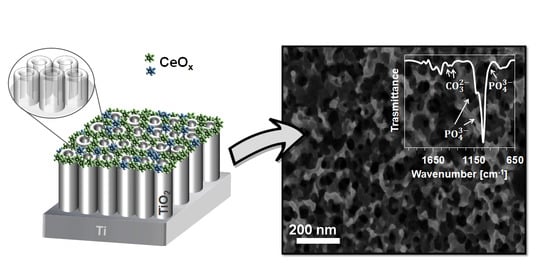
A Simple Cerium Coating Strategy for Titanium Oxide Nanotubes’ Bioactivity Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Surface Characterization
2.3. Electrochemical Measurements
2.4. Surface Wettability
2.5. In Vitro Bioactivity Test
3. Results and Discussion
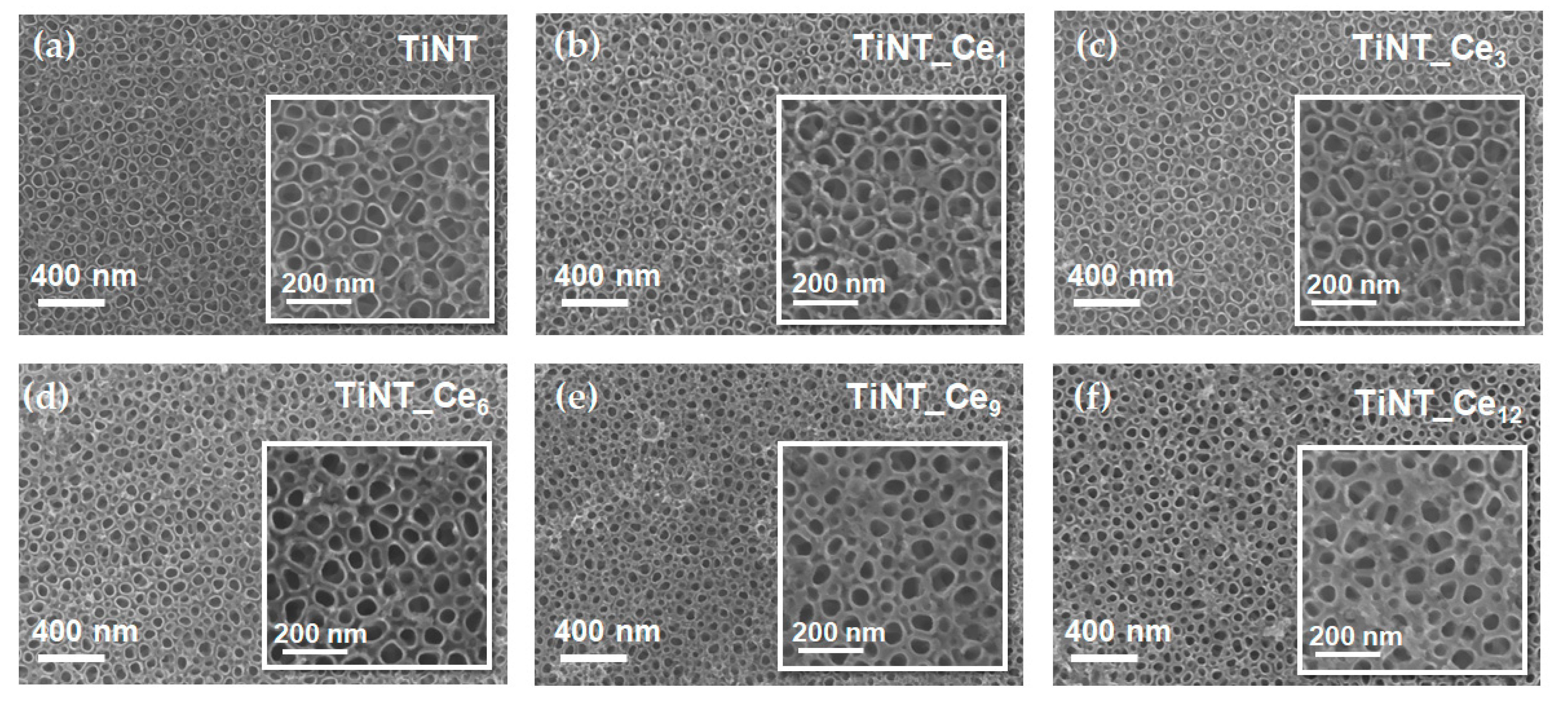
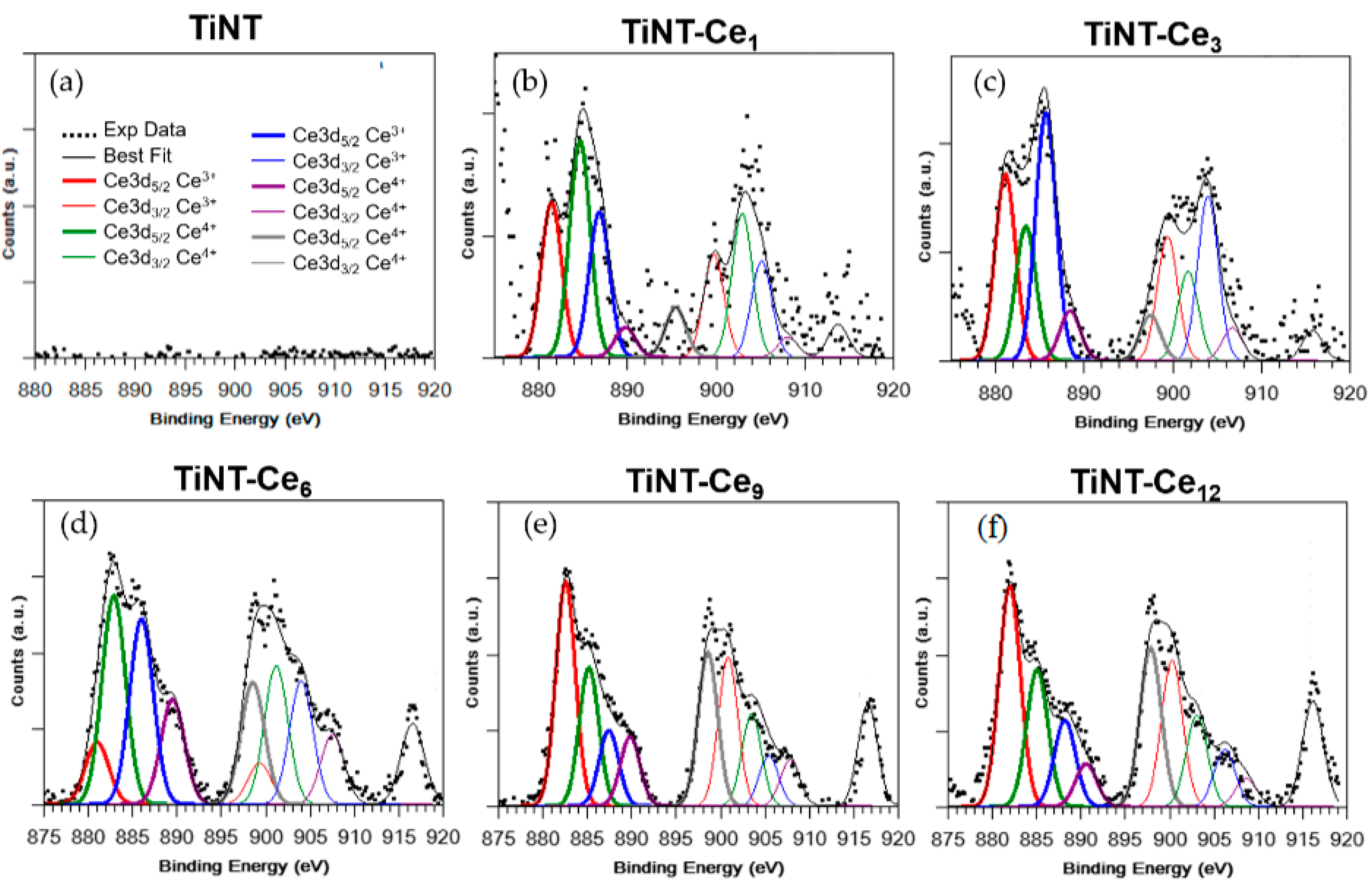
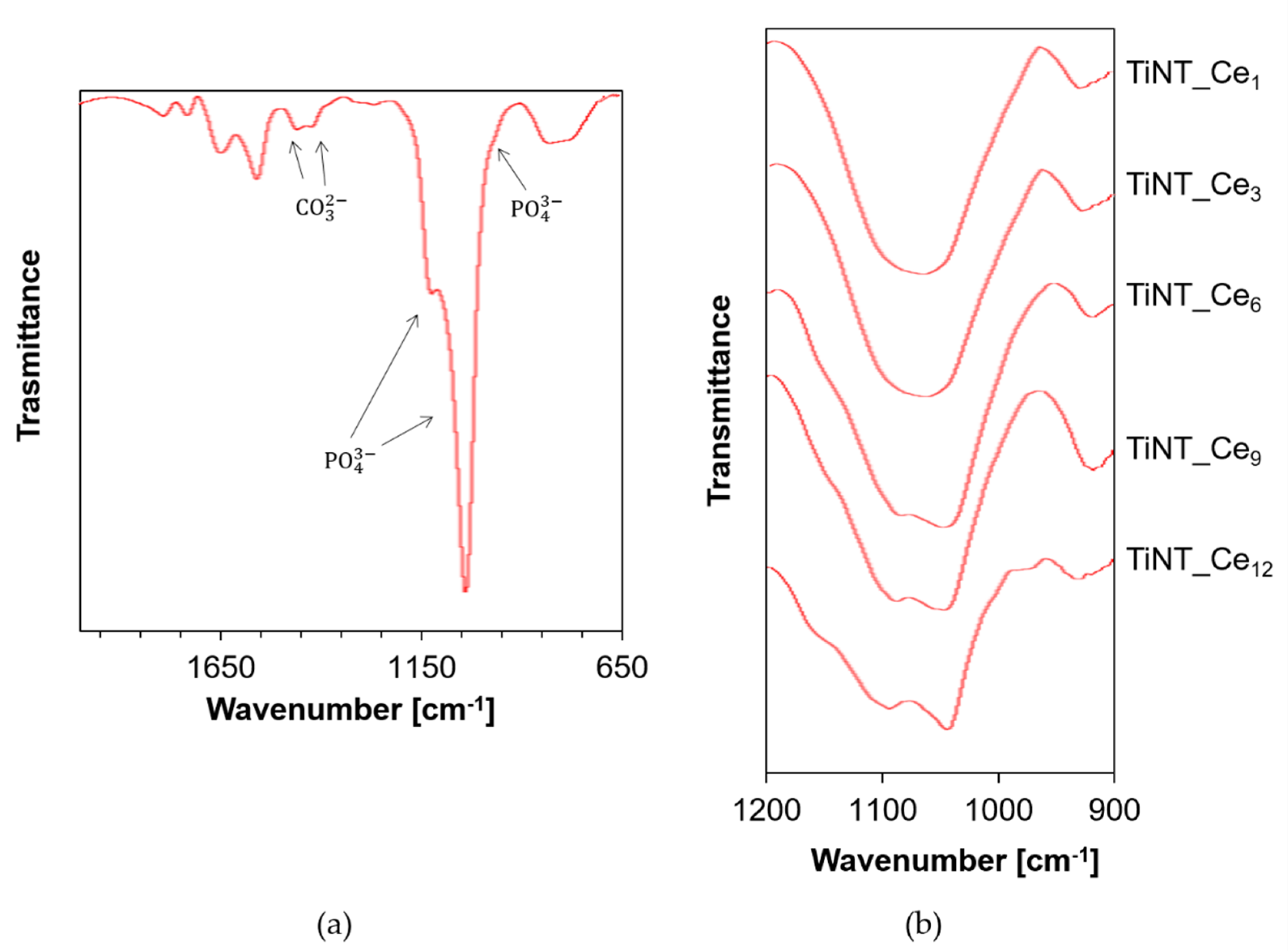
3.1. Surface Morphology and Composition
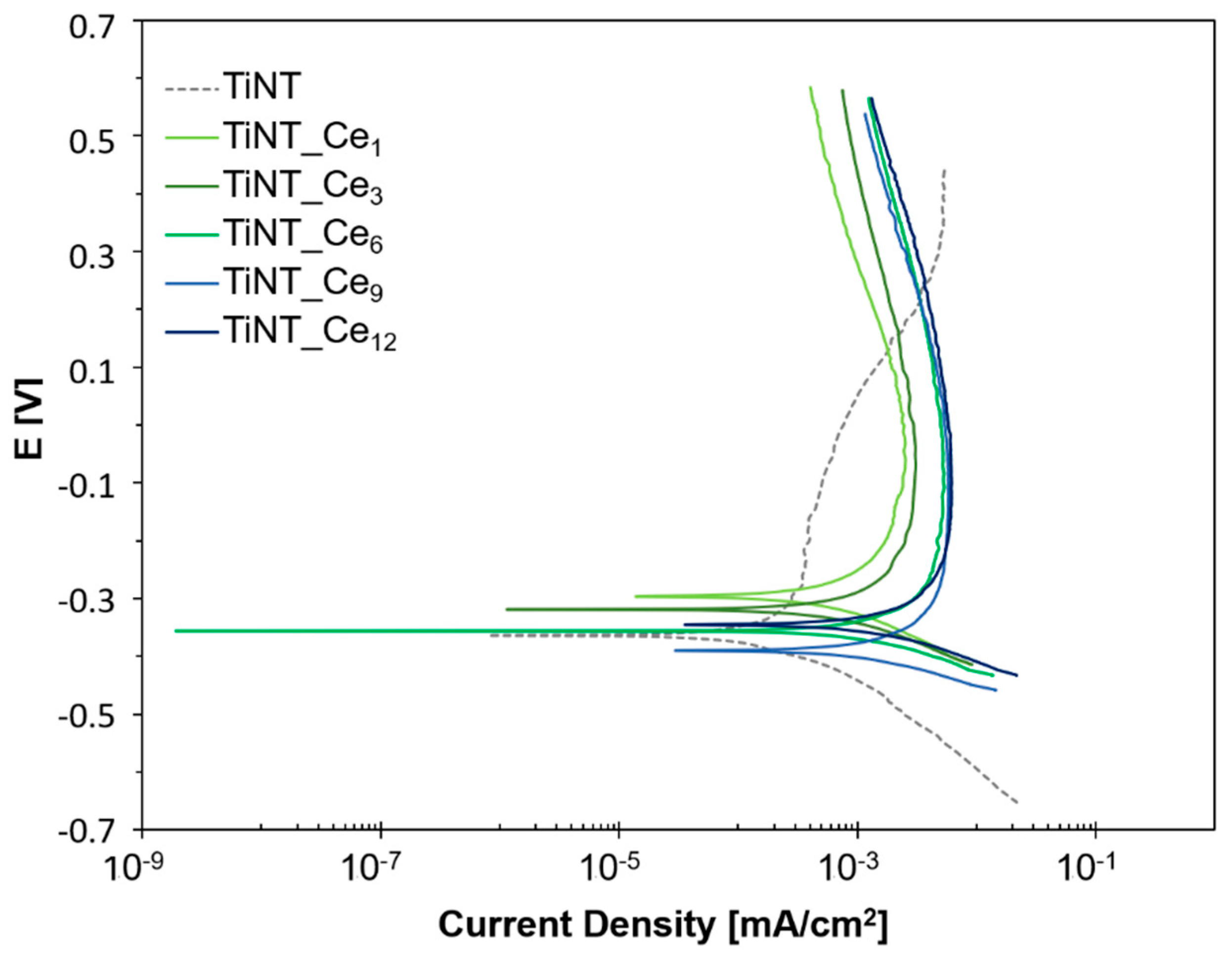
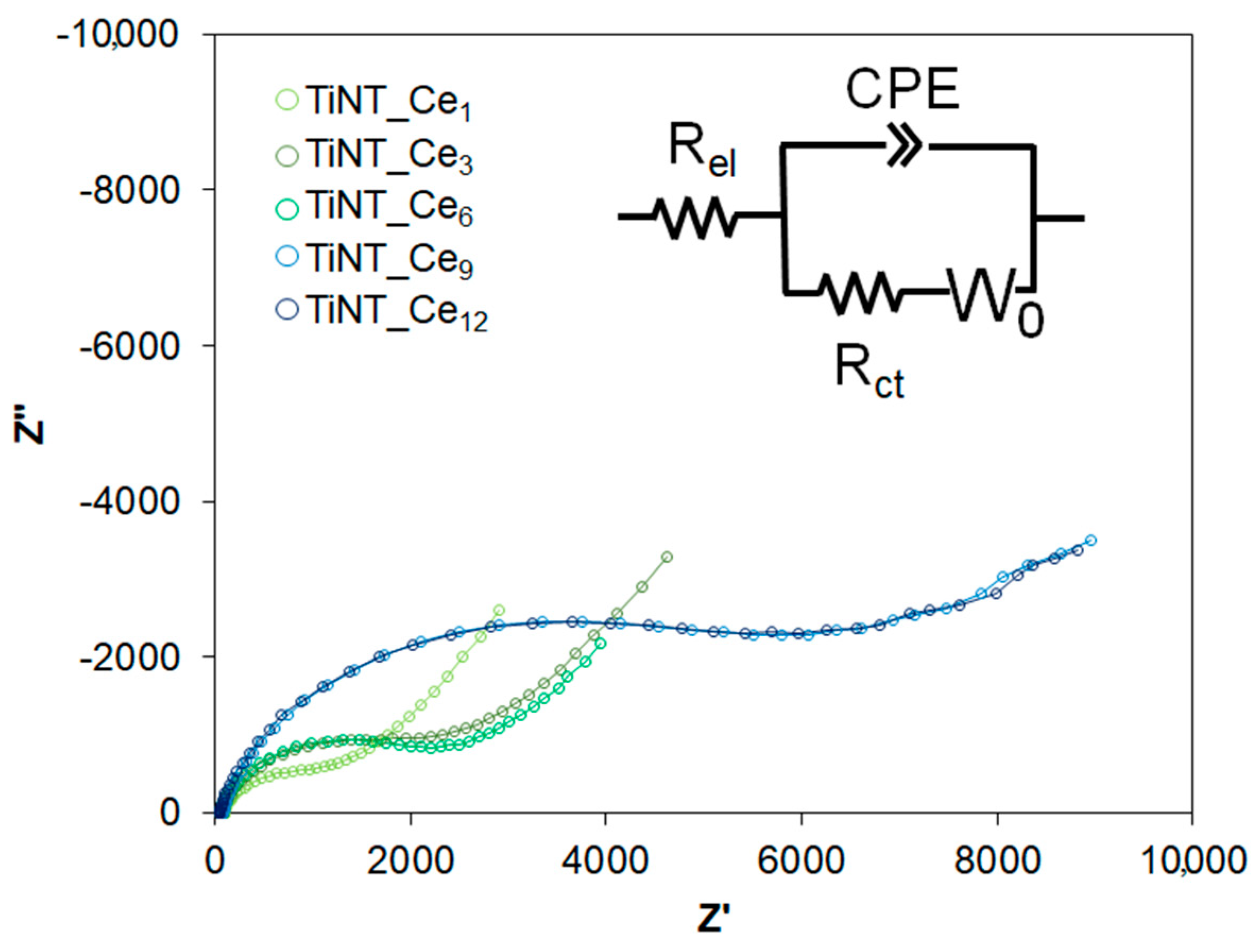
3.2. Electrochemical Behavior
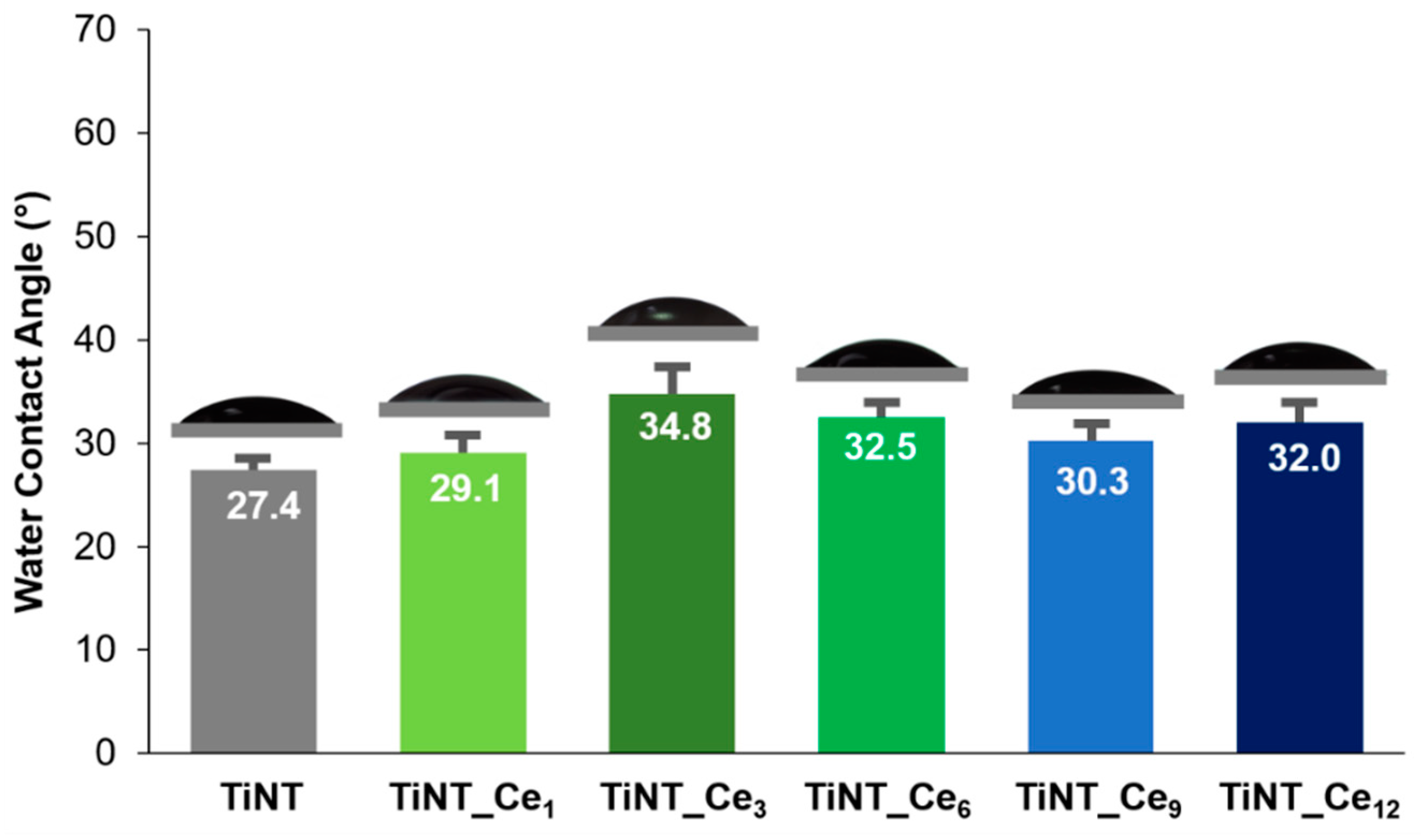
3.3. Surface Wettability
3.4. In Vitro Bioactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.Y.; Anderson, D.E. Bone and cartilage interfaces with orthopedic implants: A literature review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Zarzycki, A.; Kac, S.; Perzanowski, M.; Marszalek, M. Mechanical properties of different nanopatterned TiO2 substrates and their effect on hydrothermally synthesized bioactive hydroxyapatite coatings. Materials 2020, 13, 5290. [Google Scholar] [CrossRef]
- Stepanovska, J.; Matejka, R.; Rosina, J.; Bacakova, L. The effect of various surface treatments of Ti6Al4V on the growth and osteogenic differentiation of adipose tissue-derived stem cells. Coatings 2020, 10, 762. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Jang, J.; Kwon, T.; Bae, Y.; Suh, J. Effects of phosphoric acid treatment of titanium surfaces on surface properties, osteoblast response and removal of torque forces. Acta Biomater. 2010, 6, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Battocchio, C.; Concolato, S.; De Santis, S.; Pahlman, M.; Iuggi, G.; Santi, M.; Sotgiu, G.; Orsini, M. Chitosan functionalization of titanium and Ti6Al4V alloy with chloroacetic acid as linker agent. Mater. Sci. Eng. C 2019, 99, 1133–1140. [Google Scholar] [CrossRef]
- Wehner, C.; Behm, C.; Husejnagic, S.; Moritz, A.; Rausch-Fan, X. Effect of Multi-Phosphonate Coating of Titanium Surfaces on Osteogenic Potential. Materials 2020, 13, 5777. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, X.; Tian, A.; Wang, L.; Liu, C. Electrochemical properties of Ti3+ doped Ag-Ti nanotube arrays coated with hydroxyapatite. Appl. Surf. Sci. 2018, 436, 579–584. [Google Scholar] [CrossRef]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef]
- Calıskan, N.; Bayram, C.; Erdal, E.; Karahaliloglu, Z.; Denkbaş, E.B. Titania nanotubes with adjustable dimensions for drug reservoir sites and enhanced cell adhesion. Mater. Sci. Eng. C 2014, 35, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S.; Addai-Mensah, J.; Losich, D. Polymer micelles for delayed release of therapeutics from drug-releasing surfaces with nanotubular structures. Macromol. Biosci. 2012, 12, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Iorio, L.E.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clinic. Chem. Lab. Med. 2008, 46, 1550. [Google Scholar] [CrossRef]
- Awad, N.K.; Edwards, S.L.; Morsi, Y.S. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kalisz, M.; Grobelny, M. Influence of Cu, Au and Ag on structural and surface properties of bioactive coatings based on titanium. Mater. Sci. Eng. C 2017, 71, 1115–1121. [Google Scholar] [CrossRef]
- Lytkina, D.; Gutsalova, A.; Fedorishin, D.; Korotchenko, N.; Akhmedzhanov, R.; Kozik, V.; Kurzina, I. Synthesis and Properties of Zinc-Modified Hydroxyapatite. J. Funct. Biomater. 2020, 11, 10. [Google Scholar] [CrossRef]
- Shimazaki, T.; Miyamoto, H.; Ando, Y.; Noda, I.; Yonekura, Y.; Kawano, S.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxyapatite coating. J Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Tolkacheva, T.V.; Rau, J.V.; Buyko, E.E.; Ivanov, V.V.; Sheikin, V.V. Modification of titanium surface via Ag-, Sr- and Si-containing micro-arc calcium phosphate coating. Bioact. Mater. 2019, 4, 224–235. [Google Scholar] [CrossRef]
- Ball, J.P.; Mound, B.A.; Monsalve, A.G.; Nino, J.C.; Allen, J.B. Biocompatibility evaluation of porous ceria foams for orthopedic tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 8–15. [Google Scholar] [CrossRef]
- Gravina, A.N.; Ruso, J.M.; Laiuppa, J.A.; Santillàn, G.; Marco-Brown, J.L.; D’Elia, N.L.; Messina, P.V. Striped, bioactive Ce–TiO2 materials with peroxynitrite-scavenging activity. J. Mater. Chem. B 2014, 2, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Gravina, N.; Ruso, J.M.; Mbeh, D.A.; Yahia, L.; Sartuqui, J.; Messina, P.V. Effect of ceria on the organization and bio-ability of anatase fullerene-like crystals. RSC Adv. 2015, 5, 8077–8087. [Google Scholar] [CrossRef]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.-F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Shoko, E.; Smith, M.F.; McKenzie, R.H. Charge distribution near bulk oxygen vacancies in cerium oxides. J. Phys. Condens. Matter. 2010, 22, 223201. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Plasma sprayed cerium oxide coating inhibits H2O2-induced oxidative stress and supports cell viability. J. Mater. Sci. Mater. Med. 2016, 27, 100. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.N.; Rubert, A.A.; Bertuola, M.; Fernández Lorenzo de Mele, M. Bioactivity enhancement of cerium-containing titanium oxide nanotubes. Relationship between surface reactivity and nanostructuring process. Surf. Coat. Technol. 2019, 378, 124968. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhu, Y.F. Synthesis and characterization of CeO2-incorporated mesoporous calcium-silicate materials. Micropor. Mesopor. Mat. 2014, 197, 244–251. [Google Scholar] [CrossRef]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of catalase mimetic activity in Ce3+/Ce4+ doped bioactive glasses. J. Phys. Chem. B 2015, 119, 4009–4019. [Google Scholar] [CrossRef]
- Mandoli, C.; Pagliari, F.; Pagliari, S.; Forte, G.; Di Nardo, P.; Licoccia, S.; Traversa, E. Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 2010, 20, 1617–1624. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef]
- You, M.; Li, K.; Xie, Y.; Huang, L.; Zheng, X. The Effects of Cerium Valence States at Cerium Oxide Coatings on the Responses of Bone Mesenchymal Stem Cells and Macrophages. Biol. Trace Elem. Res. 2017, 179, 259–270. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- IInternational Standard ISO 23317:2014 Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. (2014). ICS:11:040:040. Available online: https://www.iso.org/standard/65054.html (accessed on 2 February 2021).
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 1686–1691. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J. Ti/CeOx(111) interfaces studied by XPS and STM. Surf. Sci. 2012, 606, 749–753. [Google Scholar] [CrossRef]
- Fang, J.; Bao, H.; He, B.; Wang, F.; Si, D.; Jiang, Z.; Pan, Z. Interfacial and Surface Structures of CeO2–TiO2 Mixed Oxides. J. Phys. Chem. C 2007, 111, 19078–19085. [Google Scholar] [CrossRef]
- Moeini, S.S.; Battocchio, C.; Casciardi, S.; Luisetto, I.; Lupattelli, P.; Tofani, D.; Tuti, S. Oxidized Palladium Supported on Ceria Nanorods for Catalytic Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde in Protic Solvents. Catalysts 2019, 9, 847. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Shao, P.; Yang, L.; Sun, Y.; Yang, Y.; Cui, F.; Wang, W. Modulation of coordinative unsaturation degree and valence state for cerium-based adsorbent to boost phosphate adsorption. Chem. Eng. J. 2020, 394, 124912. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Iafisco, M.; Morales, J.G.; Hernández-Hernández, M.A.; García-Ruiz, J.M.; Roveri, N. Biomimetic Carbonate–Hydroxyapatite Nanocrystals Prepared by Vapor Diffusion. Adv. Eng. Mater. 2010, 12, B218–B223. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.; Kol’tsov, A.; Kuz’mina, M.; Zorina, M.; Poritskaya, L. Ion substitutions and non-stoichiometry of carbonated apatite-(CaOH) synthesised by precipitation and hydrothermal methods. J. Mol. Struct. 2011, 992, 9–18. [Google Scholar] [CrossRef]
- De Santis, S.; Sotgiu, G.; Crescenzi, A.; Taffon, C.; Felici, A.C.; Orsini, M. On the chemical composition of psammoma bodies microcalcifications in thyroid cancer tissues. J. Pharmaceut. Biomed. 2020, 190, 113534. [Google Scholar] [CrossRef] [PubMed]
- Pleshko, N.; Boskey, A.; Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 1991, 60, 786–793. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Sfihi, H.; Barroug, A. Physico-chemical properties of nanocrystalline apatites: Implications for biominerals and biomaterials. Mater. Sci. Eng. C 2007, 27, 198–205. [Google Scholar] [CrossRef]

| Ion | Ion Concentrations (mM) |
|---|---|
| Na+ | 142.0 |
| K+ | 5.0 |
| Mg2+ | 1.5 |
| Ca2+ | 2.5 |
| Cl− | 147.8 |
| HCO3− | 4.2 |
| HPO42− | 1.0 |
| SO24− | 0.5 |
| Ecorr (V) | Icorr (μA) | Ipass-0.2 (μA) | Ipass0.3 (μA) | |
| TiNT | −0.364 | 0.57 | 0.37 | 4.87 |
| TiNT_Ce1 | −0.297 | 1.70 | 1.83 | 0.95 |
| TiNT_Ce3 | −0.320 | 1.36 | 2.81 | 1.48 |
| TiNT_Ce6 | −0.356 | 3.18 | 5.72 | 3.16 |
| TiNT_Ce9 | −0.396 | 3.18 | 5.87 | 3.18 |
| TiNT_Ce12 | −0.345 | 3.93 | 5.87 | 3.26 |
| Rel (Ω·cm2) | Rct(kΩ·cm2) | CPE | σ (kΩ·s−1/2·cm−2) | χ2 | ||
| Yo (μS·sn·cm−2) | n | |||||
| TiNT_Ce1 | 82.6 | 0.56 | 10.48 | 0.892 | 2.44 | 1.00∙10−3 |
| TiNT_Ce3 | 77.0 | 1.32 | 5.29 | 0.881 | 4.64 | 1.00∙10−4 |
| TiNT_Ce6 | 69.6 | 1.81 | 5.43 | 0.876 | 4.95 | 1.00∙10−4 |
| TiNT_Ce9 | 84.4 | 2.82 | 4.76 | 0.880 | 10.47 | 1.00∙10−4 |
| TiNT_Ce12 | 70.2 | 3.55 | 3.75 | 0.884 | 11.40 | 1.00∙10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, S.; Sotgiu, G.; Porcelli, F.; Marsotto, M.; Iucci, G.; Orsini, M. A Simple Cerium Coating Strategy for Titanium Oxide Nanotubes’ Bioactivity Enhancement. Nanomaterials 2021, 11, 445. https://doi.org/10.3390/nano11020445
De Santis S, Sotgiu G, Porcelli F, Marsotto M, Iucci G, Orsini M. A Simple Cerium Coating Strategy for Titanium Oxide Nanotubes’ Bioactivity Enhancement. Nanomaterials. 2021; 11(2):445. https://doi.org/10.3390/nano11020445
Chicago/Turabian StyleDe Santis, Serena, Giovanni Sotgiu, Francesco Porcelli, Martina Marsotto, Giovanna Iucci, and Monica Orsini. 2021. "A Simple Cerium Coating Strategy for Titanium Oxide Nanotubes’ Bioactivity Enhancement" Nanomaterials 11, no. 2: 445. https://doi.org/10.3390/nano11020445
APA StyleDe Santis, S., Sotgiu, G., Porcelli, F., Marsotto, M., Iucci, G., & Orsini, M. (2021). A Simple Cerium Coating Strategy for Titanium Oxide Nanotubes’ Bioactivity Enhancement. Nanomaterials, 11(2), 445. https://doi.org/10.3390/nano11020445