In Vitro Co-Exposure to CeO2 Nanomaterials from Diesel Engine Exhaust and Benzo(a)Pyrene Induces Additive DNA Damage in Sperm and Cumulus Cells but Not in Oocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution and Suspension Preparation Prior to Exposure
2.2. Gamete Collection
2.3. Ethical Authorization
2.4. Gamete Exposure and DNA Damage Evaluation by the Comet Assay
3. Results and Discussion
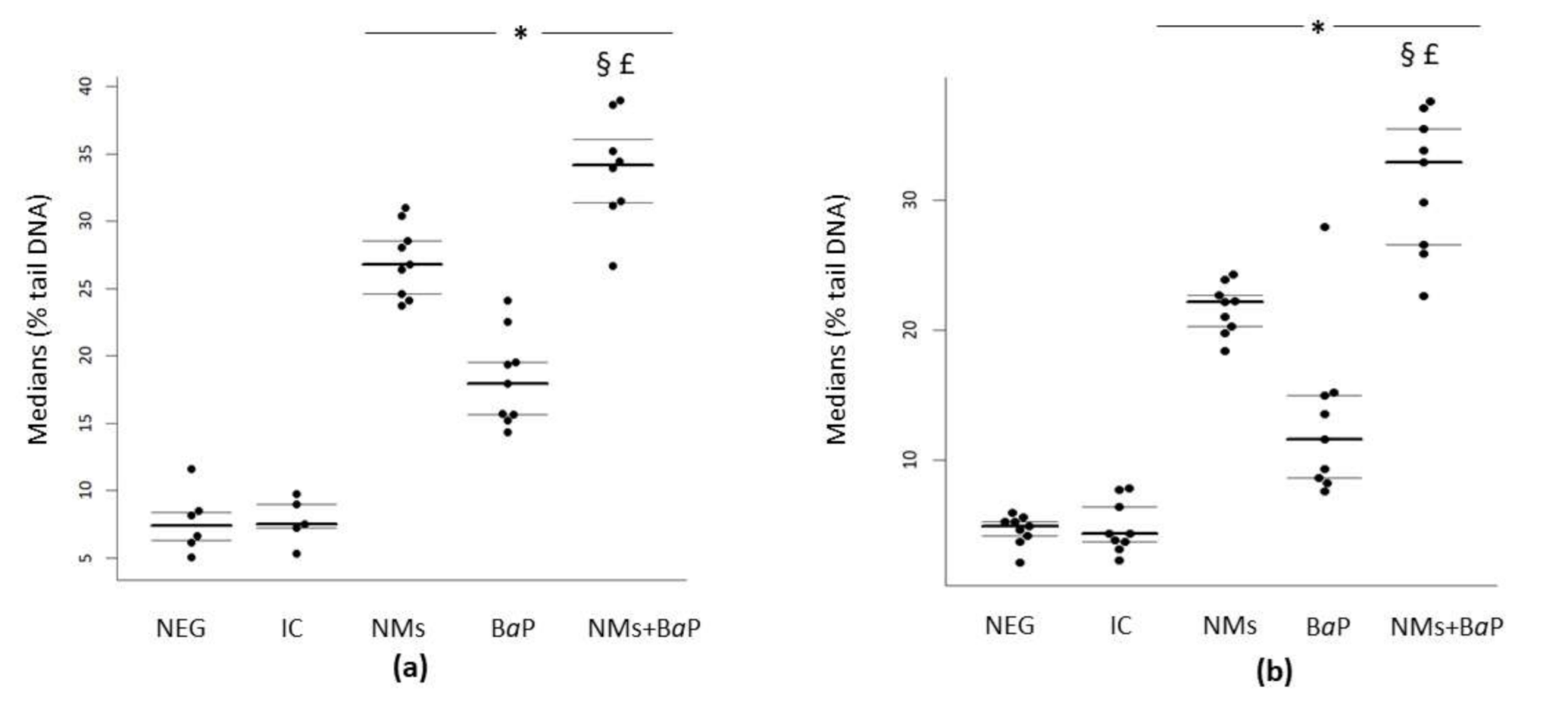
3.1. DNA Damage in Sperm Cells Induced by Aged CeO2 NMs and/or BaP
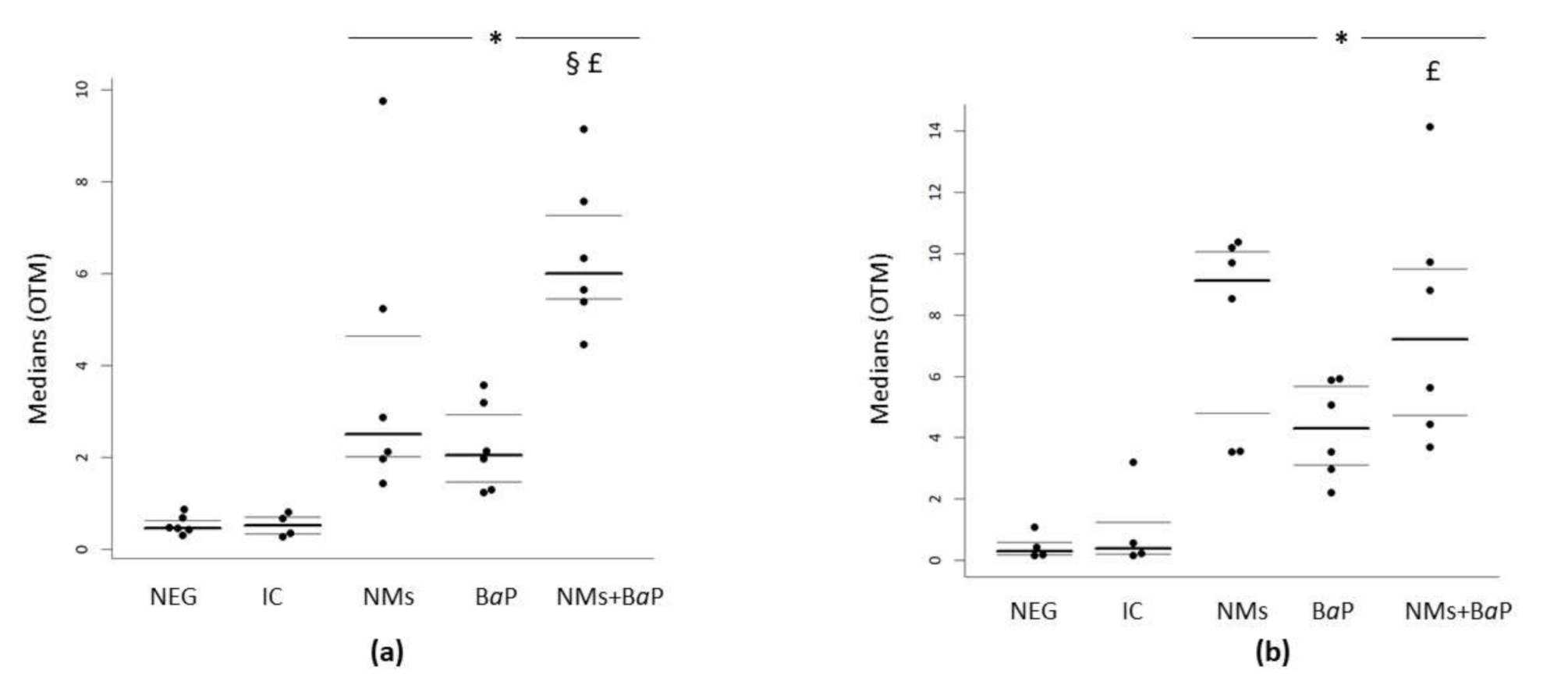
3.2. DNA Damage in COCs Induced by Aged CeO2 NMs and/or BaP
4. Conclusions
5. Limitations and Strengths
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute, H.E. Diesel Exhaust: Critical Analysis of Emissions, Exposure, and Health Effects. Available online: https://www.healtheffects.org/publication/diesel-exhaust-critical-analysis-emissions-exposure-and-health-effects (accessed on 2 January 2021).
- Kagawa, J. Health Effects of Diesel Exhaust Emissions--a Mixture of Air Pollutants of Worldwide Concern. Toxicology 2002, 181–182, 349–353. [Google Scholar] [CrossRef]
- Humans, I.W.G. on the E. of C.R. to Diesel and Gasoline Engine Exhausts and Some Nitroarenes. International Agency for Research on Cancer: Lion, France, 2014; ISBN 978-92-832-1328-4. [Google Scholar]
- Marr, L.C.; Kirchstetter, T.W.; Harley, R.A.; Miguel, A.H.; Hering, S.V.; Hammond, S.K. Characterization of Polycyclic Aromatic Hydrocarbons in Motor Vehicle Fuels and Exhaust Emissions. Environ. Sci. Technol. 1999, 33, 3091–3099. [Google Scholar] [CrossRef]
- Keith, L.H. The Source of U.S. EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, Y.; Zhang, S.; Hu, J.; Zhang, K.M.; Li, Z.; He, L.; Hao, J. Characterizing Particulate Polycyclic Aromatic Hydrocarbon Emissions from Diesel Vehicles Using a Portable Emissions Measurement System. Sci. Rep. 2017, 7, 10058. [Google Scholar] [CrossRef] [PubMed]
- European Commission Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Relating to Restrictions on the Marketing and Use of Certain Polycyclic Aromatic Hydrocarbons in Extender Oils and Tyres (Twenty-Seventh Amendment of Council Directive 76/769/EEC) EUR-Lex—52004PC0098—EN. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52004PC0098&from=HU (accessed on 2 January 2021).
- Perrin, J.; Tassistro, V.; Mandon, M.; Grillo, J.-M.; Botta, A.; Sari-Minodier, I. Tobacco Consumption and Benzo(a)Pyrene-Diol-Epoxide-DNA Adducts in Spermatozoa: In Smokers, Swim-up Procedure Selects Spermatozoa with Decreased DNA Damage. Fertil. Steril. 2011, 95, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- European Commission W.G.O.P.A.H. Ambient Air Pollution by Polycyclic Aromatic Hydrocarbons (PAH)—Cerca Con Google. Available online: https://ec.europa.eu/environment/air/pdf/annex_pah.pdf (accessed on 1 February 2021).
- Watanabe, S.; Kamiguchi, Y. Chromosome Analysis of Human Spermatozoa Following in Vitro Exposure to Cyclophosphamide, Benzo(a)Pyrene and N-Nitrosodimethylamine in the Presence of Rat Liver S9. Mutat. Res. 2001, 491, 57–63. [Google Scholar] [CrossRef]
- Einaudi, L.; Courbiere, B.; Tassistro, V.; Prevot, C.; Sari-Minodier, I.; Orsiere, T.; Perrin, J. In Vivo Exposure to Benzo(a)Pyrene Induces Significant DNA Damage in Mouse Oocytes and Cumulus Cells. Hum. Reprod. Oxf. Engl. 2014, 29, 548–554. [Google Scholar] [CrossRef]
- Sipinen, V.; Laubenthal, J.; Baumgartner, A.; Cemeli, E.; Linschooten, J.O.; Godschalk, R.W.L.; Van Schooten, F.J.; Anderson, D.; Brunborg, G. In Vitro Evaluation of Baseline and Induced DNA Damage in Human Sperm Exposed to Benzo[a]Pyrene or Its Metabolite Benzo[a]Pyrene-7,8-Diol-9,10-Epoxide, Using the Comet Assay. Mutagenesis 2010, 25, 417–425. [Google Scholar] [CrossRef]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef]
- Ginsberg, G.L.; Atherholt, T.B. Transport of DNA-Adducting Metabolites in Mouse Serum Following Benzo[a]Pyrene Administration. Carcinogenesis 1989, 10, 673–679. [Google Scholar] [CrossRef]
- Mattison, D.R.; Singh, H.; Takizawa, K.; Thomford, P.J. Ovarian Toxicity of Benzo(a)Pyrene and Metabolites in Mice. Reprod. Toxicol. 1989, 3, 115–125. [Google Scholar] [CrossRef]
- Neal, M.S.; Zhu, J.; Holloway, A.C.; Foster, W.G. Follicle Growth Is Inhibited by Benzo-[a]-Pyrene, at Concentrations Representative of Human Exposure, in an Isolated Rat Follicle Culture Assay. Hum. Reprod. Oxf. Engl. 2007, 22, 961–967. [Google Scholar] [CrossRef][Green Version]
- Mattison, D.R.; White, N.B.; Nightingale, M.R. The Effect of Benzo(a)Pyrene on Fertility, Primordial Oocyte Number, and Ovarian Response to Pregnant Mare’s Serum Gonadotropin. Pediatr. Pharmacol. 1980, 1, 143–151. [Google Scholar]
- Sheng, F.; Ji, Y.; Ma, Y.; Ding, H.; Zhang, Q.; Li, W. Polycyclic Aromatic Hydrocarbons Cause Follicle Atresia and Apoptosis in Mouse Ovarian Follicles Cultured in Vitro That Can Be Reduced with the Activator of PI3K/Akt Pathway, 740Y-P. Crit. Care Obstet. Gynecol. 2018, 4. [Google Scholar] [CrossRef]
- Neal, M.S.; Mulligan Tuttle, A.M.; Casper, R.F.; Lagunov, A.; Foster, W.G. Aryl Hydrocarbon Receptor Antagonists Attenuate the Deleterious Effects of Benzo[a]Pyrene on Isolated Rat Follicle Development. Reprod. Biomed. Online 2010, 21, 100–108. [Google Scholar] [CrossRef]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Han, R.; Savouret, J.F.; Casper, R.F. Resveratrol, a Natural Aryl Hydrocarbon Receptor Antagonist, Protects Sperm from DNA Damage and Apoptosis Caused by Benzo(a)Pyrene. Reprod. Toxicol. Elmsford N 2001, 15, 479–486. [Google Scholar] [CrossRef]
- Shi, J.P.; Evans, D.E.; Khan, A.A.; Harrison, R.M. Sources and Concentration of Nanoparticles (<10 nm Diameter) in the Urban Atmosphere. Atmos. Environ. 2001, 35, 1193–1202. [Google Scholar] [CrossRef]
- Slezakova, K.; Morais, S.; do Carmo Pereira, M. Atmospheric Nanoparticles and Their Impacts on Public Health. Curr. Top. Public Health 2013. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J.A. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef]
- Gaiser, B.K.; Fernandes, T.F.; Jepson, M.; Lead, J.R.; Tyler, C.R.; Stone, V. Assessing Exposure, Uptake and Toxicity of Silver and Cerium Dioxide Nanoparticles from Contaminated Environments. Environ. Health Glob. Access Sci. Source 2009, 8 Suppl. 1, S2. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Donaldson, K.; Duffin, R.; Tran, L.; Kelly, F.; Mudway, I.; Morin, J.-P.; Guest, R.; Jenkinson, P.; Samaras, Z.; et al. Hazard and Risk Assessment of a Nanoparticulate Cerium Oxide-Based Diesel Fuel Additive—A Case Study. Inhal. Toxicol. 2008, 20, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nazarenko, Y.; Zhang, L.; Calderon, L.; Lee, K.-B.; Garfunkel, E.; Schwander, S.; Tetley, T.D.; Chung, K.F.; Porter, A.E.; et al. Impacts of a Nanosized Ceria Additive on Diesel Engine Emissions of Particulate and Gaseous Pollutants. Environ. Sci. Technol. 2013, 47, 13077–13085. [Google Scholar] [CrossRef]
- Benameur, L.; Auffan, M.; Cassien, M.; Liu, W.; Culcasi, M.; Rahmouni, H.; Stocker, P.; Tassistro, V.; Bottero, J.-Y.; Rose, J.; et al. DNA Damage and Oxidative Stress Induced by CeO2 Nanoparticles in Human Dermal Fibroblasts: Evidence of a Clastogenic Effect as a Mechanism of Genotoxicity. Nanotoxicology 2015, 9, 696–705. [Google Scholar] [CrossRef]
- Minarchick, V.C.; Stapleton, P.A.; Sabolsky, E.M.; Nurkiewicz, T.R. Cerium Dioxide Nanoparticle Exposure Improves Microvascular Dysfunction and Reduces Oxidative Stress in Spontaneously Hypertensive Rats. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef]
- Adebayo, O.A.; Akinloye, O.; Adaramoye, O.A. Cerium Oxide Nanoparticle Elicits Oxidative Stress, Endocrine Imbalance and Lowers Sperm Characteristics in Testes of Balb/c Mice. Andrologia 2018, 50. [Google Scholar] [CrossRef]
- Cotena, M.; Auffan, M.; Robert, S.; Tassistro, V.; Resseguier, N.; Rose, J.; Perrin, J. CeO2 Nanomaterials from Diesel Engine Exhaust Induce DNA Damage and Oxidative Stress in Human and Rat Sperm In Vitro. Nanomaterials 2020, 10, 2327. [Google Scholar] [CrossRef] [PubMed]
- Préaubert, L.; Tassistro, V.; Auffan, M.; Sari-Minodier, I.; Rose, J.; Courbiere, B.; Perrin, J. Very Low Concentration of Cerium Dioxide Nanoparticles Induce DNA Damage, but No Loss of Vitality, in Human Spermatozoa. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2018, 50, 236–241. [Google Scholar] [CrossRef]
- Preaubert, L.; Courbiere, B.; Achard, V.; Tassistro, V.; Greco, F.; Orsiere, T.; Bottero, J.-Y.; Rose, J.; Auffan, M.; Perrin, J. Cerium Dioxide Nanoparticles Affect in Vitro Fertilization in Mice. Nanotoxicology 2016, 10, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Courbiere, B.; Auffan, M.; Rollais, R.; Tassistro, V.; Bonnefoy, A.; Botta, A.; Rose, J.; Orsière, T.; Perrin, J. Ultrastructural Interactions and Genotoxicity Assay of Cerium Dioxide Nanoparticles on Mouse Oocytes. Int. J. Mol. Sci. 2013, 14, 21613–21628. [Google Scholar] [CrossRef]
- Qin, F.; Shen, T.; Li, J.; Qian, J.; Zhang, J.; Zhou, G.; Tong, J. SF-1 Mediates Reproductive Toxicity Induced by Cerium Oxide Nanoparticles in Male Mice. J. Nanobiotechnology 2019, 17, 41. [Google Scholar] [CrossRef]
- Asweto, C.O.; Wu, J.; Hu, H.; Feng, L.; Yang, X.; Duan, J.; Sun, Z. Combined Effect of Silica Nanoparticles and Benzo[a]Pyrene on Cell Cycle Arrest Induction and Apoptosis in Human Umbilical Vein Endothelial Cells. Int. J. Environ. Res. Public. Health 2017, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Proux, O.; Masion, A.; Liu, W.; Benameur, L.; Ziarelli, F.; Botta, A.; Chaneac, C.; Bottero, J.-Y. Is There a Trojan-Horse Effect during Magnetic Nanoparticles and Metalloid Cocontamination of Human Dermal Fibroblasts? Environ. Sci. Technol. 2012, 46, 10789–10796. [Google Scholar] [CrossRef] [PubMed]
- Silins, I.; Högberg, J. Combined Toxic Exposures and Human Health: Biomarkers of Exposure and Effect. Int. J. Environ. Res. Public. Health 2011, 8, 629–647. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy-Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, C.E.; Ioannides, C. Metabolic Action of Polycyclic Aromatic Hydrocarbons to Mutagens in the Ames Test by Various Animal Species Including Man. Mutat. Res. Mol. Mech. Mutagen. 1989, 211, 147–151. [Google Scholar] [CrossRef]
- Auffan, M.; Tella, M.; Liu, W.; Pariat, A.; Cabié, M.; Borschneck, D.; Angeletti, B.; Landrot, G.; Mouneyrac, C.; Giamberini, L.; et al. Structural and Physical–Chemical Behavior of a CeO2 Nanoparticle Based Diesel Additive during Combustion and Environmental Release. Environ. Sci. 2017, 4, 1974–1980. [Google Scholar] [CrossRef]
- Audebert, M.; Riu, A.; Jacques, C.; Hillenweck, A.; Jamin, E.L.; Zalko, D.; Cravedi, J.-P. Use of the ΓH2AX Assay for Assessing the Genotoxicity of Polycyclic Aromatic Hydrocarbons in Human Cell Lines. Toxicol. Lett. 2010, 199, 182–192. [Google Scholar] [CrossRef]
- Alvares, A.P.; Kappas, A. Heterogeneity of Cytochrome P-450s Induced by Polychlorinated Biphenyls. J. Biol. Chem. 1977, 252, 6373–6378. [Google Scholar] [CrossRef]
- Thomas, P.E.; Reik, L.M.; Ryan, D.E.; Levin, W. Induction of Two Immunochemically Related Rat Liver Cytochrome P-450 Isozymes, Cytochromes P-450c and P-450d, by Structurally Diverse Xenobiotics. J. Biol. Chem. 1983, 258, 4590–4598. [Google Scholar] [CrossRef]
- van Leeuwen, D.M.; Gottschalk, R.W.H.; van Herwijnen, M.H.; Moonen, E.J.; Kleinjans, J.C.S.; van Delft, J.H.M. Differential Gene Expression in Human Peripheral Blood Mononuclear Cells Induced by Cigarette Smoke and Its Constituents. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 86, 200–210. [Google Scholar] [CrossRef]
- Baumgartner, A.; Kurzawa-Zegota, M.; Laubenthal, J.; Cemeli, E.; Anderson, D. Comet-Assay Parameters as Rapid Biomarkers of Exposure to Dietary/Environmental Compounds—An in Vitro Feasibility Study on Spermatozoa and Lymphocytes. Mutat. Res. 2012, 743, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Perrin, J.; Auffan, M.; Tassistro, V.; Orsière, T.; Courbiere, B. A New Approach for the Oocyte Genotoxicity Assay: Adaptation of Comet Assay on Mouse Cumulus–Oocyte Complexes. Lab. Anim. 2015. [Google Scholar] [CrossRef] [PubMed]
- Madureira, D.J.; Weiss, F.T.; Midwoud, P.V.; Helbling, D.E.; Sturla, S.J.; Schirmer, K. Systems Toxicology Approach to Understand the Kinetics of Benzo(a)Pyrene Uptake, Biotransformation, and DNA Adduct Formation in a Liver Cell Model. Available online: https://pubs.acs.org/doi/pdf/10.1021/tx400446q (accessed on 10 January 2021).
- Peters, T. Serum Albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [CrossRef]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The Extraordinary Ligand Binding Properties of Human Serum Albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef]
- Motwani, H.V.; Westberg, E.; Törnqvist, M. Interaction of Benzo[a]Pyrene Diol Epoxide Isomers with Human Serum Albumin: Site Specific Characterisation of Adducts and Associated Kinetics. Sci. Rep. 2016, 6, 36243. [Google Scholar] [CrossRef]
- Roustan, A.; Perrin, J.; Berthelot-Ricou, A.; Lopez, E.; Botta, A.; Courbiere, B. Evaluating Methods of Mouse Euthanasia on the Oocyte Quality: Cervical Dislocation versus Isoflurane Inhalation. Lab. Anim. 2012, 46, 167–169. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Baumgartner, A.; Cemeli, E.; Anderson, D. The Comet Assay in Male Reproductive Toxicology. Cell Biol. Toxicol. 2009, 25, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Berthelot-Ricou, A.; Perrin, J.; Di Giorgio, C.; De Meo, M.; Botta, A.; Courbiere, B. Comet Assay on Mouse Oocytes: An Improved Technique to Evaluate Genotoxic Risk on Female Germ Cells. Fertil. Steril. 2011, 95, 1452–1457. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- World Health Organization WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://www.who.int/publications-detail-redirect/9789241547789 (accessed on 3 January 2021).
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle Interactions with Co-Existing Contaminants: Joint Toxicity, Bioaccumulation and Risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.M.; George, J.D.; Gold, K.W.; Cibulas, W.; DeRosa, C.T. ATSDR Evaluation of Health Effects of Chemicals. IV. Polycyclic Aromatic Hydrocarbons (PAHs): Understanding a Complex Problem. Toxicol. Ind. Health 1996, 12, 742–971. [Google Scholar] [CrossRef] [PubMed]
- Oliveri Conti, G.; Calogero, A.E.; Giacone, F.; Fiore, M.; Barchitta, M.; Agodi, A.; Ferrante, M. B(a)P Adduct Levels and Fertility: A Cross-sectional Study in a Sicilian Population. Mol. Med. Rep. 2017, 15, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Senft, A.P.; Dalton, T.P.; Nebert, D.W.; Genter, M.B.; Puga, A.; Hutchinson, R.J.; Kerzee, J.K.; Uno, S.; Shertzer, H.G. Mitochondrial Reactive Oxygen Production Is Dependent on the Aromatic Hydrocarbon Receptor. Free Radic. Biol. Med. 2002, 33, 1268–1278. [Google Scholar] [CrossRef]
- Zenzes, M.T.; Puy, L.A.; Bielecki, R.; Reed, T.E. Detection of Benzo[a]Pyrene Diol Epoxide-DNA Adducts in Embryos from Smoking Couples: Evidence for Transmission by Spermatozoa. Mol. Hum. Reprod. 1999, 5, 125–131. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, P.; Han, Y.; Lu, C.; Wang, S.; Gu, A.; Fu, G.; Zhao, R.; Song, L.; Wang, X. Urinary Metabolites of Polycyclic Aromatic Hydrocarbons in Relation to Idiopathic Male Infertility. Hum. Reprod. Oxf. Engl. 2009, 24, 1067–1074. [Google Scholar] [CrossRef]
- Zhang, C.M.; Sun, Z.X.; Wang, Z.L.; Chen, J.S.; Chang, Z.; Wang, Z.; Zhu, L.; Ma, Z.H.; Peng, Y.J.; Xu, Z.A.; et al. Abnormal Methylation of Spermatozoa Induced by Benzo(a)Pyrene in Rats. Hum. Exp. Toxicol. 2019, 38, 846–856. [Google Scholar] [CrossRef]
- Mahgoub, H.A. Nanoparticles Used for Extraction of Polycyclic Aromatic Hydrocarbons. Available online: https://www.hindawi.com/journals/jchem/2019/4816849/ (accessed on 3 January 2021).
- Sun, J.D.; Wolff, R.K.; Kanapilly, G.M. Deposition, Retention, and Biological Fate of Inhaled Benzo(a)Pyrene Adsorbed onto Ultrafine Particles and as a Pure Aerosol. Toxicol. Appl. Pharmacol. 1982, 65, 231–244. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Xing, B. Adsorption of Polycyclic Aromatic Hydrocarbons by Carbon Nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Han, C.; Zhao, A.; Hahn, B.; Grecsek, H. Interaction of Engineered Nanomaterials with Hydrophobic Organic Pollutants. Nanotechnology 2016, 27, 284003. [Google Scholar] [CrossRef]
- Campen, K.A.; McNatty, K.P.; Pitman, J.L. A Protective Role of Cumulus Cells after Short-Term Exposure of Rat Cumulus Cell-Oocyte Complexes to Lifestyle or Environmental Contaminants. Reprod. Toxicol. Elmsford N 2017, 69, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of Porcine Oocytes Against Apoptotic Cell Death Caused by Oxidative Stress During In Vitro Maturation: Role of Cumulus Cells1. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef]
- Tanaka, H.; Takeo, S.; Monji, Y.; Kuwayama, T.; Iwata, H. Maternal Liver Damage Delays Meiotic Resumption in Bovine Oocytes through Impairment of Signalling Cascades Originated from Low P38MAPK Activity in Cumulus Cells. Reprod. Domest. Anim. Zuchthyg. 2014, 49, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Shaeib, F.; Khan, S.N.; Ali, I.; Thakur, M.; Saed, M.G.; Dai, J.; Awonuga, A.O.; Banerjee, J.; Abu-Soud, H.M. The Defensive Role of Cumulus Cells Against Reactive Oxygen Species Insult in Metaphase II Mouse Oocytes. Reprod. Sci. Thousand Oaks Calif. 2016, 23, 498–507. [Google Scholar] [CrossRef]
- Mattison, D.R. The Mechanisms of Action of Reproductive Toxins. Am. J. Ind. Med. 1983, 4, 65–79. [Google Scholar] [CrossRef]
- Sadeu, J.C.; Foster, W.G. Cigarette Smoke Condensate Exposure Delays Follicular Development and Function in a Stage-Dependent Manner. Fertil. Steril. 2011, 95, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Sadeu, J.C.; Foster, W.G. Effect of in Vitro Exposure to Benzo[a]Pyrene, a Component of Cigarette Smoke, on Folliculogenesis, Steroidogenesis and Oocyte Nuclear Maturation. Reprod. Toxicol. 2011, 31, 402–408. [Google Scholar] [CrossRef]
- Esengen, S.; Seçkin, U.; Borman, P.; Bodur, H.; Kutsal, Y.G.; Yücel, M. Drug Consumption in a Group of Elderly Residents of a Nursing Home: Relationship to Cognitive Impairment and Disability. J. Am. Med. Dir. Assoc. 2000, 1, 197–201. [Google Scholar]
- Siddique, S.; Sadeu, J.C.; Foster, W.G.; Feng, Y.-L.; Zhu, J. In Vitro Exposure to Cigarette Smoke Induces Oxidative Stress in Follicular Cells of F₁ Hybrid Mice. J. Appl. Toxicol. JAT 2014, 34, 224–226. [Google Scholar] [CrossRef]
- Murdoch, W.J.; Van Kirk, E.A. Estrogenic Upregulation of DNA Polymerase Beta in Oocytes of Preovulatory Ovine Follicles. Mol. Reprod. Dev. 2001, 58, 417–423. [Google Scholar] [CrossRef]
- Maman, E.; Prokopis, K.; Levron, J.; Carmely, A.; Dor, J.; Meirow, D. Does Controlled Ovarian Stimulation Prior to Chemotherapy Increase Primordial Follicle Loss and Diminish Ovarian Reserve? An Animal Study. Hum. Reprod. Oxf. Engl. 2009, 24, 206–210. [Google Scholar] [CrossRef]
- Meirow, D.; Epstein, M.; Lewis, H.; Nugent, D.; Gosden, R.G. Administration of Cyclophosphamide at Different Stages of Follicular Maturation in Mice: Effects on Reproductive Performance and Fetal Malformations. Hum. Reprod. Oxf. Engl. 2001, 16, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Rekhadevi, P.; Diggs, D.; Huderson, A.; Harris, K.; Archibong, A.; Ramesh, A. Metabolism of the Environmental Toxicant Benzo(a)Pyrene by Subcellular Fractions of Human Ovary. Hum. Exp. Toxicol. 2014, 33, 196–202. [Google Scholar] [CrossRef]
- Mattison, D.R.; Shiromizu, K.; Nightingale, M.S. Oocyte Destruction by Polycyclic Aromatic Hydrocarbons. Am. J. Ind. Med. 1983, 4, 191–202. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of Quinones in Toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Archibong, A.E.; Niaz, M.S. Ovarian Susceptibility to Benzo[a]Pyrene: Tissue Burden of Metabolites and DNA Adducts in F-344 Rats. J. Toxicol. Environ. Health A 2010, 73, 1611–1625. [Google Scholar] [CrossRef]
- Zhang, M.; Miao, Y.; Chen, Q.; Cai, M.; Dong, W.; Dai, X.; Lu, Y.; Zhou, C.; Cui, Z.; Xiong, B. BaP Exposure Causes Oocyte Meiotic Arrest and Fertilization Failure to Weaken Female Fertility. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 342–352. [Google Scholar] [CrossRef]
- Eaglesome, M.D.; Hare, W.C.; Singh, E.L. Embryo Transfer: A Discussion on Its Potential for Infectious Disease Control Based on a Review of Studies on Infection of Gametes and Early Embryos by Various Agents. Can. Vet. J. Rev. Veterinaire Can. 1980, 21, 106–112. [Google Scholar]
- Turner, K.; Horobin, R.W. Permeability of the Mouse Zona Pellucida: A Structure-Staining-Correlation Model Using Coloured Probes. J. Reprod. Fertil. 1997, 111, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T. Determination of Follicle Growth Rate in the Ovary of the Immature Mouse. J. Reprod. Fertil. 1970, 21, 81–93. [Google Scholar] [CrossRef]
- Hou, C.-C.; Zhu, J.-Q. Nanoparticles and Female Reproductive System: How Do Nanoparticles Affect Oogenesis and Embryonic Development. Oncotarget 2017, 8, 109799–109817. [Google Scholar] [CrossRef] [PubMed]
- von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid. Redox Signal. 2020, 32, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Derijck, A.; van der Heijden, G.; Giele, M.; Philippens, M.; de Boer, P. DNA Double-Strand Break Repair in Parental Chromatin of Mouse Zygotes, the First Cell Cycle as an Origin of de Novo Mutation. Hum. Mol. Genet. 2008, 17, 1922–1937. [Google Scholar] [CrossRef] [PubMed]
- Drevet, J.R.; Aitken, R.J. Oxidative Damage to Sperm DNA: Attack and Defense. Adv. Exp. Med. Biol. 2019, 1166, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.M.; Winship, A.; Liew, S.H.; Hutt, K. The Capacity of Oocytes for DNA Repair. Cell. Mol. Life Sci. CMLS 2018, 75, 2777–2792. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.; Dale, B.; Cohen, M. DNA Damage and Repair in Human Oocytes and Embryos: A Review. Zygote Camb. Engl. 2010, 18, 357–365. [Google Scholar] [CrossRef]
- van den Berg, M.M.J.; van Maarle, M.C.; van Wely, M.; Goddijn, M. Genetics of Early Miscarriage. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
| Rat Sperm | Human Sperm | |||||
|---|---|---|---|---|---|---|
| Condition | MEDIAN Values | 1st Quartile | 3rd Quartile | MEDIAN Values | 1st Quartile | 3rd Quartile |
| Negative control | 4.9 | 4.17 | 5.3 | 7.39 | 6.27 | 8.39 |
| IC | 4.34 | 3.73 | 6.4 | 7.49 | 7.20 | 8.99 |
| NMs | 22.15 | 20.3 | 22.68 | 26.78 | 24.62 | 28.55 |
| BaP | 11.64 | 8.63 | 14.99 | 17.94 | 15.64 | 19.53 |
| NMs+BaP | 32.88 | 26.57 | 35.44 | 34.19 | 31.4 | 36.06 |
| Rat Cumulus Cells | Rat Oocytes | |||||
|---|---|---|---|---|---|---|
| Condition | MEDIAN Values | 1st Quartile | 3rd Quartile | MEDIAN Values | 1st Quartile | 3rd Quartile |
| Negative control | 0.46 | 0.43 | 0.62 | 0.3 | 0.17 | 0.58 |
| IC | 0.51 | 0.33 | 0.70 | 0.39 | 0.20 | 1.23 |
| NMs | 2.49 | 2 | 4.64 | 9.12 | 4.8 | 10.07 |
| BaP | 2.04 | 1.46 | 2.92 | 4.3 | 3.12 | 5.68 |
| NMs+BaP | 5.99 | 5.44 | 7.26 | 7.22 | 4.74 | 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotena, M.; Auffan, M.; Tassistro, V.; Resseguier, N.; Rose, J.; Perrin, J. In Vitro Co-Exposure to CeO2 Nanomaterials from Diesel Engine Exhaust and Benzo(a)Pyrene Induces Additive DNA Damage in Sperm and Cumulus Cells but Not in Oocytes. Nanomaterials 2021, 11, 478. https://doi.org/10.3390/nano11020478
Cotena M, Auffan M, Tassistro V, Resseguier N, Rose J, Perrin J. In Vitro Co-Exposure to CeO2 Nanomaterials from Diesel Engine Exhaust and Benzo(a)Pyrene Induces Additive DNA Damage in Sperm and Cumulus Cells but Not in Oocytes. Nanomaterials. 2021; 11(2):478. https://doi.org/10.3390/nano11020478
Chicago/Turabian StyleCotena, Martina, Mélanie Auffan, Virginie Tassistro, Noémie Resseguier, Jérôme Rose, and Jeanne Perrin. 2021. "In Vitro Co-Exposure to CeO2 Nanomaterials from Diesel Engine Exhaust and Benzo(a)Pyrene Induces Additive DNA Damage in Sperm and Cumulus Cells but Not in Oocytes" Nanomaterials 11, no. 2: 478. https://doi.org/10.3390/nano11020478
APA StyleCotena, M., Auffan, M., Tassistro, V., Resseguier, N., Rose, J., & Perrin, J. (2021). In Vitro Co-Exposure to CeO2 Nanomaterials from Diesel Engine Exhaust and Benzo(a)Pyrene Induces Additive DNA Damage in Sperm and Cumulus Cells but Not in Oocytes. Nanomaterials, 11(2), 478. https://doi.org/10.3390/nano11020478






