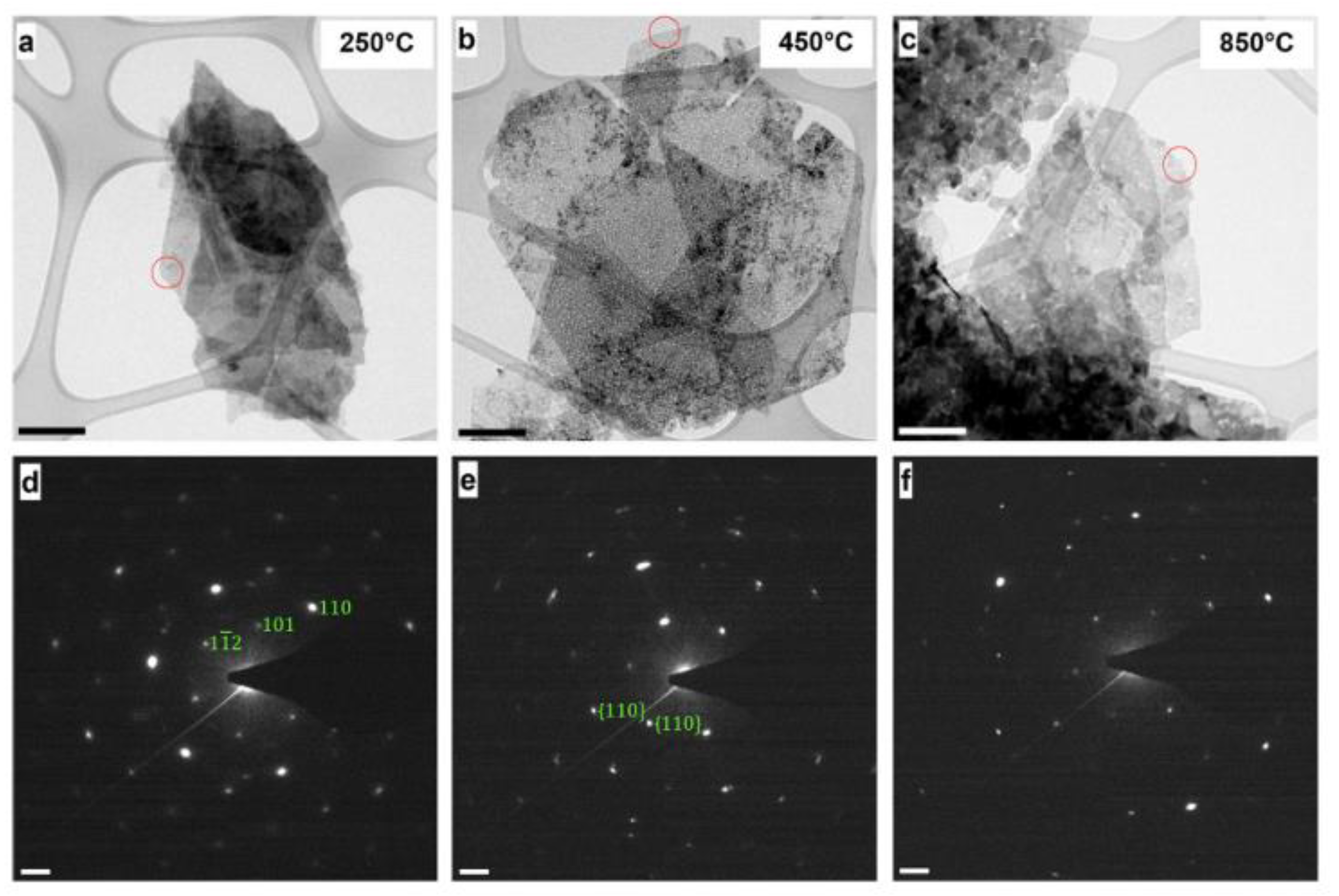
3. Characterization
4. Applications
4.1. Oxygen Evolution Reaction (OER)
| Catalyst | Conditions | Tafel Slope (mV dec−1) | Overpotential (mV) at 10 mA cm−2 |
|---|---|---|---|
| Ni/Al [4] | 1 M NaOH | 29 | 370 * |
| Ni/Fe [4] | 1 M NaOH | 25 | 320 * |
| Co/Al [4] | 1 M NaOH | 30 | 370 * |
| Co/Fe [4] | 1 M NaOH | 29 | 310 * |
| NiCo [65] | 0.1 M KOH | 113 | 290 * |
| NiMn [66] | 1 M KOH | 30 | 220 |
| CuCo [67] | 1 M KOH | 47 | 300 |
| NiCoFe [69] | 1 M KOH | 48 | 232 |
| NiFeV [70] | 1 M KOH | 42 | 192 |
| NiFeCr [71] | 1 M KOH | 69 | 280 |
| NiFe-CO32− [73] | 1 M KOH | 50 | 341 |
| NiFe-Cl− [73] | 1 M KOH | 47 | 343 |
| NiFe on nanofiber [74] | 1 M NaOH | 21 | 260 |
| NiFe-C [77] | 1 M KOH | 35 | 210 |
| Cu@NiFe [78] | 1 M KOH | 28 | 199 |
| Au/NiFe [79] | 1 M KOH | 36 | 237 |
| Ni nanoparticle/NiFe [80] | 1 M KOH | 62 | 328 |
| NiCo nanosheet [82] | 1 M KOH | 40 | 367 |
| CoAl single layer | 1 M KOH | 36 | 252 |
4.2. Other Energy Applications
4.3. Sensors
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Giorgetti, M. Layered-double-hydroxide-modified electrodes: Electroanalytical applications. Anal. Bioanal. Chem. 2013, 405, 603–614. [Google Scholar] [CrossRef]
- Pena-pereira, F.; Duarte, R.M.B.O.; Duarte, A.C. Immobilization strategies and analytical applications for metallic and metal-oxide nanomaterials on surfaces. Trends Anal. Chem. 2012, 40, 90–105. [Google Scholar] [CrossRef]
- Vlamidis, Y.; Scavetta, E.; Gazzano, M.; Tonelli, D. Iron vs Aluminum Based Layered Double Hydroxides as Water Splitting Catalysts. Electrochim. Acta 2016, 188, 653–660. [Google Scholar] [CrossRef]
- Caschera, D.; Federici, F.; Zane, D.; Focante, F.; Curulli, A.; Padeletti, G.J. Gold nanoparticles modified GC electrodes: Electrochemical behaviour dependence of different neurotransmitters and molecules of biological interest on the particles size and shape. Nanopart. Res. 2009, 11, 1925–1936. [Google Scholar] [CrossRef]
- DeRoy, A.; Forano, C.; Malki, K.E.; Besse, J.P.; Occelli, M.L.; Robson, H.E. Expanded Clays and Other Microporous Solids; Van Nostrand Reinhold: New York, NY, USA, 1992. [Google Scholar]
- Theo Kloprogge, J.; Hickey, L.; Trujillano, R.; Jesús Holgado, M.; San Román, M.S.; Rives, V.; Martens, W.N.L.; Frost, R. Characterization of Intercalated Ni/Al Hydrotalcites Prepared by the Partial Decomposition of Urea. Cryst. Growth Des. 2006, 6, 1533–1536. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Chibwe, K.; Jones, W. Intercalation of organic and inorganic anions into layered double hydroxides. J. Chem. Soc. Chem. Commun. 1989, 926–927. [Google Scholar] [CrossRef]
- Naseem, S.; Gevers, B.; Boldt, R.; Labuschagné, F.J.W.J.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.-H.; Yang, Z.; Wang, Z.; Tang, X.; Wang, T.; Fan, L.; Ooi, K. Preparation of Ni2+−Fe3+ Layered Double Hydroxide Material with High Crystallinity and Well-Defined Hexagonal Shapes. Chem. Mater. 2007, 20, 360–363. [Google Scholar] [CrossRef]
- Xu, Z.P.; Lu, G.Q.M. Hydrothermal Synthesis of Layered Double Hydroxides (LDHs) from Mixed MgO and Al2O3: LDH Formation Mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar]
- Wang, F.; Wang, T.; Sun, S.; Xu, Y.; Yu, R.; Li, H. One-step synthesis of Nickle Iron-layered double hydroxide/reduced graphene oxide/carbon nanofibres composite as electrode materials for asymmetric supercapacitor. Sci. Rep. 2018, 8, 8908. [Google Scholar] [CrossRef]
- Xu, Z.P.; Stevenson, G.S.; Lu, C.-Q.; Lu, G.Q.M.; Bartlett, P.F.; Gray, P.P. Stable Suspension of Layered Double Hydroxide Nanoparticles in Aqueous Solution. J. Am. Chem. Soc. 2005, 128, 36–37. [Google Scholar]
- Dong, H.; Chen, M.; Rahman, S.; Parekh, H.S.; Cooper, H.M.; Xu, Z.P. Engineering small MgAl-layered double hydroxide nanoparticles for enhanced gene delivery. Appl. Clay Sci. 2014, 100, 66–75. [Google Scholar] [CrossRef]
- Prince, J.; Montoya, A.; Ferrat, G.S.; Valente, J. Proposed General Sol−Gel Method to Prepare Multimetallic Layered Double Hydroxides: Synthesis, Characterization, and Envisaged Application. Chem. Mater. 2009, 21, 5826–5835. [Google Scholar] [CrossRef]
- Prevot, V.; Forano, C.P.; Besse, J. Hydrolysis in Polyol: New Route for Hybrid-Layered Double Hydroxides Preparation. Chem. Mater. 2005, 17, 6695–6701. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Graffin, P.; Tichit, D. Synthesis and characterization of sol–gel Mg/Al and Ni/Al layered double hydroxides and comparison with co-precipitated samples. Microporous Mesoporous Mater. 2000, 39, 229–247. [Google Scholar] [CrossRef]
- Aramendı, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Comparative Study of Mg/M(III) (M=Al, Ga, In) Layered Double Hydroxides Obtained by Coprecipitation and the Sol–Gel Method. J. Solid State Chem. 2002, 168, 156–161. [Google Scholar] [CrossRef]
- Tichit, D.; Lorret, O.; Coq, B.; Prinetto, F.; Ghiotti, G. Synthesis and characterization of Zn/Al and Pt/Zn/Al layered double hydroxides obtained by the sol–gel method. Microporous Mesoporous Mater. 2005, 80, 213–220. [Google Scholar] [CrossRef]
- Hu, G.; Wang, N.; O’Hare, D.; Davis, J. Synthesis of magnesium aluminium layered double hydroxides in reverse microemulsions. J. Mater. Chem. 2007, 17, 2257–2266. [Google Scholar] [CrossRef]
- Bellezza, F.; Cipiciani, A.; Costantino, U.; Nocchetti, M.; Posati, T. Hydrotalcite-Like Nanocrystals from Water-in-Oil Microemulsions. Eur. J. Inorg. Chem. 2009, 2009, 2603–2611. [Google Scholar] [CrossRef]
- Bellezza, F.; Nocchetti, M.; Posati, T.; Giovagnoli, S.; Cipiciani, A. Synthesis of colloidal dispersions of NiAl, ZnAl, NiCr, ZnCr, NiFe, and MgFe hydrotalcite-like nanoparticles. J. Colloid Interface Sci. 2012, 376, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, F.; Evans, D.G.; Duan, X. Layered double hydroxide films: Synthesis, properties and applications. Chem. Commun. 2010, 46, 5197–5210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef]
- Shao, M.; Han, J.; Shi, W.; Wei, M.; Duan, X. Layer-by-layer assembly of porphyrin/layered double hydroxide ultrathin film and its electrocatalytic behavior for H2O2. Electrochem. Commun. 2010, 12, 1077–1080. [Google Scholar] [CrossRef]
- Indira, L.; Kamath, P.V. Electrogeneration of base by cathodic reduction of anions: Novel one-step route to unary and layered double hydroxides (LDHs). J. Mater. Chem. 1994, 4, 1487–1490. [Google Scholar] [CrossRef]
- Scavetta, E.; Ballarin, B.; Giorgetti, M.; Carpani, I.; Cogo, F.; Tonelli, D. Electrodes modified by One-Step Electrosynthesis of Ni/Al-NO3 Double Layered Hydroxide. J. New Mater. Electrochem. Syst. 2004, 7, 43–50. [Google Scholar]
- Scavetta, E.; Mignani, A.; Prandstraller, D.; Tonelli, D. Electrosynthesis of Thin Films of Ni, Al Hydrotalcite Like Compounds. Chem. Mater. 2007, 4523–4529. [Google Scholar] [CrossRef]
- Scavetta, E.; Vlamidis, Y.; Posati, T.; Nocchetti, M.; Tonelli, D. Effect of the Synthesis Route and Fe Presence on the Redox Activity of Ni in Layered Double Hydroxides. ChemElectroChem 2016, 3, 1320–1328. [Google Scholar] [CrossRef]
- Scavetta, E.; Ballarin, B.; Corticelli, C.; Gualandi, I.; Tonelli, D.; Prevot, V.; Forano, C.; Mousty, C. An insight into the electrochemical behavior of Co/Al layered double hydroxide thin films prepared by electrodeposition. J. Power Sources 2012, 201, 360–367. [Google Scholar] [CrossRef]
- Basile, F.; Benito, P.; Fornasari, G.; Monti, M.; Scavetta, E.; Tonelli, D.; Vaccari, A. Novel Rh-based structured catalysts for the catalytic partial oxidation of methane. Catal. Today 2010, 157, 183–190. [Google Scholar] [CrossRef]
- Musella, E.; Gualandi, I.; Scavetta, E.; Gazzano, M.; Rivalta, A.; Venuti, E.; Christian, M.; Morandi, V.; Tonelli, D. Electrochemical Approach for the Production of Layered Double Hydroxides with a Well-Defined Co/Me(III) Ratio. Chem. A Eur. J. 2019, 25, 16301–16310. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Vlamidis, Y.; Mazzei, L.; Musella, E.; Giorgetti, M.; Christian, M.; Morandi, V.; Scavetta, E.; Tonelli, D. Ni/Al Layered Double Hydroxide and Carbon Nanomaterial Composites for Glucose Sensing. ACS Appl. Nano Mater. 2019, 2, 143–155. [Google Scholar] [CrossRef]
- Musella, E.; Gualandi, I.; Scavetta, E.; Rivalta, A.; Venuti, E.; Christian, M.; Morandi, V.; Mullaliu, A.; Giorgetti, M.; Tonelli, D. Newly developed electrochemical synthesis of Co-based layered double hydroxides: Toward noble metal-free electro-catalysis. J. Mater. Chem. A 2019, 7, 11241–11249. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Z.; Han, X.; Huang, H.; Zhao, C.; Yang, J.; Qiu, J. NiCo-layered double hydroxides vertically assembled on carbon fiber papers as binder-free high-active electrocatalysts for water oxidation. Carbon N. Y. 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, F.; Chen, W.; Ma, Y.; Wu, Y.; Gu, X.; Qin, Y.; Tao, P.; Song, C.; Shang, W.; et al. In Situ Vertical Growth of Fe–Ni Layered Double-Hydroxide Arrays on Fe–Ni Alloy Foil: Interfacial Layer Enhanced Electrocatalyst with Small Overpotential for Oxygen Evolution Reaction. ACS Energy Lett. 2018, 3, 2357–2365. [Google Scholar] [CrossRef]
- Kanezaki, E. Thermal behavior of the hydrotalcite-like layered structure of Mg and Al-layered double hydroxides with interlayer carbonate by means of in situ powder HTXRD and DTA/TG. Solid State Ion. 1998, 106, 279–284. [Google Scholar] [CrossRef]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Chapter 14.1—Layered Double Hydroxides (LDH). In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar]
- Duan, X.; Evans, D.G. Layered Double Hydroxides, Structure and Bonding; Springer: Berlin, Germany, 2005. [Google Scholar]
- Musella, E.; Gualandi, I.; Ferrari, G.; Mastroianni, D.; Scavetta, E.; Giorgetti, M.; Migliori, A.; Christian, M.; Morandi, V.; Denecke, R.; et al. Electrosynthesis of Ni/Al layered double hydroxide and reduced graphene oxide composites for the development of hybrid capacitors. Electrochim. Acta 2021, 365, 137294. [Google Scholar] [CrossRef]
- Hu, J.; Lei, G.; Lu, Z.; Liu, K.; Sang, S.; Liu, H. Alternating assembly of Ni–Al layered double hydroxide and graphene for high-rate alkaline battery cathode. Chem. Commun. 2015, 51, 9983–9986. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Z.; Wang, R.; Wang, T. Layered double hydroxide/carbon nanotubes composite as a high performance anode material for Ni–Zn secondary batteries. Electrochim. Acta 2013, 111, 581–587. [Google Scholar] [CrossRef]
- Kloprogge, T.J.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32-, NO3-, SO42- and ClO4- in Mg/Al-hydrotalcite. Mineral. Soc. Am. 2015, 87, 623–629. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Yang, Y.; Zhang, X.; Zhu, R.; Yu, H. Insight into the adsorption mechanisms of aqueous hexavalent chromium by EDTA intercalated layered double hydroxides: XRD, FTIR, XPS, and zeta potential studies. New J. Chem. 2019, 43, 15915–15923. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Wang, Q.; Hare, D.O. Recent Advances in the Synthesis and Application of Layered Double Hydroxide ( LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, G.; Zheng, C.; Xiang, X.; Li, F. Facile Sodium Alginate Assisted Assembly of Ni−Al Layered Double Hydroxide Nanostructures. Ind. Eng. Chem. Res. 2010, 49, 2759–2767. [Google Scholar] [CrossRef]
- Prevot, V.; Caperaa, N.; Taviot-Guého, C.; Forano, C. Glycine-Assisted Hydrothermal Synthesis of NiAl-Layered Double Hydroxide Nanostructures. Cryst. Growth Des. 2009, 9, 3646–3654. [Google Scholar] [CrossRef]
- Prevot, V.; Szczepaniak, C.; Jaber, M. Aerosol-assisted self-assembly of hybrid Layered Double Hydroxide particles into spherical architectures. J. Colloid Interface Sci. 2011, 356, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, R.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Hollow nanoshell of layered double hydroxide. Chem. Commun. 2006, 3125–3127. [Google Scholar] [CrossRef]
- Géraud, E.; Rafqah, S.; Sarakha, M.; Forano, C.; Prevot, V.; Leroux, F. Three Dimensionally Ordered Macroporous Layered Double Hydroxides: Preparation by Templated Impregnation/Coprecipitation and Pattern Stability upon Calcination. Chem. Mater. 2008, 20, 1116–1125. [Google Scholar] [CrossRef]
- Gunawan, P.; Xu, R. Direct Assembly of Anisotropic Layered Double Hydroxide (LDH) Nanocrystals on Spherical Template for Fabrication of Drug-LDH Hollow Nanospheres. Chem. Mater. 2009, 21, 781–783. [Google Scholar] [CrossRef]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W. Hierarchical Hollow Nanoprisms Based on Ultrathin Ni-Fe Layered Double Hydroxide Nanosheets with Enhanced Electrocatalytic Activity towards Oxygen Evolution. Angew. Chemie Int. Ed. 2018, 57, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.; Jaskaniec, S.; McCarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. npj 2D Mater. Appl. 2018, 2, 11. [Google Scholar] [CrossRef]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells-Fundamentals and Applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium−Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Fabbri, E.; Habereder, A.; Waltar, K.; Kötz, R.; Schmidt, T.J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthick, K.; Kundu, S. Evolution of layered double hydroxides (LDH) as high performance water oxidation electrocatalysts: A review with insights on structure, activity and mechanism. Mater. Today Energy 2017, 6, 1–26. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application (Adv. Energy Mater. 17/2019). Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel–cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction. J. Power Sources 2015, 278, 445–451. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Li, X.; Xin, M.; Cai, T.; Xing, W.; Chai, Y.; Xue, Q.; Yan, Z. Bifuntional petaloid nickel manganese layered double hydroxides decorated on a freestanding carbon foam for flexible asymmetric supercapacitor and oxygen evolution. Electrochim. Acta 2017, 252, 275–285. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Chen, L.; Wei, X.; Shi, J.; He, M. Facile synthesis of Cu doped cobalt hydroxide (Cu-Co(OH)2) nano-sheets for efficient electrocatalytic oxygen evolution. J. Mater. Chem. A 2017, 5, 22568–22575. [Google Scholar] [CrossRef]
- Long, X.; Xiao, S.; Wang, Z.; Zheng, X.; Yang, S. Co intake mediated formation of ultrathin nanosheets of transition metal LDH—an advanced electrocatalyst for oxygen evolution reaction. Chem. Commun. 2015, 51, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Pan, X.; Long, X.; Yi, Z. Room-Temperature Synthesis FeNiCo Layered Double Hydroxide as an Excellent Electrochemical Water Oxidation Catalyst. J. Electrochem. Soc. 2017, 164, H755–H759. [Google Scholar] [CrossRef]
- Li, P.; Duan, X.; Kuang, Y.; Li, Y.; Zhang, G.; Liu, W.; Sun, X. Tuning Electronic Structure of NiFe Layered Double Hydroxides with Vanadium Doping toward High Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2018, 8, 1703341. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.; Shearer, M.J.; Sheng, H.; Li, W.; Chen, J.; Xiao, P.; Zhang, Y.; Hamers, R.J.; Jin, S. Highly Active Trimetallic NiFeCr Layered Double Hydroxide Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Zhou, D.; Cai, Z.; Bi, Y.; Tian, W.; Luo, M.; Zhang, Q.; Xie, Q.; Wang, J.; Li, Y.; Kuang, Y.; et al. Effects of redox-active interlayer anions on the oxygen evolution reactivity of NiFe-layered double hydroxide nanosheets. Nano Res. 2018, 11, 1358–1368. [Google Scholar] [CrossRef]
- Dang, L.; Liang, H.; Zhuo, J.; Lamb, B.K.; Sheng, H.; Yang, Y.; Jin, S. Direct Synthesis and Anion Exchange of Noncarbonate-Intercalated NiFe-Layered Double Hydroxides and the Influence on Electrocatalysis. Chem. Mater. 2018, 30, 4321–4330. [Google Scholar] [CrossRef]
- Chen, R.; Sun, G.; Yang, C.; Zhang, L.; Miao, J.; Tao, H.; Yang, H.; Chen, J.; Chen, P.; Liu, B. Achieving stable and efficient water oxidation by incorporating NiFe layered double hydroxide nanoparticles into aligned carbon nanotubes. Nanoscale Horiz. 2016, 1, 156–160. [Google Scholar] [CrossRef]
- Long, X.; Li, J.; Xiao, S.; Yan, K.; Wang, Z.; Chen, H.; Yang, S. A Strongly Coupled Graphene and FeNi Double Hydroxide Hybrid as an Excellent Electrocatalyst for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 7584–7588. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Duan, J.; Jaroniec, M.; Qiao, S.Z. Three-Dimensional N-Doped Graphene Hydrogel/NiCo Double Hydroxide Electrocatalysts for Highly Efficient Oxygen Evolution. Angew. Chem. Int. Ed. 2013, 52, 13567–13570. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Tu, W.; Sheng, Y.; Du, Y.; Kraft, M.; Borgna, A.; Xu, R. A Highly Efficient Oxygen Evolution Catalyst Consisting of Interconnected Nickel–Iron-Layered Double Hydroxide and Carbon Nanodomains. Adv. Mater. 2018, 30, 1705106. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Xi, L.; Yu, Y.; Chen, N.; Sun, S.; Wang, W.; Lange, K.M.; Zhang, B. Single-Atom Au/NiFe Layered Double Hydroxide Electrocatalyst: Probing the Origin of Activity for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 3876–3879. [Google Scholar] [CrossRef]
- Gao, X.; Long, X.; Yu, H.; Pan, X.; Yi, Z. Ni Nanoparticles Decorated NiFe Layered Double Hydroxide as Bifunctional Electrochemical Catalyst. J. Electrochem. Soc. 2017, 164, H307–H310. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Liang, H.; Meng, F.; Cabán-Acevedo, M.; Li, L.; Forticaux, A.; Xiu, L.; Wang, Z.; Jin, S. Hydrothermal Continuous Flow Synthesis and Exfoliation of NiCo Layered Double Hydroxide Nanosheets for Enhanced Oxygen Evolution Catalysis. Nano Lett. 2015, 15, 1421–1427. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Lu, Q.; Chen, B.; Chen, J.; Huang, Y.; Ma, Q.; Tan, C.; Yang, J.; Cao, X.; et al. Self-Assembly of Single-Layer CoAl-Layered Double Hydroxide Nanosheets on 3D Graphene Network Used as Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. Adv. Mater. 2016, 28, 7640–7645. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.-H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.-G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Hou, C.-C.; Wang, C.-J.; Yang, X.; Shi, R.; Chen, Y. Ir4+-Doped NiFe LDH to expedite hydrogen evolution kinetics as a Pt-like electrocatalyst for water splitting. Chem. Commun. 2018, 54, 6400–6403. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated Hydrogen Evolution Kinetics on NiFe-Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites. Adv. Mater. 2018, 30, 1706279. [Google Scholar] [CrossRef]
- Li, D.; Hao, G.; Guo, W.; Liu, G.; Li, J.; Zhao, Q. Highly efficient Ni nanotube arrays and Ni nanotube arrays coupled with NiFe layered-double-hydroxide electrocatalysts for overall water splitting. J. Power Sources 2020, 448, 227434. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zhang, B.; Yang, H.G. Defect-Rich Ultrathin Cobalt–Iron Layered Double Hydroxide for Electrochemical Overall Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 34474–34481. [Google Scholar] [CrossRef]
- Arif, M.; Yasin, G.; Shakeel, M.; Mushtaq, M.A.; Ye, W.; Fang, X.; Ji, S.; Yan, D. Hierarchical CoFe-layered double hydroxide and g-C 3 N 4 heterostructures with enhanced bifunctional photo/electrocatalytic activity towards overall water splitting. Mater. Chem. Front. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Bhowmik, T.; Kundu, M.K.; Barman, S. CoFe Layered Double Hydroxide Supported on Graphitic Carbon Nitrides: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen Evolution and Hydrogen Evolution Reactions. ACS Appl. Energy Mater. 2018, 1, 1200–1209. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A Heterostructure Coupling of Exfoliated Ni–Fe Hydroxide Nanosheet and Defective Graphene as a Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, J.-G.; Lv, L.; Li, Z.; Ao, X.; Xu, C.; Xue, X.; Hong, G.; Wang, C. Engineering hierarchical CoSe/NiFe layered-double-hydroxide nanoarrays as high efficient bifunctional electrocatalyst for overall water splitting. J. Power Sources 2019, 425, 138–146. [Google Scholar] [CrossRef]
- Dutta, S.; Indra, A.; Feng, Y.; Song, T.; Paik, U. Self-Supported Nickel Iron Layered Double Hydroxide-Nickel Selenide Electrocatalyst for Superior Water Splitting Activity. ACS Appl. Mater. Interfaces 2017, 9, 33766–33774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, F.; Zou, H.; Yuan, Y.; Wang, H.; Xia, J.; Wang, Z. Preparation of a Pt/NiFe layered double hydroxide/reduced graphene oxide composite as an electrocatalyst for methanol oxidation. J. Electroanal. Chem. 2018, 818, 198–203. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Xu, K.Q.; Xia, J.; Liu, Q.; Wang, Z. Highly dispersed ultrafine Pt nanoparticles on nickel-cobalt layered double hydroxide nanoarray for enhanced electrocatalytic methanol oxidation. Int. J. Hydrog. Energy 2018, 43, 16302–16310. [Google Scholar] [CrossRef]
- Ballarin, B.; Mignani, A.; Scavetta, E.; Giorgetti, M.; Tonelli, D.; Boanini, E.; Mousty, C.; Prevot, V. Synthesis Route to Supported Gold Nanoparticle Layered Double Hydroxides as Efficient Catalysts in the Electrooxidation of Methanol. Langmuir 2012, 28, 15065–15074. [Google Scholar] [CrossRef]
- Zhao, J.; Kong, X.; Shi, W.; Shao, M.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. Self-assembly of layered double hydroxide nanosheets/Au nanoparticles ultrathin films for enzyme-free electrocatalysis of glucose. J. Mater. Chem. 2011, 21, 13926–13933. [Google Scholar] [CrossRef]
- Khalafallah, D.; Xiaoyu, L.; Zhi, M.; Hong, Z. 3D Hierarchical NiCo Layered Double Hydroxide Nanosheet Arrays Decorated with Noble Metal Nanoparticles for Enhanced Urea Electrocatalysis. ChemElectroChem 2020, 7, 163–174. [Google Scholar] [CrossRef]
- Xie, J.; Qu, H.; Lei, F.; Peng, X.; Liu, W.; Gao, L.; Hao, P.; Cui, G.; Tang, B. Partially amorphous nickel–iron layered double hydroxide nanosheet arrays for robust bifunctional electrocatalysis. J. Mater. Chem. A 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Zeng, M.; Wu, J.; Li, Z.; Wu, H.; Wang, J.; Wang, H.; He, L.; Yang, X. Interlayer Effect in NiCo Layered Double Hydroxide for Promoted Electrocatalytic Urea Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 4777–4783. [Google Scholar] [CrossRef]
- Ballarin, B.; Gazzano, M.; Seeber, R.; Tonelli, D.; Vaccari, A. Electrodes coated by hydrotalcite-like clays. Effect of the metals and the intercalated anions on ion accumulation and retention capability. J. Electroanal. Chem. 1998, 445, 27–37. [Google Scholar] [CrossRef]
- Ballarin, B.; Seeber, R.; Tonelli, D.; Vaccari, A. Electrocatalytic properties of nickel(II) hydrotalcite-type anionic clay: Application to methanol and ethanol oxidation. J. Electroanal. Chem. 1999, 463, 123–127. [Google Scholar] [CrossRef]
- Scavetta, E.; Tonelli, D. Amperometric Sensors Based on Synthetic Hydrotalcites and Their Application for Ethanol Detection in Beer. Electroanalysis 2005, 17, 363–370. [Google Scholar] [CrossRef]
- Scavetta, E.; Stipa, S.; Tonelli, D. Electrodeposition of a nickel-based hydrotalcite on Pt nanoparticles for ethanol and glucose sensing. Electrochem. Commun. 2007, 9, 2838–2842. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Zhou, T.; Zhang, Y.; Chen, L. A novel nonenzymatic electrochemical glucose sensor modified with Ni/Al layered double hydroxide. Electrochim. Acta 2013, 109, 532–535. [Google Scholar] [CrossRef]
- Ai, H.; Huang, X.; Zhu, Z.; Liu, J.; Chi, Q.; Li, Y.; Li, Z.; Ji, X. Short communication A novel glucose sensor based on monodispersed Ni/Al layered double hydroxide and chitosan. Biosens. Bioelect. 2008, 24, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Liang, W.; Chen, L.; Li, Y.; He, X. Non-enzymatic glucose sensor with high sensitivity based on Cu-Al layered double hydroxides. Sens. Actuators B Chem. 2018, 273, 41–47. [Google Scholar] [CrossRef]
- Wang, H.; Xiang, X.; Li, F. Facile synthesis and novel electrocatalytic performance of nanostructured Ni–Al layered double hydroxide / carbon nanotube composites. J. Mater. Chem. 2010, 20, 3944–3952. [Google Scholar] [CrossRef]
- Carpani, I.; Tonelli, D. Electrooxidation of Aliphatic and Aromatic Amines at a Ni, Al Based Hydrotalcite Modified Electrode. Electroanalysis 2006, 24, 2421–2425. [Google Scholar] [CrossRef]
- Gualandi, I.; Solito, A.G.; Scavetta, E.; Tonelli, D. Electrochemical Pretreatment of Pt Surface: Modification with Co/Al Layered Double Hydroxide for Analytical Applications. Electroanalysis 2012, 24, 857–864. [Google Scholar] [CrossRef]
- Khenifi, A.; Derriche, Z.; Forano, C.; Prevot, V.; Mousty, C.; Scavetta, E.; Ballarin, B.; Guadagnini, L.; Tonelli, D. Glyphosate and glufosinate detection at electrogenerated NiAl-LDH thin films. Anal. Chim. Acta 2009, 654, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chang, Q.; Liu, Q.; Yang, G.; Guan, H.; Chen, G.; Dong, C. Highly sensitive nonenzymatic H2O2 sensor based on NiFe-layered double hydroxides nanosheets grown on Ni foam. Surf. Interfaces 2018, 12, 102–107. [Google Scholar] [CrossRef]
- Gualandi, I.; Scavetta, E.; Zappoli, S.; Tonelli, D. Electrocatalytic oxidation of salicylic acid by a cobalt hydrotalcite-like compound modified Pt electrode. Biosens. Bioelectron. 2011, 26, 3200–3206. [Google Scholar] [CrossRef]
- Scavetta, E.; Casagrande, A.; Gualandi, I.; Tonelli, D. Analytical performances of Ni LDH films electrochemically deposited on Pt surfaces: Phenol and glucose detection. J. Electroanal. Chem. 2014, 722–723, 15–22. [Google Scholar] [CrossRef]
- Zhan, T.; Song, Y.; Tan, Z.; Hou, W. Electrochemical bisphenol A sensor based on exfoliated Ni2Al-layered double hydroxide nanosheets modified electrode. Sens. Actuators B Chem. 2017, 238, 962–971. [Google Scholar] [CrossRef]
- Hai, B.; Zou, Y. Carbon cloth supported NiAl-layered double hydroxides for flexible application and highly sensitive electrochemical sensors. Sens. Actuators B Chem. 2015, 208, 143–150. [Google Scholar] [CrossRef]
- Gualandi, I.; Scavetta, E.; Vlamidis, Y.; Casagrande, A.; Tonelli, D. Co/Al layered double hydroxide coated electrode for in flow amperometric detection of sugars. Electrochim. Acta 2015, 173, 67–75. [Google Scholar] [CrossRef]
- Fu, R.; Lu, Y.; Ding, Y.; Li, L.; Ren, Z.; Si, X.; Wu, Q. A novel non-enzymatic glucose electrochemical sensor based on CNF@Ni-Co layered double hydroxide modified glassy carbon electrode. Microchem. J. 2019, 150, 104106. [Google Scholar] [CrossRef]
- Eshghi, A.; Kheirmand, M. Graphene/Ni–Fe layered double hydroxide nano composites as advanced electrode materials for glucose electro oxidation. Int. J. Hydrog. Energy 2017, 42, 15064–15072. [Google Scholar] [CrossRef]
- Rezaei, B.; Khosropour, H.; Ensafi, A.A.; Dinari, M.; Nabiyan, A. A new electrochemical sensor for the simultaneous determination of guanine and adenine: Using a NiAl-layered double hydroxide/graphene oxide-multi wall carbon nanotube modified glassy carbon electrode. RSC Adv. 2015, 5, 75756–75765. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, Z.; Ashraf, G.; Wang, J.; Luo, H.; Chen, X.; Xiao, F.; Liu, H. Hierarchical CNTs@CuMn Layered Double Hydroxide Nanohybrid with Enhanced Electrochemical Performance in H2S Detection from Live Cells. Anal. Chem. 2019, 91, 3912–3920. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, J.E.; Zhang, L.; Chen, X.; Zhang, H.; Zhang, F. Nanoscale Facile synthesis of NiAl-layered double hydroxide/graphene hybrid with enhanced electrochemical properties for detection of dopamine. Nanoscale 2011, 3, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, B.; Fang, L.; Fan, S.; Wu, F.; Hu, B.; Meng, F. Highly Sensitive Nonenzymatic Glucose Sensor Based on 3D Ultrathin NiFe Layered Double Hydroxide Nanosheets. Electroanalysis 2017, 29, 1755–1761. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Ji, X.; Jiang, J.; Ding, R.; Hu, Y.; Hu, A.; Huang, X. Ni/Al layered double hydroxide nanosheet film grown directly on Ti substrate and its application for a nonenzymatic glucose sensor. Sens. Actuators B Chem. 2010, 147, 241–247. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, J.; Han, J.; Zhang, D.; Wei, M.; Duan, X. Fabrication of Naphthol green B/layered double hydroxide nanosheets ultrathin film and its application in electrocatalysis. Electrochim. Acta 2011, 56, 1123–1129. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Xie, D.; Gu, Y.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H.; Wong, P.K. NiFe-Layered Double Hydroxide Nanosheet Arrays Supported on Carbon Cloth for Highly Sensitive Detection of Nitrite. ACS Appl. Mater. Interfaces 2018, 10, 6541–6551. [Google Scholar] [CrossRef] [PubMed]


| Onset potential | The potential at which the current starts to increase due to the occurrence of water oxidation. |
| Overpotential at 10 mA cm−2 | The difference between the potential at which a current density of 10 mA cm−2 is recorded and the thermodynamic potential for O2 + 4e− + 4 H+ ⇄ 2H2O |
| Mass and specific activities | Current densities divided by the catalyst loading (expressed as mass or volume) |
| Turnover frequency (TOF) | The moles number of O2 generated per time unit at a defined overpotential |
| Faradaic efficiency | The percentage of charge that flows at the electrode, and is used to produce O2 |
| Long-term stability test | Long-time experiment under OER conditions to verify the mantainance of performance |
| Catalyst | Conditions | Voltage * (V) | OER | HER | ||
|---|---|---|---|---|---|---|
| Overpotential (mV) at 10 mA cm−2 | Tafel Slope (mV dec−1) | Overpotential (mV) at 10 mA cm−2 | Tafel Slope (mV dec−1) | |||
| Cu nanowires NiFe [78] | 1 M KOH | 1.54 | 199 | 28 | 116 | 59 |
| Ir4+ doped NiFe [85] | 1 M KOH | 1.41 | 200 | - | 34 | 32 |
| NiFe [84] | 1 M NaOH | 1.70 | 240 | - | 210 | - |
| NiFeRu [86] | 1 M KOH | 1.52 | 225 | - | 29 | 31 |
| Ni nanotubes NiFe [87] | 1 M KOH | 1.51 | 191 | 41 | 101 | 101 |
| Defective CoFe [88] | 1 M KOH | 1.63 | 300 | 40 | 255 | 95 |
| CoFe C3N4 [89] | 1 M KOH | 1.82 | 275 | 58 | 417 | 77 |
| Co0.4Fe0.6/g-CNx [90] | 1 M KOH | 1.61 | 280 | 29 | 270 | 79 |
| CoSe/NiFe [92] | 1 M KOH | 1.53 | 201 | 39 | 98 | 89 |
| NiFe/NiSe [93] | 1 M KOH | 1.53 | 240 ** | 66 | 270 ** | 70 |
| Sensitivity | The slope of the calibration line (the first derivative for a curve). The calibration line is obtained by plotting the current vs. the analyte concentrations of standard solutions. |
| Limit of detection | The lowest quantity of the analyte that can be distinguished from the blank (absence of analyte) at a fixed statistical confidence level. It is the analyte concentration (or amount) that generates a signal equal to the blank signal plus n times its standard deviation (usually 3). |
| Range of linearity | The concentration range wherein the response can be approximated by a line. The lower limit is usually the limit of detection (LoD) value. The upper limit is the concentration value at which the calibration curve departs from linearity (limit of linearity; LoL). |
| Response time | The time required to reach 90% of the signal. It is evaluated by measuring the signal after a variation of the concentration. |
| Repeatability/Reproducibility | Evaluated repeating the same measurements with the same sensor/different sensors, and usually expressed as standard deviation. |
| Lifetime | Evaluated by analyzing the same solution or acquiring calibration lines over time, usually for weeks or months. It is the time after which the signal is decreased by a fixed value (for example 10%). |
| Electrode Modifier | Analyte | Tecnhique | LoD | Conditions |
|---|---|---|---|---|
| NiAl [102] | Methanol, Ethanol | CV | 3 ppm | 0.1 M NaOH |
| NiAl [103] | Ethanol | CV | 5 mM | 0.1 M NaOH |
| Pt nanoparticles + NiAl [104] | Ethanol | A | 0.05 mM | 0.1 M NaOH |
| Glucose | A | 0.025 mM | 0.1 M NaOH | |
| NiAl [105] | Glucose | CV | - | 0.1 M NaOH |
| NiAl + chitosane [106] | Glucose | CA | 0.01 mM | 0.1 M NaOH |
| CuAl [107] | Glucose | A | 0.02 µM | 0.1 M NaOH |
| NiAl CNT [108] | Glucose | CV | - | 0.1 M NaOH |
| NiAl [109] | Alphatic ammines | A | 0.7 µM | 0.1 M NaOH |
| Aromatic ammines | A | 6 µM | 0.1 M NaOH | |
| NiAl [110] | Phenol | A | 1 µM | 0.1 M NaOH |
| Glucose | A | 0.01 mM | 0.1 M NaOH | |
| NiAl [111] | Glyphosate | A | 1 µM | 0.1 M NaOH |
| Glufosinate | A | 5 µM | 0.1 M NaOH | |
| NiFe [112] | H2O2 | A | 0.5 µM | 0.1 M NaOH |
| CoAl [113] | Salycilyc Acid | A | 0.2 µM | 0.1 M NaOH |
| CoAl [114] | Aniline | A | 0.02 µM | 0.1 M NaOH |
| Phenol | A | 0.3 µM | 0.1 M NaOH | |
| NiAl [115] | Bisphenol A | DPV | 7 nM | 0.1 M pH 8.5 phosphate buffer solution |
| NiAl on carbon cloth [116] | Glucose | A | 0.2 µM | 0.1 M NaOH |
| CoAl [117] | Glucose (+ other sugar) | A | 10 µM | 0.1 M NaOH |
| CNF@NiCo [118] | Glucose | A | 0.03 µM | 0.1 M NaOH |
| NiAl/Electrochemical reduced Graphene Oxide [34] | Glucose | A | 0.6 µM | 0.1 M NaOH |
| NiAl/GO [120] | Guanine | LSV | 3 nM | 0.1 M pH 7 posphate buffer solution |
| Adenine | LSV | 20 nM | 0.1 M pH 7 posphate buffer solution | |
| CNTs@CuMn [121] | H2S | A | 0.3 nM | 0.1 M Posphate buffer saline |
| NiAl/graphene [122] | Dopamine | CV | 0.1 mM | 0.1 M NaOH |
| Ultrathin NiFe [123] | Glucose | A | 0.6 µM | 0.1 M NaOH |
| NiAl nanosheets [124] | Glucose | A | 5 µM | 0.1 M NaOH |
| CoAl/Naphthol Green B [125] | H2O2 | A | 0.9 µM | 0.1 M NaOH |
| NiFe Carbon cloth [126] | NO2− | A | 0.02 µM | Posphate Buffer Saline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonelli, D.; Gualandi, I.; Musella, E.; Scavetta, E. Synthesis and Characterization of Layered Double Hydroxides as Materials for Electrocatalytic Applications. Nanomaterials 2021, 11, 725. https://doi.org/10.3390/nano11030725
Tonelli D, Gualandi I, Musella E, Scavetta E. Synthesis and Characterization of Layered Double Hydroxides as Materials for Electrocatalytic Applications. Nanomaterials. 2021; 11(3):725. https://doi.org/10.3390/nano11030725
Chicago/Turabian StyleTonelli, Domenica, Isacco Gualandi, Elisa Musella, and Erika Scavetta. 2021. "Synthesis and Characterization of Layered Double Hydroxides as Materials for Electrocatalytic Applications" Nanomaterials 11, no. 3: 725. https://doi.org/10.3390/nano11030725
APA StyleTonelli, D., Gualandi, I., Musella, E., & Scavetta, E. (2021). Synthesis and Characterization of Layered Double Hydroxides as Materials for Electrocatalytic Applications. Nanomaterials, 11(3), 725. https://doi.org/10.3390/nano11030725









