Porous Hybrids Structure between Silver Nanoparticle and Layered Double Hydroxide for Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ag/3-MPA
2.3. Hybridization of Ag@LDH and Prepartion of Ag@LDO
2.4. Characterization
2.5. Surface-Enhanced Raman Scattering Spectroscopy (SERS)
3. Results and Discussion
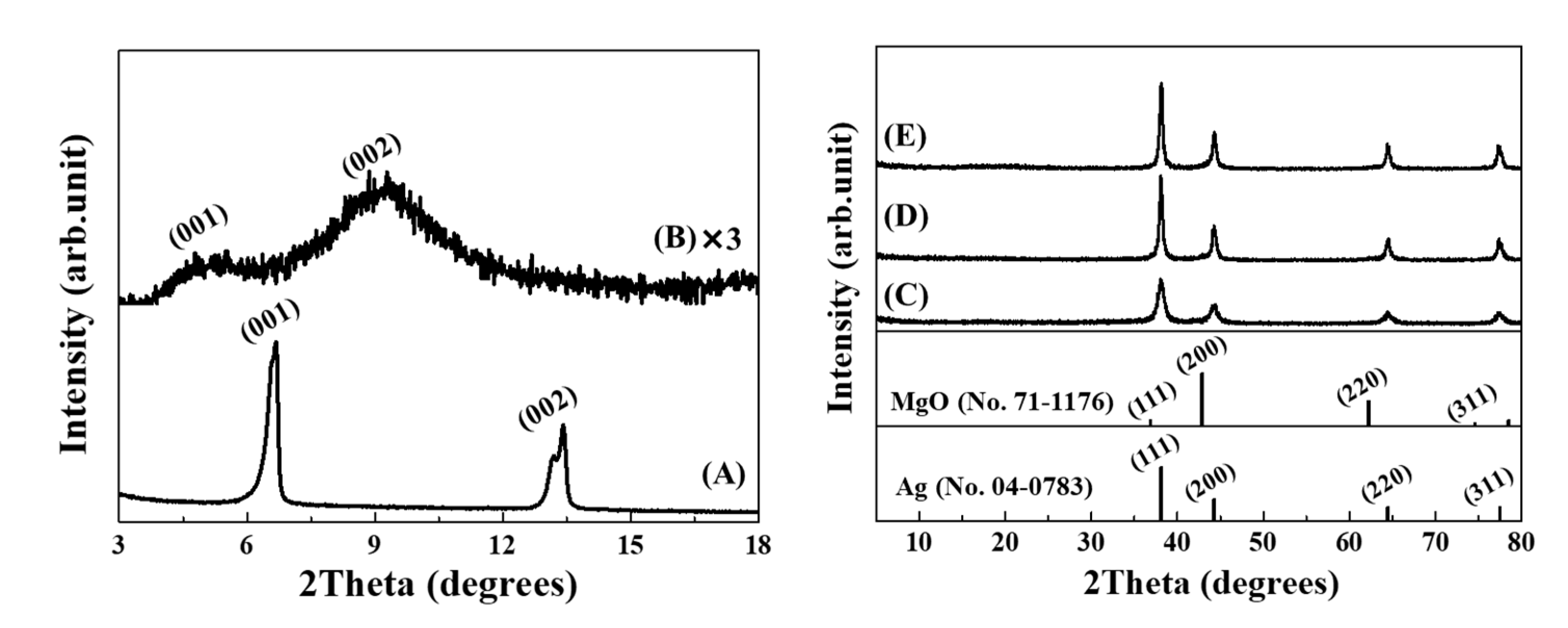
3.1. Crystal Strucutre of Ag@LDO
3.2. Pore Structure of Ag@LDO
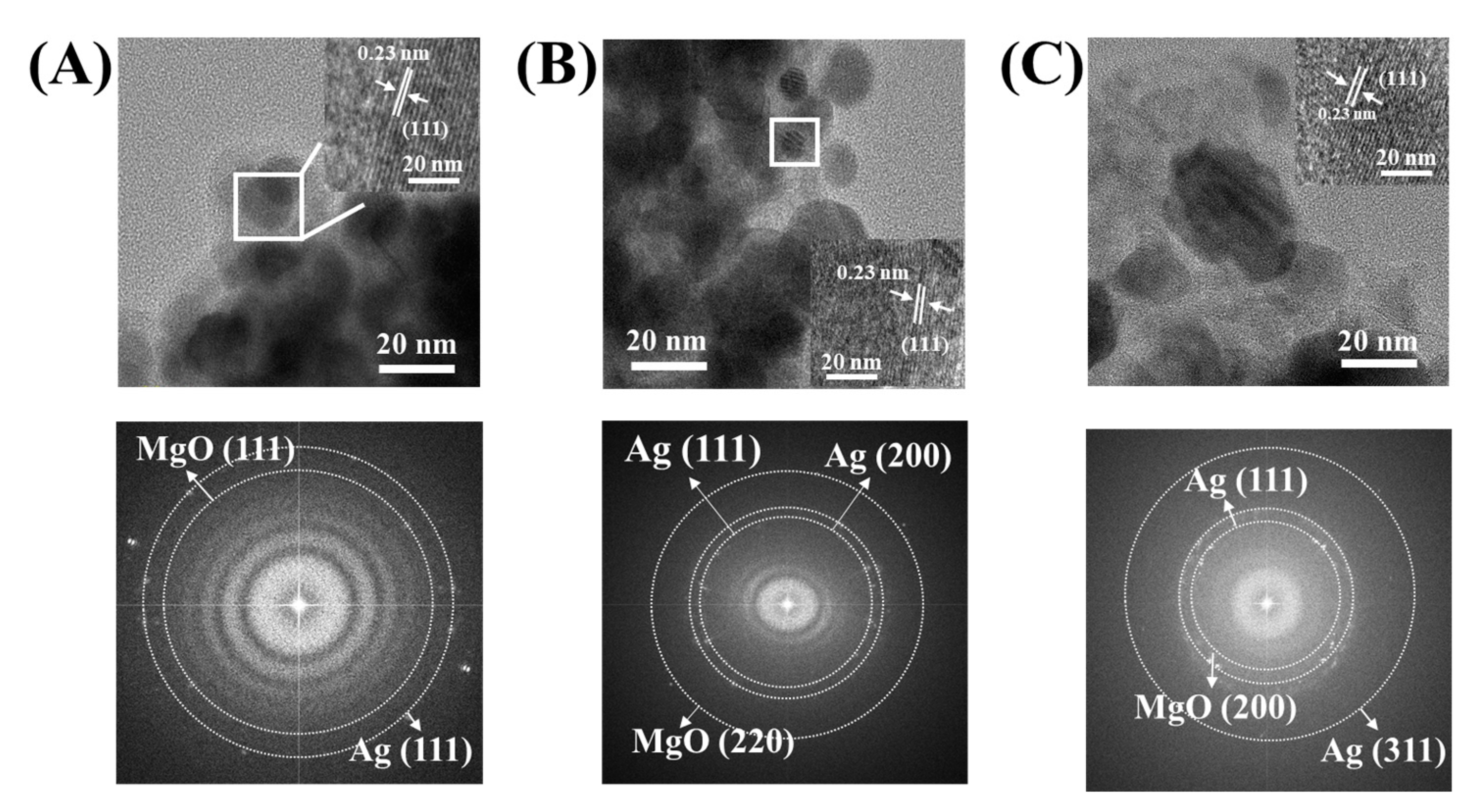
3.3. Microscopic Structures of Ag@LDO
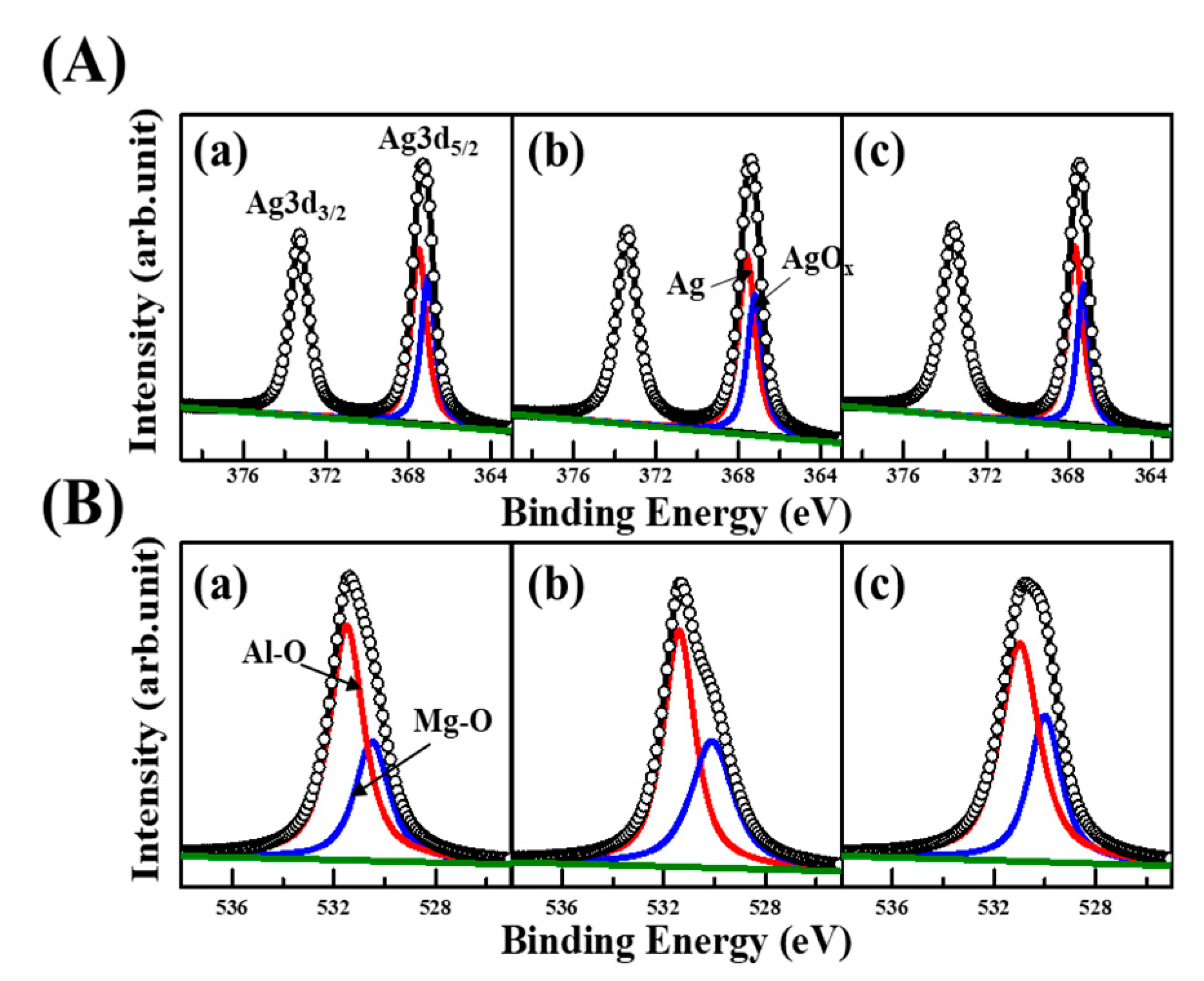
3.4. Chemical Environments of Ag@LDO
3.5. Electronic Structure of Ag@LDO
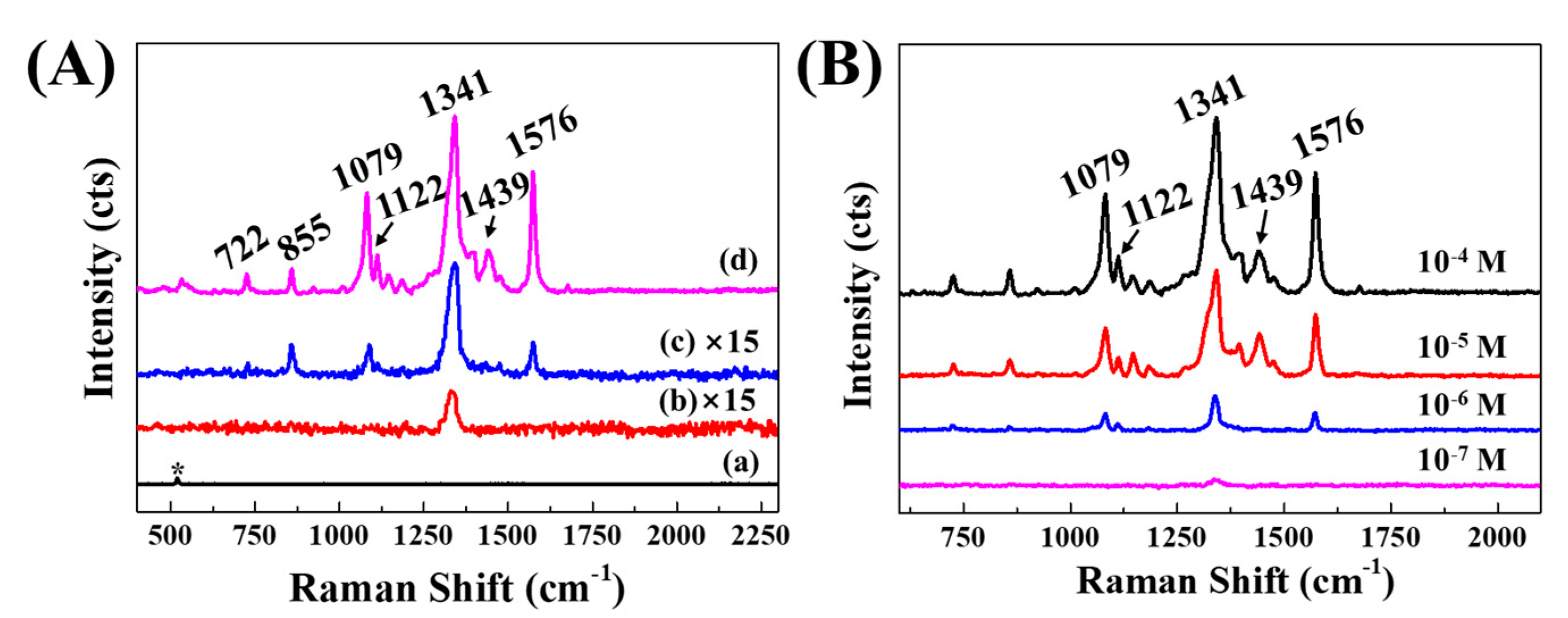
3.6. SERS Effect of Ag@LDO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Istiqola, A.; Syafiuddin, A. A review of silver nanoparticles in food packaging technologies: Regulation, methods, properties, migration, and future challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef]
- Lem, K.W.; Choudhury, A.; Lakhani, A.A.; Kuyate, P.; Haw, J.R.; Lee, D.S.; Iqbal, Z.; Brumlik, C.J. Use of Nanosilver in Consumer Products. Recent Pat. Nanotechnol. 2012, 6, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. Fabrication of cotton fabrics with durable antibacterial activities finishing by Ag nanoparticles. Text. Res. J. 2019, 89, 867–880. [Google Scholar] [CrossRef]
- Kejlová, K.; Kašpárková, V.; Krsek, D.; Jírová, D.; Kolářová, H.; Dvořáková, M.; Tománková, K.; Mikulcová, V. Characteristics of silver nanoparticles in vehicles for biological applications. Int. J. Pharm. 2015, 496, 878–885. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, G.; Dong, H.; Chen, Y.; Zhang, J.; Yan, M.; Zhu, Y.; Yuan, Y.; Xie, Y.; Huang, Z. The impact of silver nanoparticles on the co-composting of sewage sludge and agricultural waste: Evolutions of organic matter and nitrogen. Bioresour. Technol. 2017, 230, 132–139. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; Moustafa, Y.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Hashem, A.I. Methylene Blue Catalytic Degradation Using Silver and Magnetite Nanoparticles Functionalized with a Poly (ionic liquid) Based on Quaternized Dialkylethanolamine with 2-Acrylamido-2-methylpropane Sulfonate-co-Vinylpyrrolidone. ACS Omega 2020, 5, 2829–2842. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, G.; Singh, G.; Kumar, M.; Bhalla, V. Silver nanoparticles: Facile synthesis and their catalytic application for the degradation of dyes. RSC Adv. 2015, 5, 25781–25788. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Park, W.H. Facile Interpretation of Catalytic Reaction between Organic Dye Pollutants and Silver Nanoparticles with Different Shapes. J. Nanomater. 2019, 2019, 3257892. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Shvalya, V.; Filipič, G.; Zavašnik, J.; Abdulhalim, I.; Cvelbar, U. Surface-enhanced Raman spectroscopy for chemical and biological sensing using nanoplasmonics: The relevance of interparticle spacing and surface morphology. Appl. Phys. Rev. 2020, 7, 031307. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, T.-H.; Wu, Y.; Li, W.; Zeng, Q.-G.; Long, L.; Li, Z.-Y. Roadmap for single-molecule surface-enhanced Raman spectroscopy. Adv. Photonics 2020, 2, 014002. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef]
- Palanisamy, S.; Yan, L.; Zhang, X.; He, T. Surface enhanced Raman scattering-active worm-like Ag clusters for sensitive and selective detection of dopamine. Anal. Methods 2015, 7, 3438–3447. [Google Scholar] [CrossRef]
- Li, W.; Camargo, P.H.C.; Lu, X.; Xia, Y. Dimers of Silver Nanospheres: Facile Synthesis and Their Use as Hot Spots for Surface-Enhanced Raman Scattering. Nano Lett. 2009, 9, 485–490. [Google Scholar] [CrossRef]
- Mulvihill, M.J.; Ling, X.Y.; Henzie, J.; Yang, P. Anisotropic Etching of Silver Nanoparticles for Plasmonic Structures Capable of Single-Particle SERS. J. Am. Chem. Soc. 2010, 132, 268–274. [Google Scholar] [CrossRef]
- Sonntag, M.D.; Klingsporn, J.M.; Zrimsek, A.B.; Sharma, B.; Ruvuna, L.K.; Van Duyne, R.P. Molecular plasmonics for nanoscale spectroscopy. Chem. Soc. Rev. 2014, 43, 1230–1247. [Google Scholar] [CrossRef]
- Fang, J.; Liu, S.; Li, Z. Polyhedral silver mesocages for single particle surface-enhanced Raman scattering-based biosensor. Biomaterials 2011, 32, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Leis, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Silver Nanostars with High SERS Performance. J. Phys. Chem. C 2013, 117, 7791–7795. [Google Scholar] [CrossRef]
- Rekha, C.R.; Nayar, V.U.; Gopchandran, K.G. Synthesis of highly stable silver nanorods and their application as SERS substrates. J. Sci. Adv. Mater. Devices 2018, 3, 196–205. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Chapter 7—Metallic colloids and other SERS substrates. In Principles of Surface-Enhanced Raman Spectroscopy; Le Ru, E.C., Etchegoin, P.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 367–413. [Google Scholar]
- Cho, W.J.; Kim, Y.; Kim, J.K. Ultrahigh-Density Array of Silver Nanoclusters for SERS Substrate with High Sensitivity and Excellent Reproducibility. ACS Nano 2012, 6, 249–255. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core–Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Yan, X.; Wang, M.; Sun, X.; Wang, Y.; Shi, G.; Ma, W.; Hou, P. Sandwich-like Ag@Cu@CW SERS substrate with tunable nanogaps and component based on the Plasmonic nanonodule structures for sensitive detection crystal violet and 4-aminothiophenol. Appl. Surf. Sci. 2019, 479, 879–886. [Google Scholar] [CrossRef]
- Potara, M.; Baia, M.; Farcau, C.; Astilean, S. Chitosan-coated anisotropic silver nanoparticles as a SERS substrate for single-molecule detection. Nanotechnology 2012, 23, 055501. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Zeiri, L.; Manna, J.; Nandi, S.; Jelinek, R. Carbon-Dot/Silver-Nanoparticle Flexible SERS-Active Films. ACS Appl. Mater. Interfaces 2016, 8, 25637–25643. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, F.; Chen, Z.; Hou, X.; Liu, Q.; Long, Z. AuNPs/COFs as a new type of SERS substrate for sensitive recognition of polyaromatic hydrocarbons. Chem. Commun. 2017, 53, 11044–11047. [Google Scholar] [CrossRef]
- Gwak, G.-H.; Kim, M.-K.; Lee, W.-J.; Jeung, D.-G.; Park, J.K.; Paek, S.-M.; Oh, J.-M. Facile Synthetic Route To Prepare Ultrathin Silver Nanosheets by Reducing Silver Thiolates in Interlayer Surface of Layered Double Hydroxides. Inorg. Chem. 2020, 59, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Gwak, G.-H.; Yamaguchi, T.; Kim, M.-K.; Park, J.K.; Oh, J.-M. Silver nanoplate-pillared mesoporous nano-clays for surface enhanced raman scattering. J. Ind. Eng. Chem. 2020, 89, 250–256. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: Recent developments and applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Lee, S.J.; Han, S.W.; Choi, H.J.; Kim, K. Structure and Thermal Behavior of a Layered Silver Carboxylate. J. Phys. Chem. B 2002, 106, 2892–2900. [Google Scholar] [CrossRef]
- Veselska, O.; Dessal, C.; Melizi, S.; Guillou, N.; Podbevšek, D.; Ledoux, G.; Elkaim, E.; Fateeva, A.; Demessence, A. New Lamellar Silver Thiolate Coordination Polymers with Tunable Photoluminescence Energies by Metal Substitution. Inorg. Chem. 2019, 58, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, A. Structures of monolayers formed from different HS—(CH2)2—X thiols on gold, silver and copper: Comparitive studies by surface-enhanced Raman scattering. J. Raman Spectrosc. 2003, 34, 853–862. [Google Scholar] [CrossRef]
- Yang, W.; Kim, Y.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. A study by in situ techniques of the thermal evolution of the structure of a Mg–Al–CO3 layered double hydroxide. Chem. Eng. Sci. 2002, 57, 2945–2953. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; Hussein, M.Z.b.; Zakaria, A. Improvement of the crystallinity and photocatalytic property of zinc oxide as calcination product of Zn–Al layered double hydroxide. J. Alloys Compd. 2012, 539, 154–160. [Google Scholar] [CrossRef]
- Kovanda, F.; Grygar, T.; Dorničák, V.t. Thermal behaviour of Ni–Mn layered double hydroxide and characterization of formed oxides. Solid State Sci. 2003, 5, 1019–1026. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing: Reading, MA, USA, 1956. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Höller, R.P.M.; Jahn, I.J.; Cialla-May, D.; Chanana, M.; Popp, J.; Fery, A.; Kuttner, C. Biomacromolecular-Assembled Nanoclusters: Key Aspects for Robust Colloidal SERS Sensing. ACS Appl. Mater. Interfaces 2020, 12, 57302–57313. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Puebla, R.A.; Contreras-Caceres, R.; Pastoriza-Santos, I.; Perez-Juste, J.; Liz-Marzan, L.M. Au@pNIPAM Colloids as Molecular Traps for Surface-Enhanced, Spectroscopic, Ultra-Sensitive Analysis. Angew. Chem. Int. Ed. 2009, 48, 138. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.E. X-ray Diffraction; Dover Publications: Mineola, NY, USA, 1990. [Google Scholar]
- Hanawalt, J.; Rinn, H.; Frevel, L. Chemical analysis by X-ray diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Wan, C.; Tian, R.; Kondou, M.; Yang, R.; Zong, P.; Koumoto, K. Ultrahigh thermoelectric power factor in flexible hybrid inorganic-organic superlattice. Nat. Commun. 2017, 8, 1024. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Inayat, A.; Ofili, J.; Schwieger, W. Thermal decomposition, gas phase hydration and liquid phase reconstruction in the system Mg/Al hydrotalcite/mixed oxide: A comparative study. Appl. Clay Sci. 2010, 50, 176–181. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Dobešová, J.; Machovič, V.; Bezdička, P.; Obalová, L.; Jirátová, K.; Grygar, T. Mixed oxides obtained from Co and Mn containing layered double hydroxides: Preparation, characterization, and catalytic properties. J. Solid State Chem. 2006, 179, 812–823. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Nishi, Y.; Inagaki, M. Chapter 11—Gas Adsorption/Desorption Isotherm for Pore Structure Characterization. In Materials Science and Engineering of Carbon; Inagaki, M., Kang, F., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 227–247. [Google Scholar] [CrossRef]
- Jung, J.-S.; Ko, S.-J.; Lee, H.-B.; Lee, S.-B.; Kim, H.-J.; Oh, J.-M. Hierarchical ag nanostructures fabricated from silver coordination polymers for antibacterial surface. Polymers 2019, 11, 1055. [Google Scholar] [CrossRef]
- Kim, M.-K.; Gwak, G.-H.; Oh, J.-M. Fibrous Silver Particles Prepared from Layered Silver Alkanethiolates and Their Catalytic Property. J. Nanosci. Nanotechnol. 2017, 17, 3581–3587. [Google Scholar] [CrossRef]
- Habibi, A.; Jalaly, M.; Rahmanifard, R.; Ghorbanzadeh, M. The effect of calcination conditions on the crystal growth and battery performance of nanocrystalline Li(Ni1/3Co1/3Mn1/3)O2 as a cathode material for Li-ion batteries. New J. Chem. 2018, 42, 19026–19033. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.I.; Dieringer, J.A.; Van Duyne, R.P. Creating, Characterizing, and Controlling Chemistry with SERS Hot Spots. Phys. Chem. Chem. Phys. 2013, 15, 21. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, S.; Kim, J.-M.; Choi, S.; Kim, J.; Park, S.-J.; Park, D.; Nam, J.-M.; Park, S. Synthesis and Single-Particle Surface-Enhanced Raman Scattering Study of Plasmonic Tripod Nanoframes with Y-Shaped Hot-Zones. Nano Lett. 2020, 20, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-C.; Chen, D.-H. Microwave-assisted green synthesis of Ag/reduced graphene oxide nanocomposite as a surface-enhanced Raman scattering substrate with high uniformity. Nanoscale Res. Lett. 2014, 9, 193. [Google Scholar] [CrossRef]
- Maiti, N.; Thomas, S.; Debnath, A.; Kapoor, S. Raman and XPS study on the interaction of taurine with silver nanoparticles. RSC Adv. 2016, 6, 56406–56411. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, Y.; Kim, K. Dodecanethiol-Derivatized Au/Ag Bimetallic Nanoparticles: TEM, UV/VIS, XPS, and FTIR Analysis. J. Colloid Interface Sci. 1998, 208, 272–278. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Durucan, C.; Akkopru, B. Effect of calcination on microstructure and antibacterial activity of silver-containing silica coatings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Hashimoto, S.; Tomita, T.; Matsuo, S.; Makita, Y. Examination of Silver Nanoparticle Fabrication by Pulsed-Laser Ablation of Flakes in Primary Alcohols. J. Phys. Chem. C 2008, 112, 1321–1329. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, W.; El-Sayed, M.A. On the Universal Scaling Behavior of the Distance Decay of Plasmon Coupling in Metal Nanoparticle Pairs: A Plasmon Ruler Equation. Nano Lett. 2007, 7, 2080–2088. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Radecka, M.; Zakrzewska, K.; Czternastek, H.; Stapiński, T.; Debrus, S. The influence of thermal annealing on the structural, electrical and optical properties of TiO2-x thin films. Appl. Surf. Sci. 1993, 65–66, 227–234. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Singh, M.K.; Mathpal, M.C.; Mishra, S.K.; Agarwal, A. Study of structural transformation in TiO2 nanoparticles and its optical properties. J. Alloys Compd. 2013, 549, 114–120. [Google Scholar] [CrossRef]
- Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Structural and Optical Properties of Ag Nanoparticles Synthesized by Thermal Treatment Method. Materials 2017, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Xie, W.C.; Liu, G.K.; Yan, R.W.; Wu, D.Y.; Tang, J. The discovery of the hydrogen bond from p-Nitrothiophenol by Raman spectroscopy: Guideline for the thioalcohol molecule recognition tool. Sci. Rep. 2016, 6, 31981. [Google Scholar] [CrossRef]
- Fleger, Y.; Mastai, Y.; Rosenbluh, M.; Dressler, D.H. Surface enhanced Raman spectroscopy of aromatic compounds on silver nanoclusters. Surf. Sci. 2009, 603, 788–793. [Google Scholar] [CrossRef]
- Itoh, T.; Maeda, T.; Kasuya, A. In situ surface-enhanced Raman scattering spectroelectrochemistry of oxygen species. Faraday Discuss. 2006, 132, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Li, X. Optimal Size of Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy under Different Conditions. J. Nanomater. 2013, 2013, 790323. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Prietzel, C.; Reinecke, A.; Koetz, J. “Green” gold nanotriangles: Synthesis, purification by polyelectrolyte/micelle depletion flocculation and performance in surface-enhanced Raman scattering. RSC Adv. 2016, 6, 33561–33568. [Google Scholar] [CrossRef]
- Li, M.; Kang, J.W.; Dasari, R.R.; Barman, I. Shedding Light on the Extinction-Enhancement Duality in Gold Nanostar-Enhancement Raman Spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 14115–14119. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.-Y.; Zhou, L.; Lu, F.; Cai, N.; Li, J.-M. Highly sensitive SERS monitoring of catalytic reaction by bifunctional Ag-Pd triangular nanoplates. J. Saudi Chem. Soc. 2019, 23, 887–895. [Google Scholar] [CrossRef]




| Ag@LDO400 | Ag@LDO500 | Ag@LDO600 | |
|---|---|---|---|
| ISERS/counts | 77 | 1126 | 4338 |
| IRS·CRS−1/counts·M−1 | 524.81 | 524.81 | 524.81 |
| SEF | 1.5 × 103 | 2.1 × 104 | 8.3 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-B.; Paek, S.-M.; Oh, J.-M. Porous Hybrids Structure between Silver Nanoparticle and Layered Double Hydroxide for Surface-Enhanced Raman Spectroscopy. Nanomaterials 2021, 11, 447. https://doi.org/10.3390/nano11020447
Lee S-B, Paek S-M, Oh J-M. Porous Hybrids Structure between Silver Nanoparticle and Layered Double Hydroxide for Surface-Enhanced Raman Spectroscopy. Nanomaterials. 2021; 11(2):447. https://doi.org/10.3390/nano11020447
Chicago/Turabian StyleLee, Su-Bin, Seung-Min Paek, and Jae-Min Oh. 2021. "Porous Hybrids Structure between Silver Nanoparticle and Layered Double Hydroxide for Surface-Enhanced Raman Spectroscopy" Nanomaterials 11, no. 2: 447. https://doi.org/10.3390/nano11020447
APA StyleLee, S.-B., Paek, S.-M., & Oh, J.-M. (2021). Porous Hybrids Structure between Silver Nanoparticle and Layered Double Hydroxide for Surface-Enhanced Raman Spectroscopy. Nanomaterials, 11(2), 447. https://doi.org/10.3390/nano11020447






