Nanostructure of Porous Si and Anodic SiO2 Surface Passivation for Improved Efficiency Porous Si Solar Cells
Abstract
1. Introduction
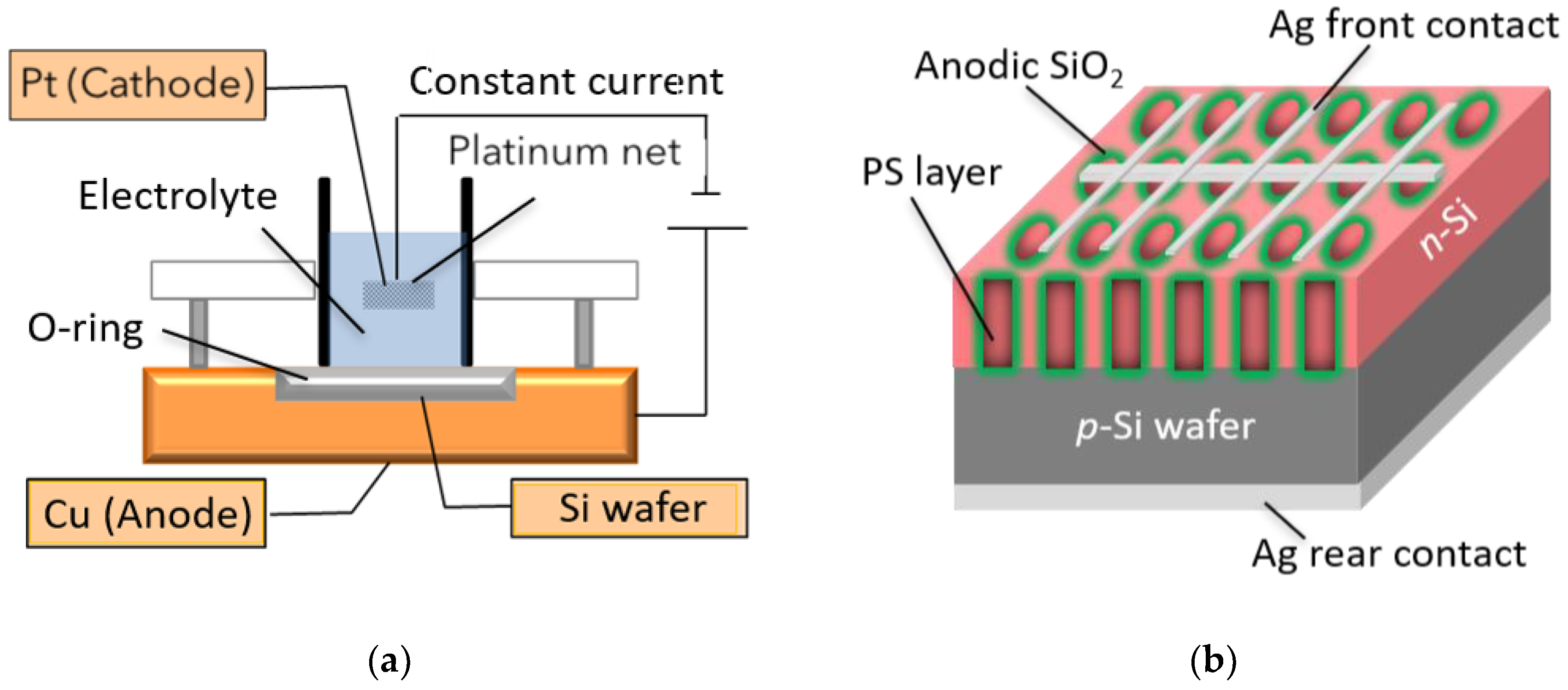
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Nanostructured PS and Depth of P-N Interface
3.2. Electrochemical Passivation Effect in Solar Cells with Thin PS Layer of 146 nm
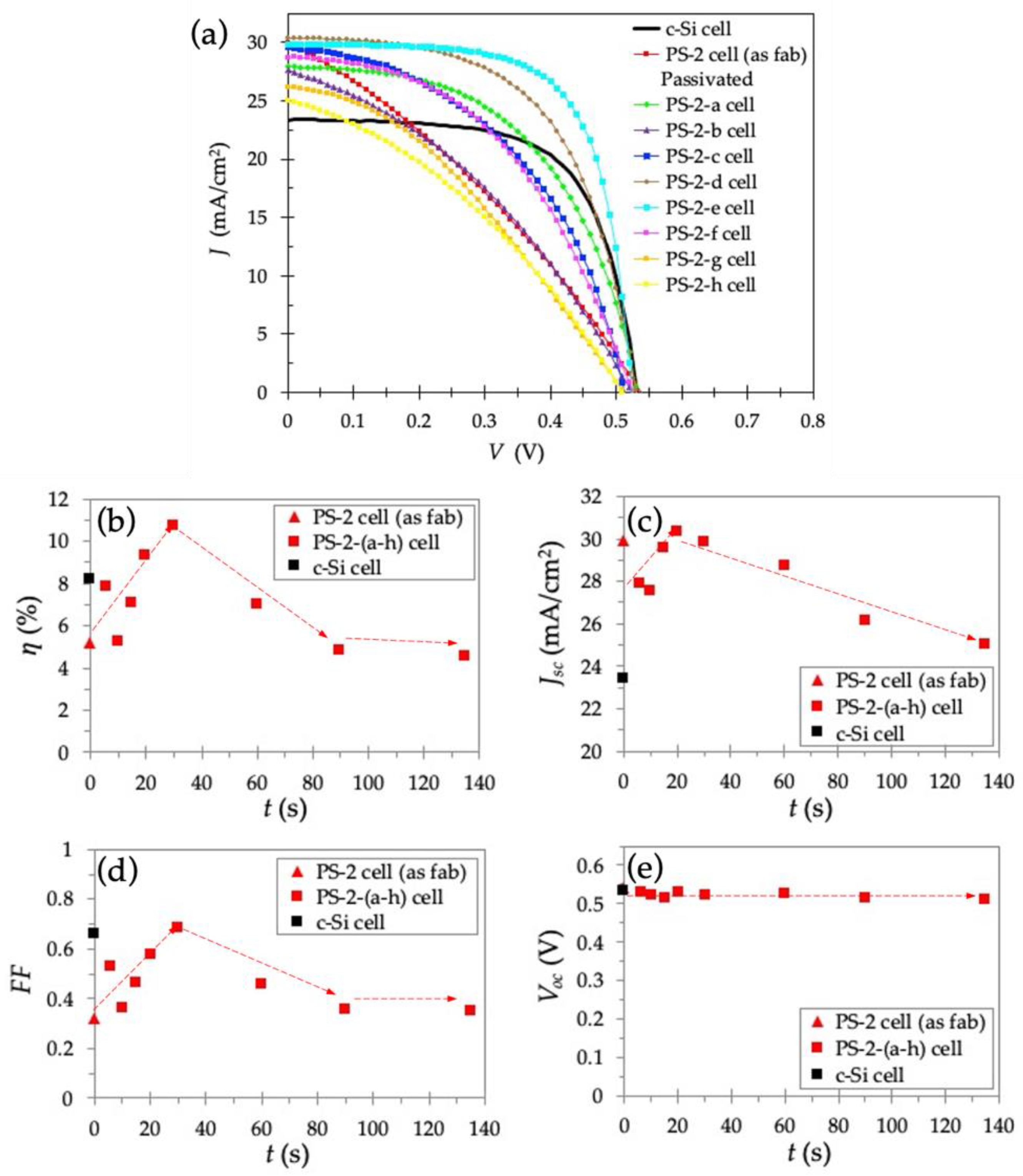
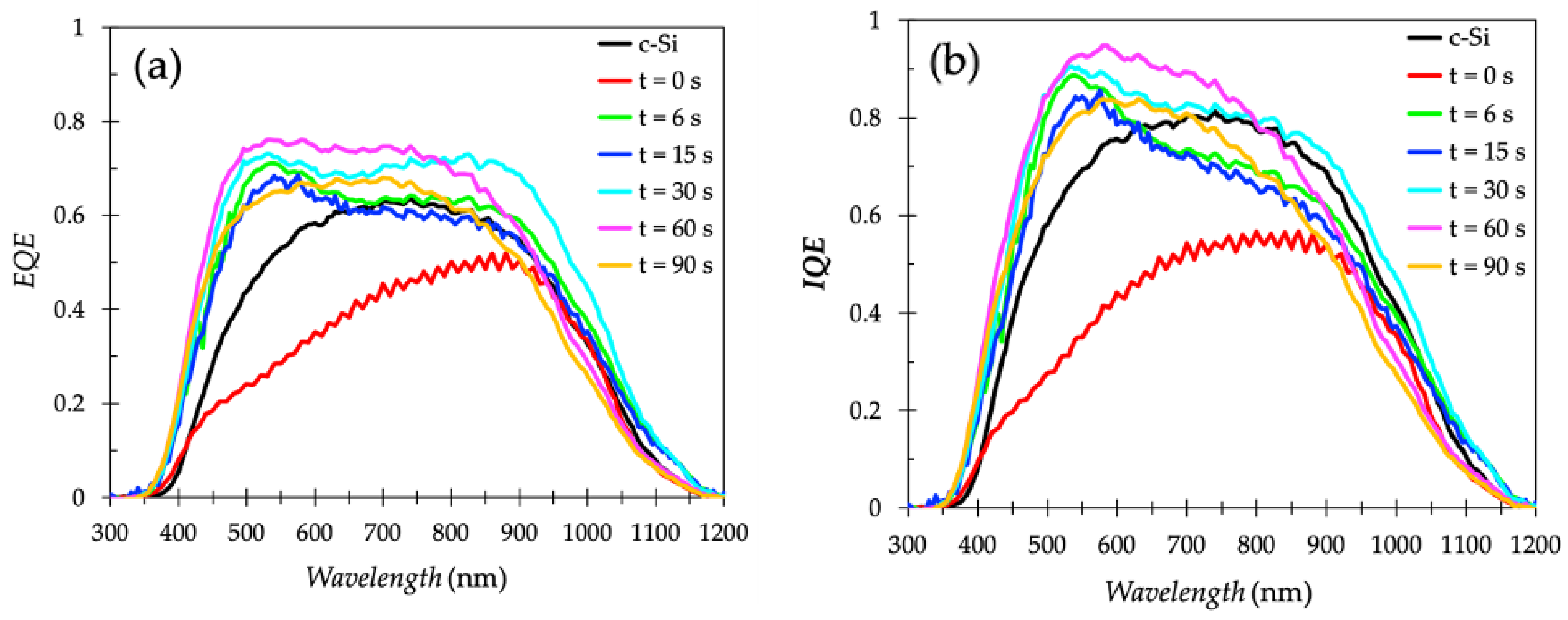
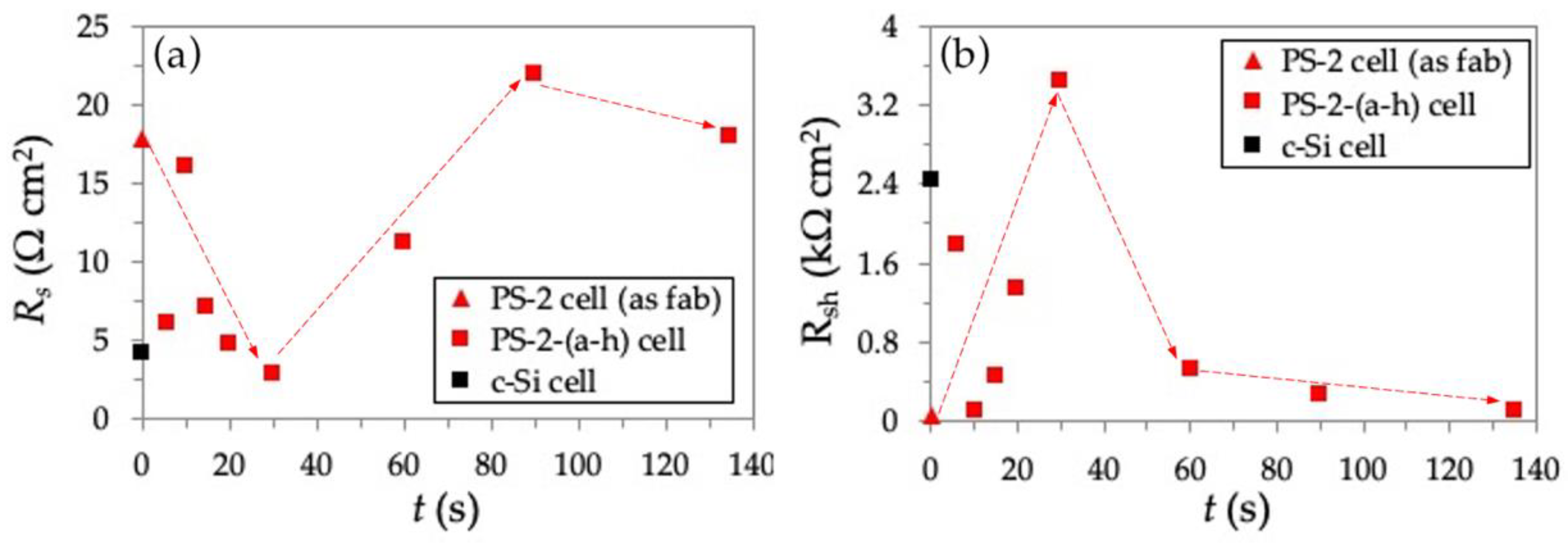
3.3. Electrochemical Passivation Effect in Solar Cells with Thick PS Layer of 191–255 nm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon nanostructures for photonics and photovoltaics. Nat. Nanotechnol. 2014, 9, 19–32. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photo over 26%. Nat. Energy. 2017, 2, 17032. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Akita, H.; Ihara, M. Epitaxial growth of silicon nanowire arrays at wafer-scale using high-speed rotating-disk CVD for improved light-trapping. CrystEngComm 2016, 18, 6153. [Google Scholar] [CrossRef]
- Wang, L.-X.; Zhou, Z.-Q.; Hao, H.-C.; Lu, M. A porous Si- emitter crystalline-Si solar cell with 18.97% efficiency. Nanotechnology 2016, 27, 425207. [Google Scholar] [CrossRef] [PubMed]
- Lukianov, A.; Ihara, M. Free-standing epitaxial silicon thin films for solar cells grown on double porous layers of silicon and electrochemically oxidized porous silicon dioxide. Thin Solid Films 2018, 648, 1–7. [Google Scholar] [CrossRef]
- Garnett, E.; Yang, P. Light Trapping in Silicon Nanowire Solar Cells. Nano Lett. 2010, 10, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Kelzenberg, M.D.; Boettcher, S.W.; Petykiewicz, J.A.; Turner-Evans, D.B.; Putnam, M.C.; Warren, E.L.; Spurgeon, J.M.; Briggs, R.M.; Lewis, N.S.; Atwater, H.A. Enhanced absorption and carrier collection in Si wire arrays for photovoltaic applications. Nat. Mater. 2010, 9, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Dzhafarov, T. Silicon Solar Cells with Nanoporous Silicon Layer. In Solar Cells—Research and Application Perspectives; Morales-Acevedo, A., Ed.; IntechOpen: London, UK, 2013; p. 42. ISBN 978-953-51-6313-8. [Google Scholar]
- Martí, A.; Araújo, G.L. Limiting efficiencies for photovoltaic energy conversion in multigap systems. Sol. Energy Mater. Sol. Cells 1996, 43, 203–222. [Google Scholar] [CrossRef]
- Chen, H.; Hou, X.; Li, G.; Zhang, F.; Yu, M.; Wang, X. Passivation of porous silicon by wet thermal oxidation. J. Appl. Phys. 1996, 79, 3282–3285. [Google Scholar] [CrossRef]
- Li, G.; Hou, X.; Yuan, S.; Chen, H.; Zhang, F.; Fan, H.; Wang, X. Passivation of light-emitting porous silicon by rapid thermal treatment in NH3. J. Appl. Phys. 1996, 80, 5967–5970. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Lai, H.; Li, C.; Wang, J. Investigation of passivation of porous silicon at room temperature. Solid State Commun. 2007, 142, 358–362. [Google Scholar] [CrossRef]
- Shiraz, H.G. Effect of anodization time on photovoltaic properties of nanoporous silicon based solar cells. Sustain. Energy Fuels 2017, 1, 652. [Google Scholar] [CrossRef]
- Khezami, L.; Jemai, A.B.; Alhathlool, R.; Rabha, M.B. Electronic quality improvement of crystalline silicon by stain etch-ing-based PS nanostructures for solar cells application. Sol. Energy 2016, 129, 38. [Google Scholar] [CrossRef]
- Aziz, W.J.; Ramizy, A.; Ibrahim, K.; Hassan, Z.; Omar, K. The effect of anti-reflection coating of porous silicon on solar cells efficiency. Optik 2011, 122, 1462–1465. [Google Scholar] [CrossRef]
- Hoex, B.B.; Gielis, J.J.; Van De Sanden, M.R.; Kessels, W.E. On the c-Si surface passivation mechanism by the negative-charge-dielectric Al2O3. J. Appl. Phys. 2008, 104, 113703. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, S.; Hua, X.; Lin, X.; Kong, X.; Dai, N.; Shen, W. An effective way to simultaneous realization of excellent optical and electrical performance in large-scale Si nano/microstructures. Prog. Photovoltaics: Res. Appl. 2014, 23, 964–972. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, S.; Tan, M.; Shen, W. SiO2 passivation layer grown by liquid phase deposition for silicon solar cell application. Front. Energy 2017, 11, 52–59. [Google Scholar] [CrossRef]
- Grant, N.E.; Kho, T.; Fong, K.; Franklin, E.; McIntosh, K.; Stocks, M.; Wan, Y.; Wang, E.-C.; Zin, N.; Murphy, J.; et al. Anodic oxidations: Excellent process durability and surface passivation for high efficiency silicon solar cells. Sol. Energy Mater. Sol. Cells 2019, 203, 110155. [Google Scholar] [CrossRef]
- Oh, J.; Yuan, H.-C.; Branz, H.M. An 18.2%-efficient black-silicon solar cell achieved through control of carrier recombination in nanostructures. Nat. Nanotechnol. 2012, 7, 743–748. [Google Scholar] [CrossRef]
- Patil, J.J.; Smith, B.D.; Grossman, J.C. Ultra-high aspect ratio functional nanoporous silicon via nucleated catalysts. RSC Adv. 2017, 7, 11537–11542. [Google Scholar] [CrossRef]
- Ben Slama, S.; Hajji, M.; Ezzaouia, H. Crystallization of amorphous silicon thin films deposited by PECVD on nickel-metalized porous silicon. Nanoscale Res. Lett. 2012, 7, 464. [Google Scholar] [CrossRef]
- Porous Silicon Homepage. Available online: https://www.poroussilicon.com/wafer-etching.html (accessed on 18 January 2021).
- Gautier, G.; Defforge, T.; Desplobain, S.; Billoué, J.; Capelle, M.; Povéda, P.; Vanga, K.; Lu, B.; Bardet, B.; Lascaud, J.; et al. Porous silicon in microelectronics: From academic studies to industry. ECS Trans. 2015, 69, 123. [Google Scholar] [CrossRef]
- Boehringer, M.; Artmann, H.; Witt, K. Porous Silicon in a Semiconductor Manufacturing Environment. J. Microelectromechanical Syst. 2012, 21, 1375–1381. [Google Scholar] [CrossRef]
- Hasegawa, K.; Takazawa, C.; Fujita, M.; Noda, S.; Ihara, M. Critical effect of nanometer-size surface roughness of a porous Si seed layer on the defect density of epitaxial Si films for solar cells by rapid vapor deposition. CrystEngComm 2018, 20, 1774–1778. [Google Scholar] [CrossRef]
- Shibahara, R.; Hasegawa, K.; Fave, A.; Fourmond, E.; Noda, S.; Ihara, M. Electrical Properties of Monocrystalline Thin Film Si for Solar Cells Fabricated By Rapid Vapor Deposition with Nano-Surface Controlling Double Layer Porous Si in H2; ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- Lukianov, A.; Murakami, K.; Takazawa, C.; Ihara, M. Formation of the seed layers for layer-transfer process silicon solar cells by zone-heating recrystallization of porous silicon structures. Appl. Phys. Lett. 2016, 108, 213904. [Google Scholar] [CrossRef]
- Bruggeman, D. Calculation of different physical constants of heterogen substances: Dielectric constants and conductibility of mixtures from isotrop substances. Ann. Phys. 1935, 416, 665–679. [Google Scholar] [CrossRef]
- Kriegler, R.J. Semiconductor Silicon; Huff, H.R., Burgess, R.R., Eds.; Electrochemical Society: Princeton, NJ, USA, 1973; p. 363. [Google Scholar]
- Beale, M.; Benjamin, J.; Uren, M.; Chew, N.; Cullis, A. An experimental and theoretical study of the formation and microstructure of porous silicon. J. Cryst. Growth 1985, 73, 622–636. [Google Scholar] [CrossRef]
- Deal, B.E. Thermal Oxidation Kinetics of Silicon in Pyrogenic H2O and 5% HCI/H2O Mixtures. J. Electrochem. Soc. 1978, 125, 576. [Google Scholar] [CrossRef]
- Patridge, S.L. Silicon-on-insulator technology. IEE Proc. E 1986, 133, 66. [Google Scholar]
- Knoops, H.C.M.; Potts, S.E.; Bol, A.A.; Kessels, W.M.M. Atomic Layer Deposition. Handb. Cryst. Growth 2015, 3, 1101–1134. [Google Scholar]
- Breitenstein, O.; Rakotoniaina, J.P.; Al Rifai, M.H.; Werner, M. Shunt types in crystalline silicon solar cells. Prog. Photovolt. Res. Appl. 2004, 12, 529–538. [Google Scholar] [CrossRef]
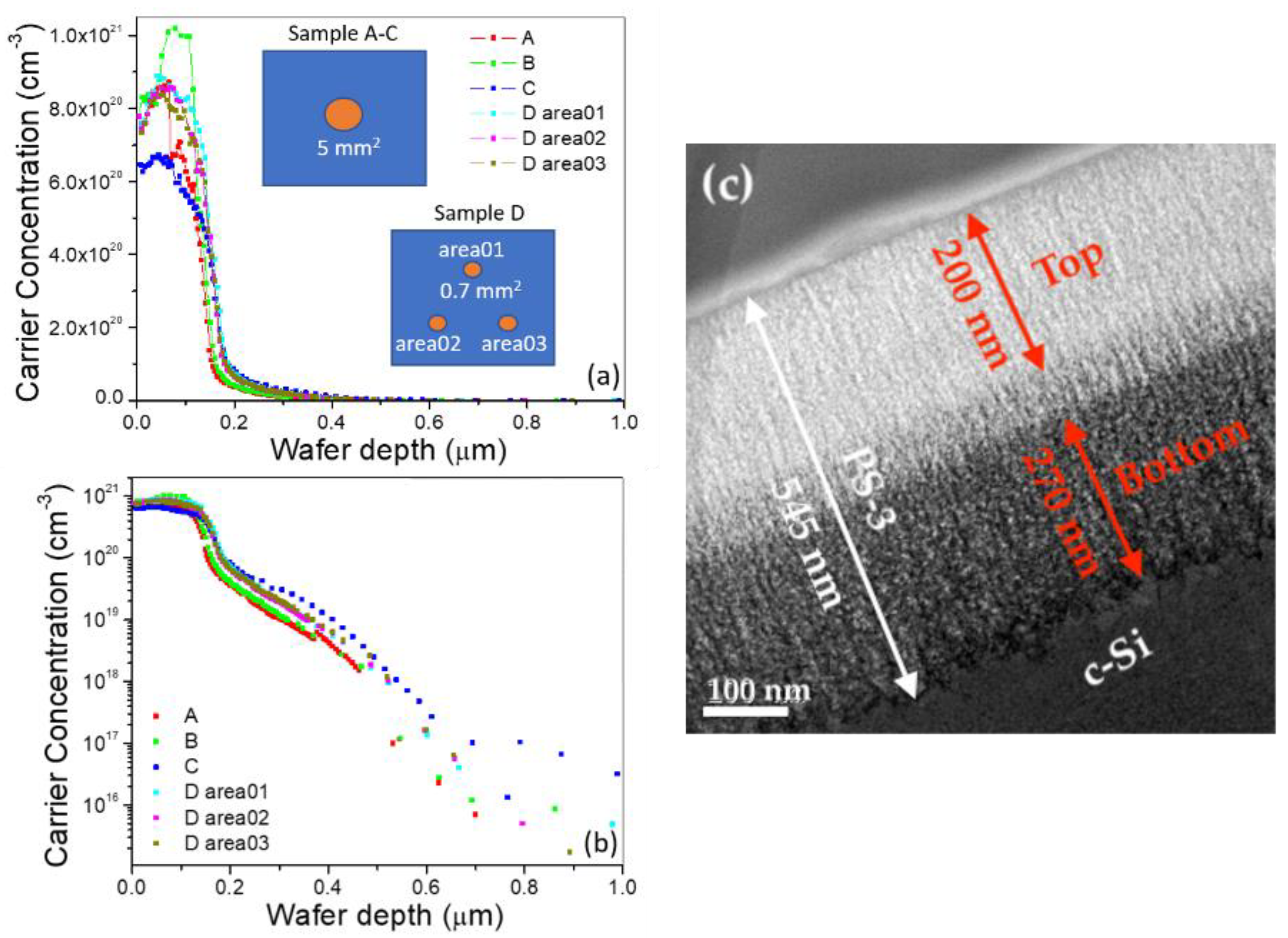


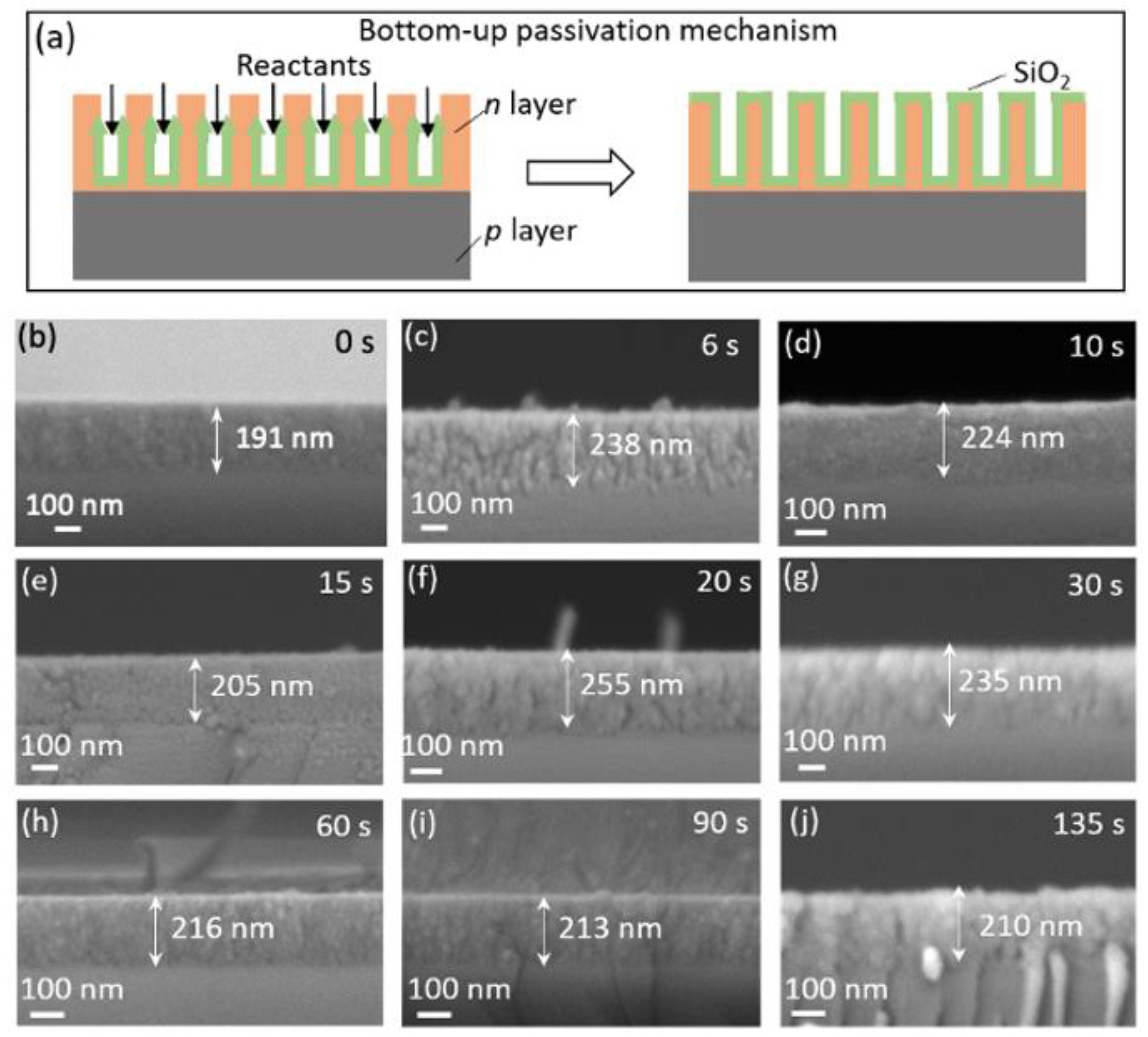
| Samples | Current Density (mA/cm2) | Etching Duration (s) | Passivation Duration (s) | PS Layer Thickness (nm) | Volume Ratio of SiO2 to PS Layer (%) | Experiment | Section |
|---|---|---|---|---|---|---|---|
| Samples A,B,C | 0 | 0 | 0 | 0 | 0 | ECV 5 mm2 | Results and Discussion part 3.1 |
| Sample D | 0 | 0 | 0 | 0 | 0 | ECV 0.7 mm2 | |
| PS-1 layer | 75 | 6 | 0 | 146 | 0 | TEM HRTEM | |
| PS-2 layer | 75 | 6 | 0 | 176 | 0 | ||
| PS-3 layer | ① 75 ② 5 | ① 10 ② 300 | 0 | 545 | 0 | ||
| c-Si cell | 0 | 0 | 0 | 0 | 0 | Reflectivity J–V curve EQE | Results and Discussion part 3.2 |
| As-fabricated PS-1 cell | 75 | 6 | 0 | 150 | 0 | ||
| Passivated PS-1 cell | 75 | 6 | - | - | - | ||
| As-fabricated PS-2 cell | 75 | 6 | 0 | 191 | 0 | FESEM Reflectivity J–V curve EQE IQE | Results and Discussion part 3.3 |
| Passivated PS-2-a cell | 75 | 6 | 6 | 239 | 0.6 | ||
| Passivated PS-2-b cell | 75 | 6 | 10 | 224 | 0.9 | ||
| Passivated PS-2-c cell | 75 | 6 | 15 | 205 | 1.4 | ||
| Passivated PS-2-d cell | 75 | 6 | 20 | 255 | 1.9 | ||
| Passivated PS-2-e cell | 75 | 6 | 30 | 235 | 2.8 | ||
| Passivated PS-2-f cell | 75 | 6 | 60 | 216 | 5.6 | ||
| Passivated PS-2-g cell | 75 | 6 | 90 | 213 | 8.5 | ||
| Passivated PS-2-h cell | 75 | 6 | 135 | 210 | 12.7 |
| c-Si Cell | As-Fabricated PS-1 Cell | Passivated PS-1 Cell | |
|---|---|---|---|
| Jsc (mA/cm2) 1 | 23.7 | 27.5 | 30.5 |
| Voc (V) | 0.510 | 0.510 | 0.510 |
| FF | 0.660 | 0.300 | 0.550 |
| η (%) 1 | 7.93 | 4.15 | 8.54 |
| Samples | t (s) | PS Layer Thickness (nm) | Voc (V) | Jsc (mA/cm2) 1 | FF | η (%) 1 |
|---|---|---|---|---|---|---|
| c-Si cell | 0 | 0 | 0.532 | 23.4 | 0.657 | 8.16 |
| As fabricated PS-2 cell | 0 | 191 | 0.539 | 29.9 | 0.320 | 5.15 |
| Passivated PS-2-a cell | 6 | 239 | 0.532 | 27.9 | 0.530 | 7.86 |
| Passivated PS-2-b cell | 10 | 224 | 0.524 | 27.6 | 0.364 | 5.25 |
| Passivated PS-2-c cell | 15 | 205 | 0.514 | 29.6 | 0.466 | 7.08 |
| Passivated PS-2-d cell | 20 | 255 | 0.531 | 30.4 | 0.578 | 9.32 |
| Passivated PS-2-e cell | 30 | 235 | 0.523 | 29.8 | 0.685 | 10.70 |
| Passivated PS-2-f cell | 60 | 216 | 0.526 | 28.7 | 0.462 | 6.97 |
| Passivated PS-2-g cell | 90 | 213 | 0.513 | 26.2 | 0.357 | 4.79 |
| Passivated PS-2-h cell | 135 | 210 | 0.511 | 25.0 | 0.354 | 4.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundarapura, P.; Zhang, X.-M.; Yogai, R.; Murakami, K.; Fave, A.; Ihara, M. Nanostructure of Porous Si and Anodic SiO2 Surface Passivation for Improved Efficiency Porous Si Solar Cells. Nanomaterials 2021, 11, 459. https://doi.org/10.3390/nano11020459
Sundarapura P, Zhang X-M, Yogai R, Murakami K, Fave A, Ihara M. Nanostructure of Porous Si and Anodic SiO2 Surface Passivation for Improved Efficiency Porous Si Solar Cells. Nanomaterials. 2021; 11(2):459. https://doi.org/10.3390/nano11020459
Chicago/Turabian StyleSundarapura, Panus, Xiao-Mei Zhang, Ryoji Yogai, Kazuki Murakami, Alain Fave, and Manabu Ihara. 2021. "Nanostructure of Porous Si and Anodic SiO2 Surface Passivation for Improved Efficiency Porous Si Solar Cells" Nanomaterials 11, no. 2: 459. https://doi.org/10.3390/nano11020459
APA StyleSundarapura, P., Zhang, X.-M., Yogai, R., Murakami, K., Fave, A., & Ihara, M. (2021). Nanostructure of Porous Si and Anodic SiO2 Surface Passivation for Improved Efficiency Porous Si Solar Cells. Nanomaterials, 11(2), 459. https://doi.org/10.3390/nano11020459







