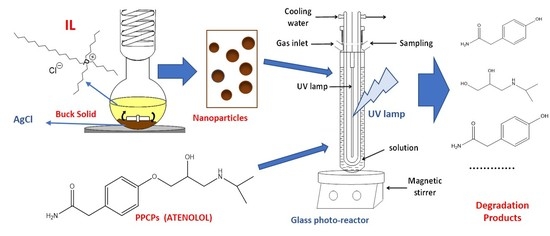
Nanomaterial Synthesis in Ionic Liquids and Their Use on the Photocatalytic Degradation of Emerging Pollutants
Abstract
1. Introduction
2. Materials and Methods
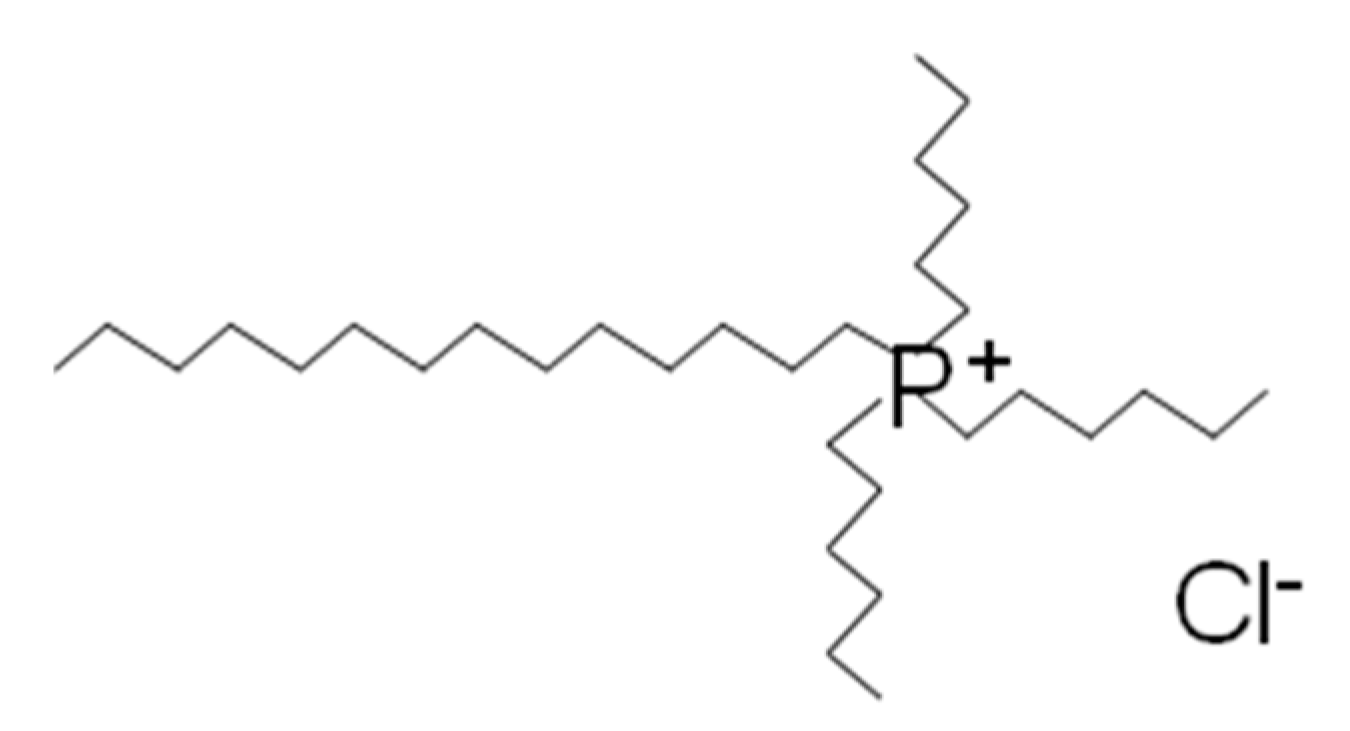
2.1. Materials
2.2. Preparation of AgCl Nanoparticles
2.3. Preparation of AgCl@Fe3O4 and TiO2@Fe3O4 Magnetic Nanocomposites
2.4. Characterization of the Nanomaterials
2.5. Experimental Set-Up for Photocatalytic Degradation of ATL
2.6. Analytical Method
3. Results and Discussion
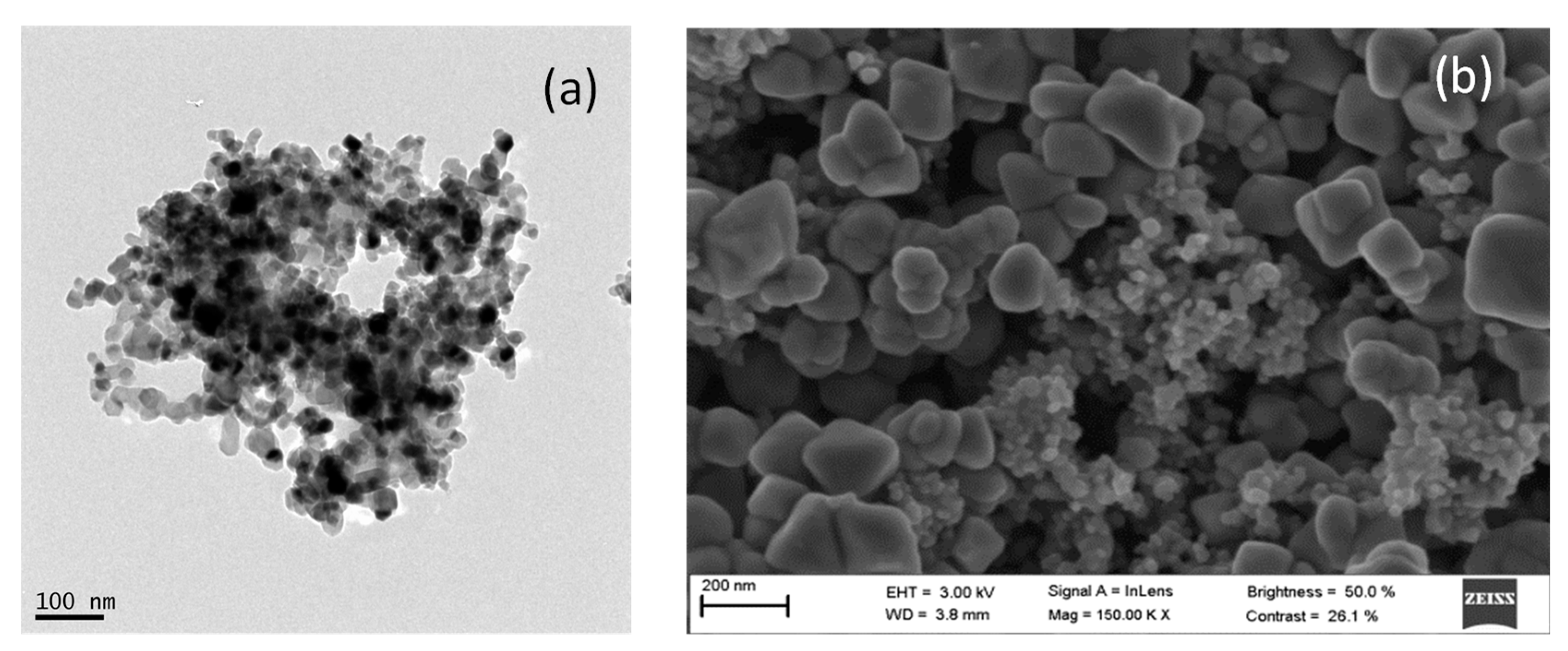
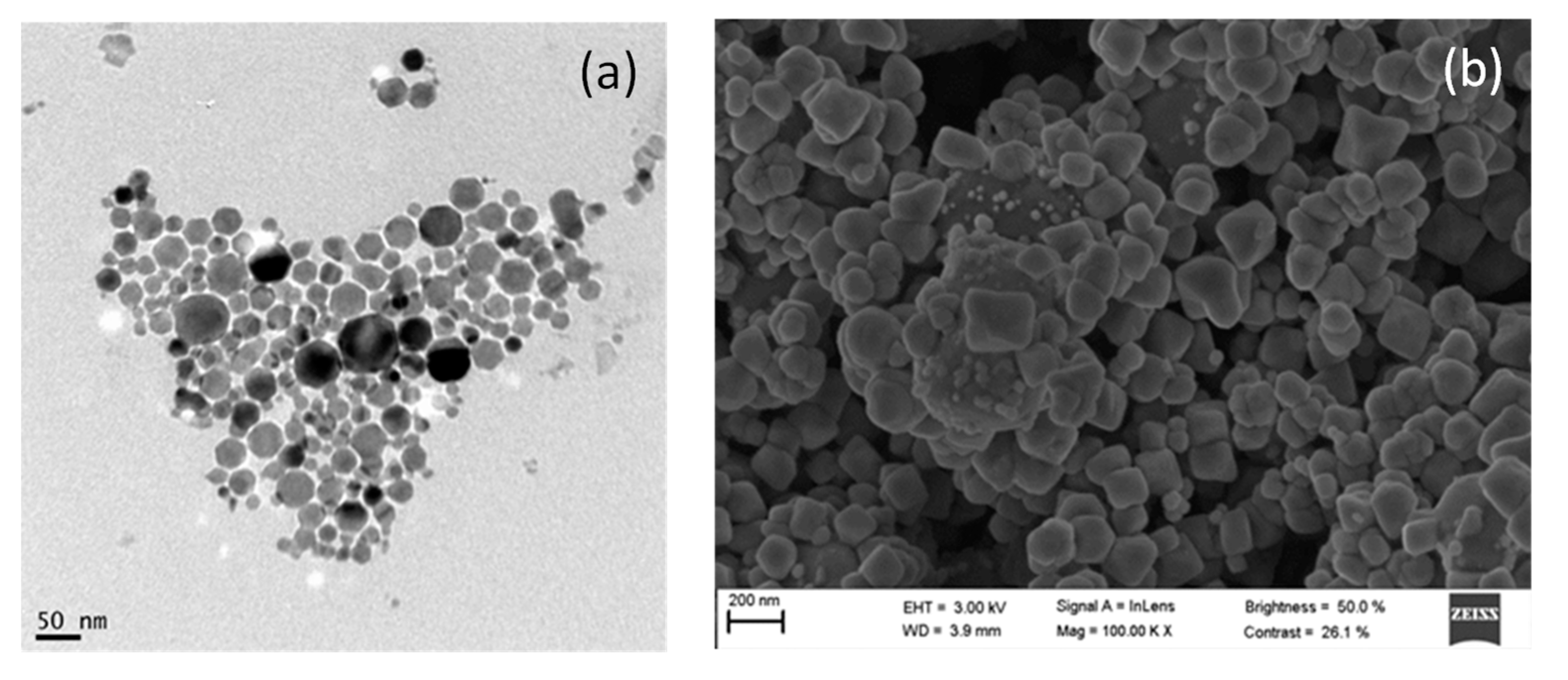
3.1. Characterization of AgCl Nanoparticles
3.2. Characterization of TiO2@Fe3O4 and AgCl@Fe3O4 Nanocomposites
3.3. Photocatalytic Degradation of ATL with AgCl Nanoparticles
3.3.1. Degradation Tests
3.3.2. Influence of Operational Parameters
AgCl Concentration
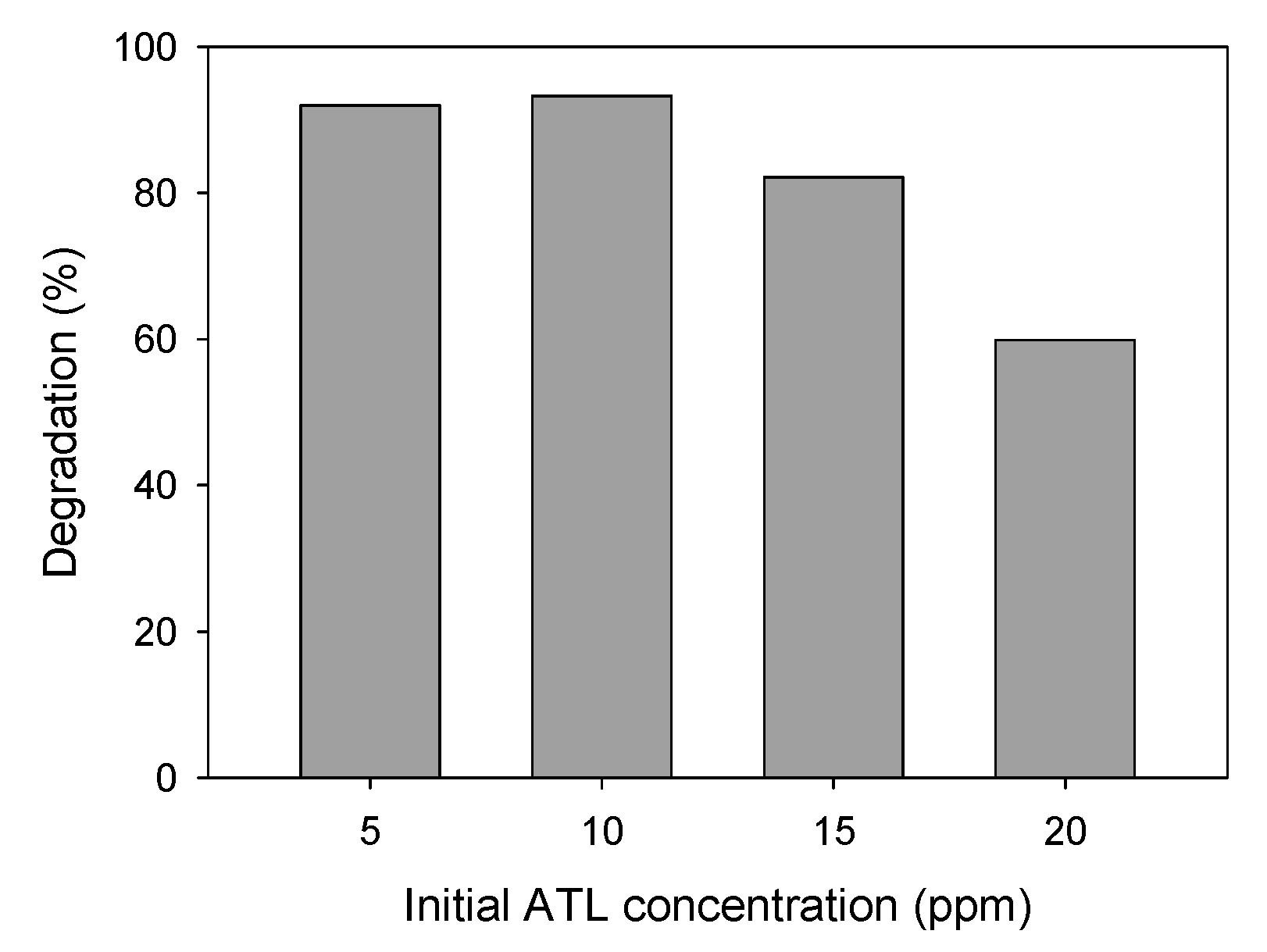
ATL Initial Concentration
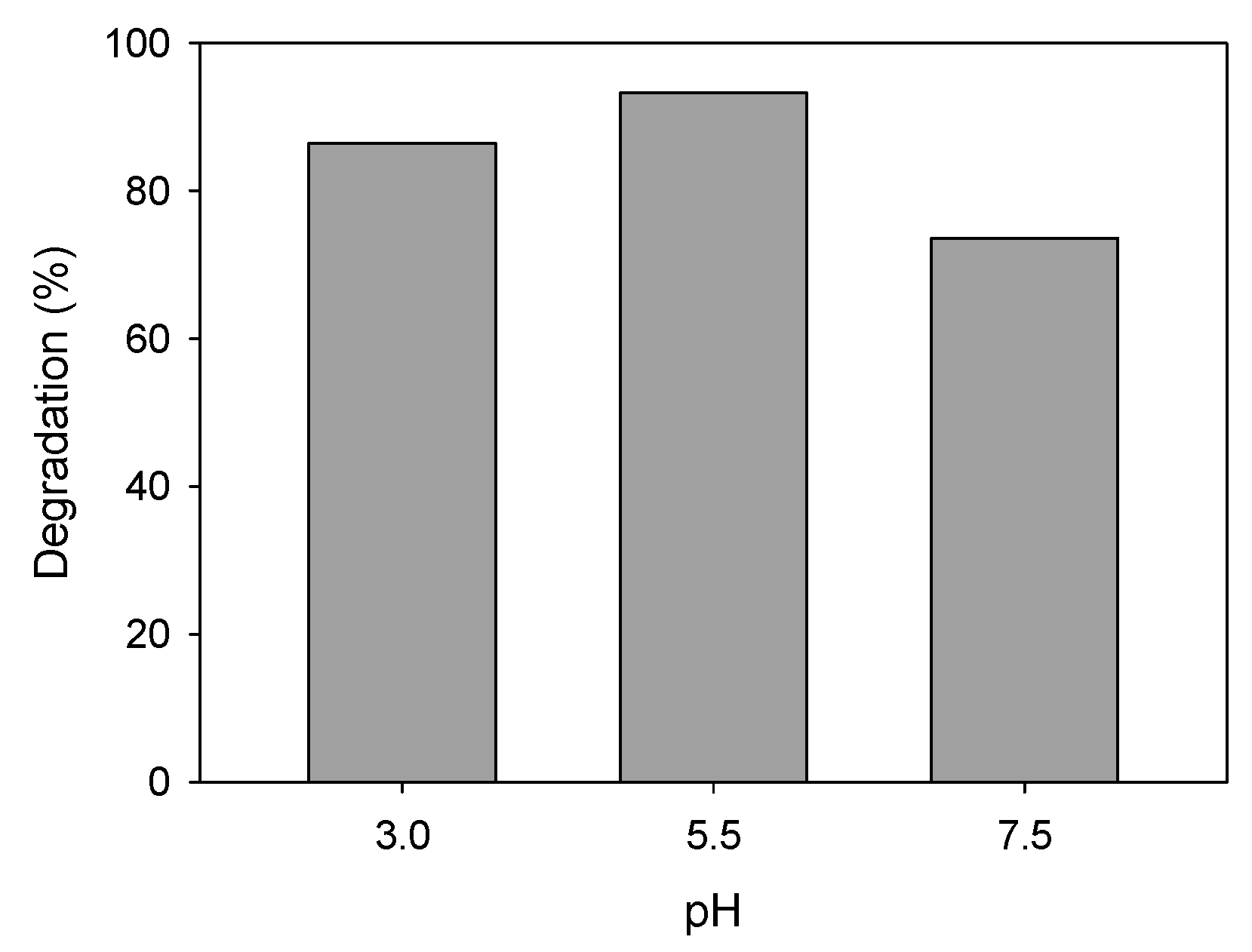
pH Solution
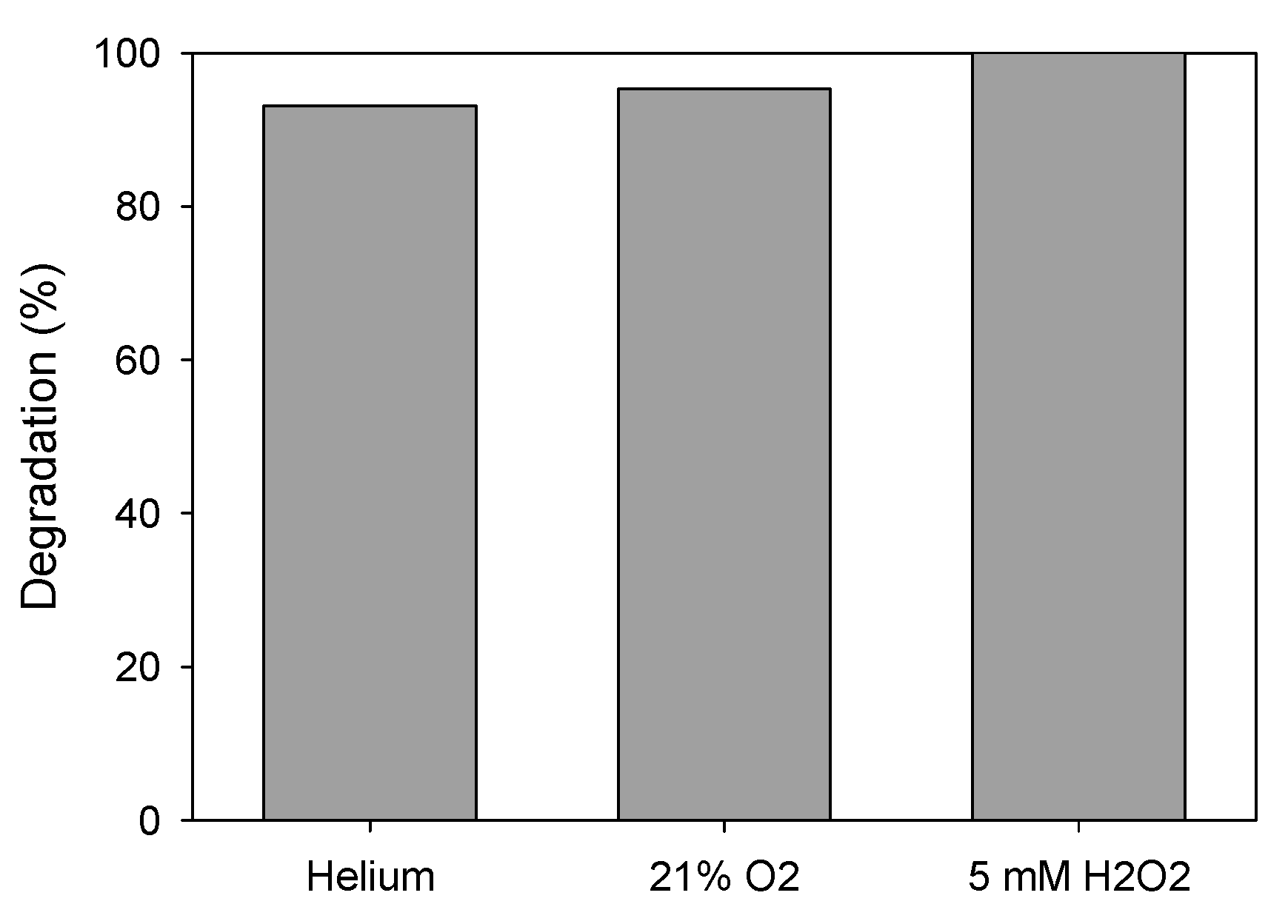
Addition of Oxidizing Agents
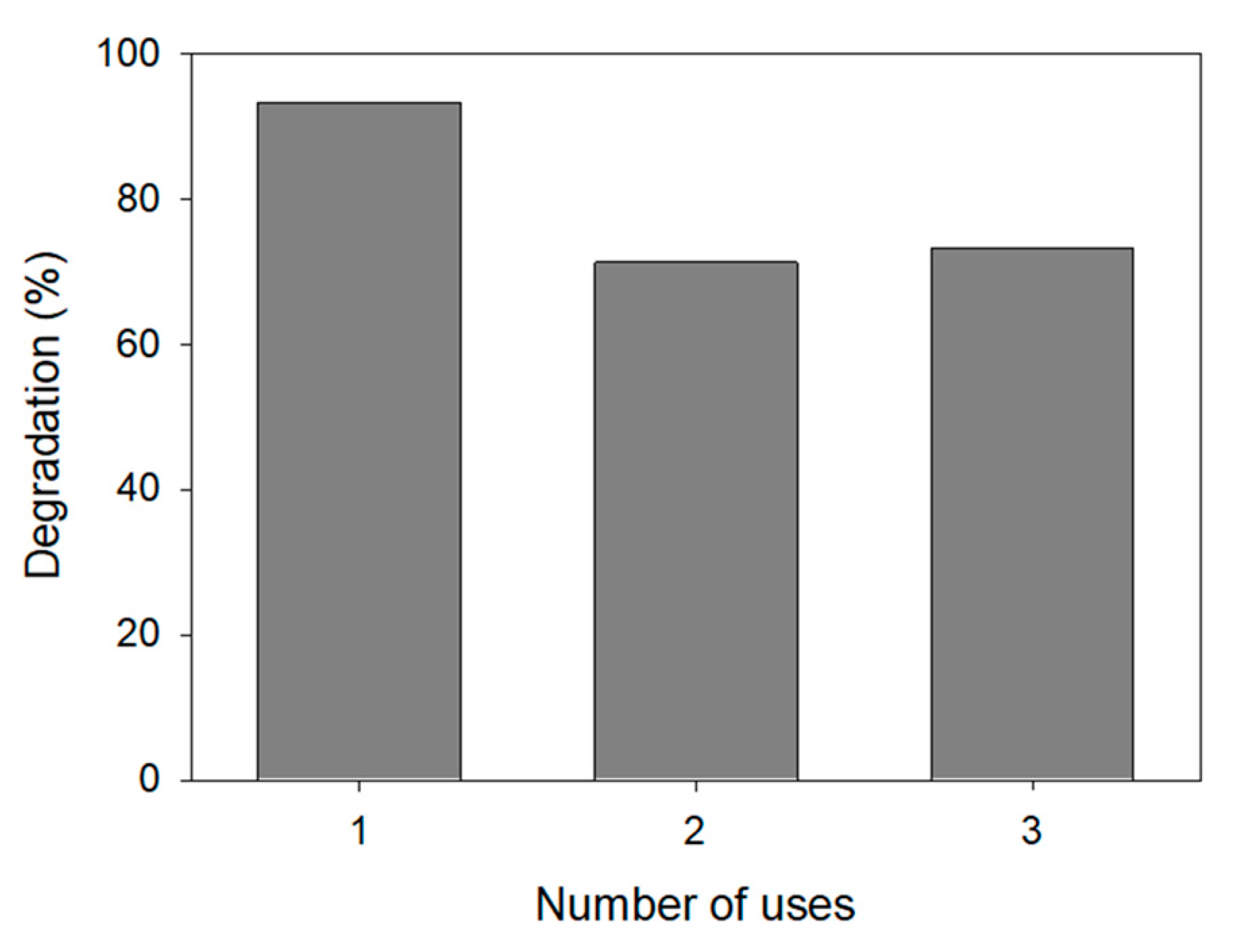
3.3.3. Durability of the Nanophotocatalyst
3.3.4. Kinetics
3.3.5. Degradation Products
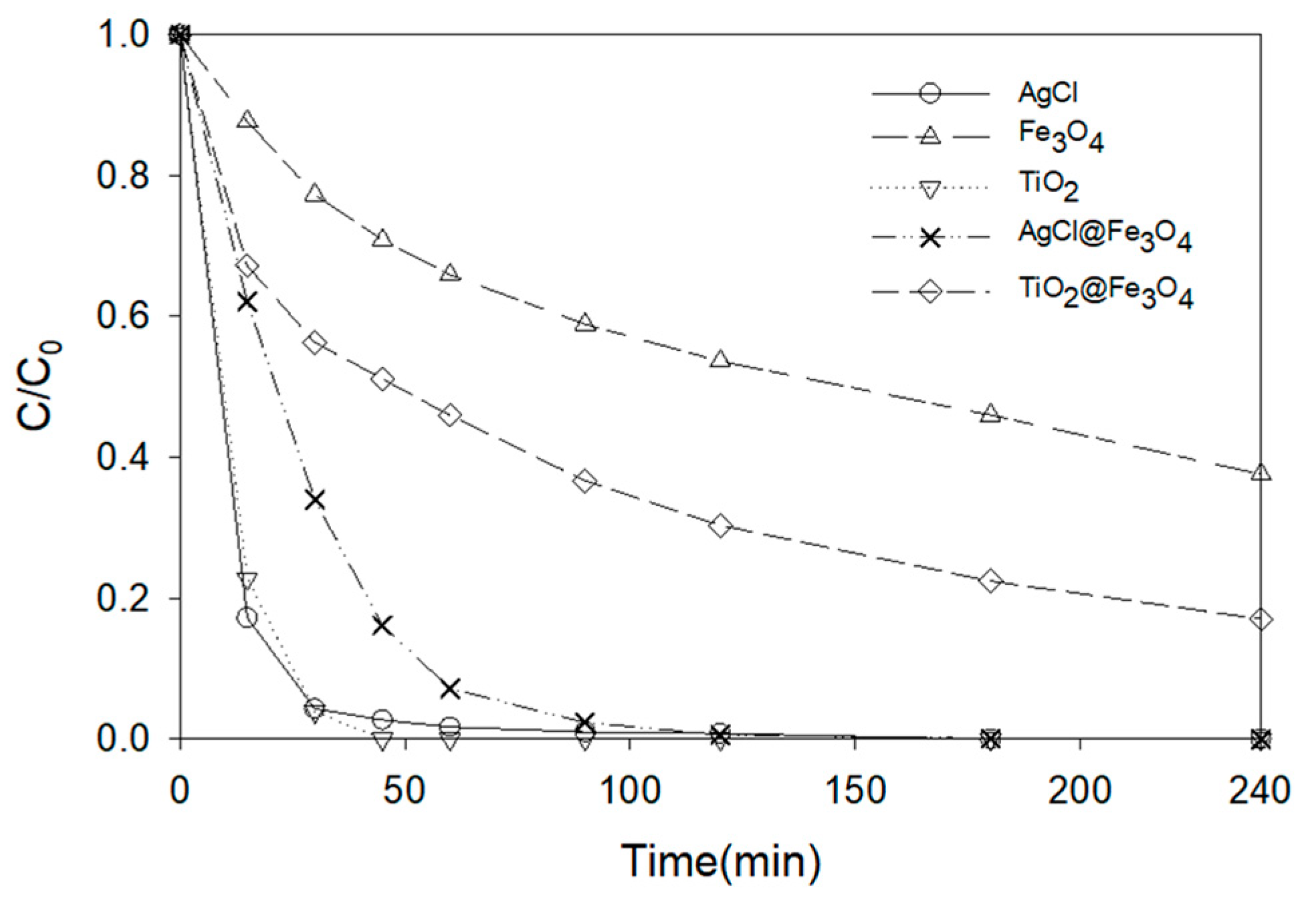
3.4. Photocatalytic Degradation of ATL with AgCl@Fe3O4 or TiO2@Fe3O4 Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ. Pollut. 2006, 144, 184–189. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef]
- Recsetara, M.S.; Fitzsimmonsb, K.M.; Cuello, J.L.; Hoppe-Jones, C.; Snyderc, S.A. Evaluation of a recirculating hydroponic bed bioreactor for removal of contaminants of emerging concern from tertiary-treated wastewater effluent. Chemosphere 2021, 128121. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.G.J.; Klumpp, E.; Umlauf, G.; Nadal, M.; Van Der Ploeg, M.; Van De Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Roberts, J.; Kumar, A.; Du, J.; Hepplewhite, C.; Ellis, D.J.; Christy, A.G.; Beavis, S.G. Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow. Sci. Total Environ. 2016, 541, 1625–1637. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.N.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceu-ticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Grassi, M.; Rizzo, L.; Farina, A. Endocrine disruptors compounds, pharmaceuticals and personal care products in urban wastewater: Implications for agricultural reuse and their removal by adsorption process. Environ. Sci. Pollut. Res. 2013, 20, 3616–3628. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.-M.; Jang, M.; Park, C.M.; Muñoz-Senmache, J.C.; Hernández-Maldonado, A.J.; Heyden, A.; Yu, M.; Yoon, Y. Removal of contaminants of emerging concern by metal-organic framework nanoadsorbents: A review. Chem. Eng. J. 2019, 369, 928–946. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and de-nitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef]
- Gardner, M.; Jones, V.; Comber, S.; Scrimshaw, M.D.; Coello-Garcia, T.; Cartmell, E.; Lester, J.; Ellor, B. Performance of UK wastewater treatment works with respect to trace contaminants. Sci. Total Environ. 2013, 456, 359–369. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Utset, B.; Garcia, J.; Casado, J.; Domènech, X.; Peral, J. Replacement of H2O2 by O2 in Fenton and photo-Fenton reactions. Chemosphere 2000, 41, 1187–1192. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone Sci. Eng. 2019, 41, 3–16. [Google Scholar] [CrossRef]
- Chu, K.H.; Al-Hamadani, Y.; Park, C.M.; Lee, G.; Jang, M.; Jang, A.; Her, N.; Son, A.; Yoon, Y. Ultrasonic treatment of endocrine disrupting compounds, pharmaceuticals, and personal care products in water: A review. Chem. Eng. J. 2017, 327, 629–647. [Google Scholar] [CrossRef]
- Pérez, G.; Fernández-Alba, A.; Urtiaga, A.; Ortiz, I. Electro-oxidation of reverse osmosis concentrates generated in tertiary water treatment. Water Res. 2010, 44, 2763–2772. [Google Scholar] [CrossRef]
- Urtiaga, A.; Pérez, G.; Ibáñez, R.; Ortiz, I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 2013, 331, 26–34. [Google Scholar] [CrossRef]
- Duan, X.D.; He, X.X.; Wang, D.; Mezyk, S.P.; Otto, S.C.; Marfil-Vega, R.; Mills, M.A.; Dionysiou, D.D. Decomposition of Iodinated Pharmaceuticals by UV-254 nm-assisted Advanced Oxidation Processes. J. Hazard. Mater. 2017, 323, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Baena-Nogueras, R.M.; González-Mazo, E.; Lara-Martín, P.A. Degradation kinetics of pharmaceuticals and personal care products in surface waters: Photolysis vs biodegradation. Sci. Total. Environ. 2017, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process. Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.; Michael, C.; Xekoukoulotakis, N.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 1737–1746. [Google Scholar] [CrossRef]
- Martínez, C.; Canle, M.; Fernández, M.; Santaballa, J.; Faria, J. Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes–anatase composites. Appl. Catal. B Environ. 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R.; Manikandan, G.; Gomathisankar, P. Photodegradation of carboxylic acids on Al2O3 and SiO2 nanoparticles. Indian J. Chem. Part A Inorg. Phys. Theor. Anal. 2011, 50, 163–170. [Google Scholar]
- Jia, Y.; Zhou, L.; Ferronato, C.; Yang, X.; Salvador, A.; Zeng, C.; Chovelon, J.-M. Photocatalytic degradation of atenolol in aqueous titanium dioxide suspensions: Kinetics, intermediates and degradation pathways. J. Photochem. Photobiol. A Chem. 2013, 254, 35–44. [Google Scholar] [CrossRef]
- Bodzek, M.; Rajca, M. Photocatalysis in the treatment and disinfection of water. Part I. Theoretical backgrounds/Foto-kataliza w oczyszczaniu i dezynfekcji wody część i. podstawy teoretyczne. Ecol. Chem. Eng. S 2012, 489. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-H.; Salabas, E.-L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C.Z. Facile method to synthesize magnetic iron oxides/TiO2 hybrid nanoparticles and their photodegradation application of methylene blue. Nanoscale Res. Lett. 2011, 6, 533. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, P.M.; Jaramillo, J.; López-Piñero, F.; Plucinski, P.K. Preparation and characterization of magnetic TiO2 nano-particles and their utilization for the degradation of emerging pollutants in water. Appl. Catal. B Environ. 2010, 100, 338–345. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A. Ultrasonic-assisted preparation of novel ternary ZnO/AgI/Fe3O4 nanocomposites as magnetically separable visible-light-driven photocatalysts with excellent activity. J. Colloid Interface Sci. 2016, 461, 144–153. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Li, X.; Wang, D.; Wei, B.; Song, H.; Li, X.; Fu, S. Preparation and photocatalytic properties of magnetically reusable Fe3O4@ZnO core/shell nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2016, 75, 66–71. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Q.; Ju, M.; Li, W. New Developments in Material Preparation Using a Combination of Ionic Liquids and Microwave Irradiation. Nanomaterials 2019, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Ruby, C.; Sonkaria, S.; Taubert, A. A green and sustainable nanotechnology: Role of ionic liquids. Int. J. Precis. Eng. Manuf. 2012, 13, 1207–1213. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Nanoparticles in ionic liquids: Interactions and organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef]
- Antonietti, M.; Kuang, D.; Smarsly, B.; Zhou, Y. Ionic Liquids for the Convenient Synthesis of Functional Nanoparticles and Other Inorganic Nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4988–4992. [Google Scholar] [CrossRef]
- Scholten, J.D.; Prechtl, M.H.G.; Dupont, J. Formation of Nanoparticles Assisted by Ionic Liquids. In Handbook of Green Chemistry; Wiley: Hoboken, NJ, USA, 2010; Volume 8, pp. 1–31. [Google Scholar]
- Duan, X.; Ma, J.-M.; Lian, J.; Zheng, W. The art of using ionic liquids in the synthesis of inorganic nanomaterials. CrystEngComm 2014, 16, 2550–2559. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, B.; Rodríguez-Palmeiro, I.; Rodil, R.; Rodil, E.; Arce, A.; Soto, A. Synthesis of AgCl nanoparticles in ionic liquid and their application in photodegradation of Orange II. J. Mater. Sci. 2015, 50, 3576–3585. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Pathak, R.N. Ionic Liquid Based Silica Tuned Silver Nanoparticles: Novel Approach for Fabrication. Synth. React. Inorg. Met. Chem. 2016, 46, 1265–1271. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. Synthesis of very small TiO2 nanocrystals in a room-temperature ionic liquid and their self-assembly toward mesoporous spherical aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, Z.; Jiang, W.; Jiang, W.; Zhang, W.; Liu, J. Synthesis of CeO2 nanosheets with a room temperature ionic liquid assisted method. J. Adv. Ceram. 2016, 5, 111–116. [Google Scholar] [CrossRef]
- Chen, J.; Xia, J.; Di, J.; Ji, M.; Li, H.; Xu, H.; Zhang, Q.; Lu, J. Reactable ionic liquid assisted synthesis of BiPO4 and the influences of solvent on structure, morphology and photocatalytic performance. Colloids Surf. A Phys. Chem. Eng. Asp. 2016, 488, 110–117. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, J.; Chen, J.; Di, J.; Ji, M.; Xu, L.; Chen, Z.; Li, H. Facile fabrication of g-C3N4/BiPO 4 hybrid materials via a reactable ionic liquid for the photocatalytic degradation of antibiotic ciprofloxacin. J. Photochem. Photobiol. A Chem. 2017, 339, 59–66. [Google Scholar] [CrossRef]
- Hu, Q.; Ji, M.; Di, J.; Wang, B.; Xia, J.; Zhao, Y.; Li, H. Ionic liquid-induced double regulation of carbon quantum dots modified bismuth oxychloride/bismuth oxybromide nanosheets with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2018, 519, 263–272. [Google Scholar] [CrossRef]
- Yin, S.; Ding, Y.; Hu, Q.; Wu, T.; Li, M.; Chen, Y.; Shao, Y.; Di, J.; Wang, B.; Xia, J.; et al. CQDs modified PbBiO2Cl nanosheets with improved molecular oxygen activation ability for photodegradation of organic contaminants. J. Photochem. Photobiol. A Chem. 2019, 382, 111921. [Google Scholar]
- Nan, Q.; Huang, S.; Zhou, Y.; Zhao, S.; He, M.; Wang, Y.; Li, S.; Huang, T.; Pan, W. Ionic liquid-assisted synthesis of porous BiOBr microspheres with enhanced visible light photocatalytic performance. Appl. Organomet. Chem. 2018, 32, e4596. [Google Scholar] [CrossRef]
- Arce, A.; Soto, A.; Rodil, E.; Rodríguez-Cabo, B. Method for the Preparation of Nanoparticles in Ionic Liquids. Patent US20130221289A1, 29 August 2013. [Google Scholar]
- Kim, S.W.; Chung, H.; Kwon, J.-H.; Yoon, H.G.; Kim, W. Facile Synthesis of Silver Chloride Nanocubes and Their Derivatives. Bull. Korean Chem. Soc. 2010, 31, 2918–2922. [Google Scholar] [CrossRef]
- Song, J.; Roh, J.; Lee, I.; Jang, J. Low temperature aqueous phase synthesis of silver/silver chloride plasmonic nanoparticles as visible light photocatalysts. Dalton Trans. 2013, 42, 13897–13904. [Google Scholar] [CrossRef] [PubMed]
- Husein, M.M.; Rodil, E.; Vera, J.H. A novel method for the preparation of silver chloride nanoparticles starting from their solid powder using microemulsions. J. Colloid Interface Sci. 2005, 288, 457–467. [Google Scholar] [CrossRef]
- Petit, C.; Lixon, P.; Pileni, M.P. In situ synthesis of silver nanocluster in AOT reverse micelles. J. Phys. Chem. 1993, 97, 12974–12983. [Google Scholar] [CrossRef]
- Choi, M.; Shin, K.H.; Jang, J. Plasmonic photocatalyticsystem using silver chloride/silver nanostructures under visible light. J. Colloid Interface Sci. 2010, 341, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, W.; Jiang, L. AgCl and Ag/AgCl hollow spheres based on self-assemblies of a multi-amine head surfactant. J. Colloid Interface Sci. 2009, 338, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, G.; Chen, D.; Lv, X.; Li, J. Tuning photoelectrochemical performances of Ag@TiO2 nanocomposites via reduction/oxidation of Ag. Chem. Mater. 2008, 20, 6543–6549. [Google Scholar] [CrossRef]
- Ma, J.; Guo, S.; Guo, X.; Ge, H. A mild synthetic route to Fe3O4@TiO2-Au composites: Preparation, characterization and photocatalytic activity. Appl. Surf. Sci. 2015, 353, 1117–1125. [Google Scholar] [CrossRef]
- Torres, J.D.; Faria, E.A.; Souza, J.R.; Prado, A.G. Preparation of photoactive chitosan–niobium (V) oxide composites for dye degradation. J. Photochem. Photobiol. A Chem. 2006, 182, 202–206. [Google Scholar] [CrossRef]
- Prado, A.G.; Bolzon, L.B.; Pedroso, C.P.; Moura, A.O.; Costa, L.L. Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl. Catal. B Environ. 2008, 82, 219–224. [Google Scholar] [CrossRef]
- Bhatia, V.; Malekshoar, G.; Dhir, A.; Ray, A.K. Enhanced photocatalytic degradation of atenolol using graphene TiO2 composite. J. Photochem. Photobiol. A Chem. 2017, 332, 182–187. [Google Scholar] [CrossRef]
- Li, F.; Kang, Y.; Chen, M.; Liu, G.; Lv, W.; Yao, K.; Chen, P.; Huang, H. Photocatalytic degradation and removal mechanism of ibuprofen via monoclinic BiVO4 under simulated solar light. Chemosphere 2016, 150, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Quintana, J.B.; Cela, R. Transformation of phenazone-type drugs during chlorination. Water Res. 2012, 46, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Quintana, J.B.; Cela, R. Reaction of β-blockers and β-agonist pharmaceuticals with aqueous chlorine. Investigation of kinetics and by-products by liquid chromatography quadrupole time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 2385–2395. [Google Scholar]
- Salgado, R.; Pereira, V.; Carvalho, G.; Soeiro, R.; Gaffney, V.; Almeida, C.; Cardoso, V.V.; Ferreira, E.; Benoliel, M.; Ternes, T.; et al. Photodegradation kinetics and transformation products of ketoprofen, diclofenac and atenolol in pure water and treated wastewater. J. Hazard. Mater. 2013, 244, 516–527. [Google Scholar] [CrossRef]
- Zeng, C.; Ji, Y.; Zhou, L.; Zhang, Y.; Yang, X. The role of dissolved organic matters in the aquatic photodegradation of atenolol. J. Hazard. Mater. 2012, 239, 340–347. [Google Scholar] [CrossRef]
- Yang, H.; Ao, Z.; Li, G.; Song, W.; Cooper, W.J.; Luo, H.; Guo, X. Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: A case of β-blockers. J. Hazard. Mater. 2010, 179, 834–839. [Google Scholar] [CrossRef] [PubMed]



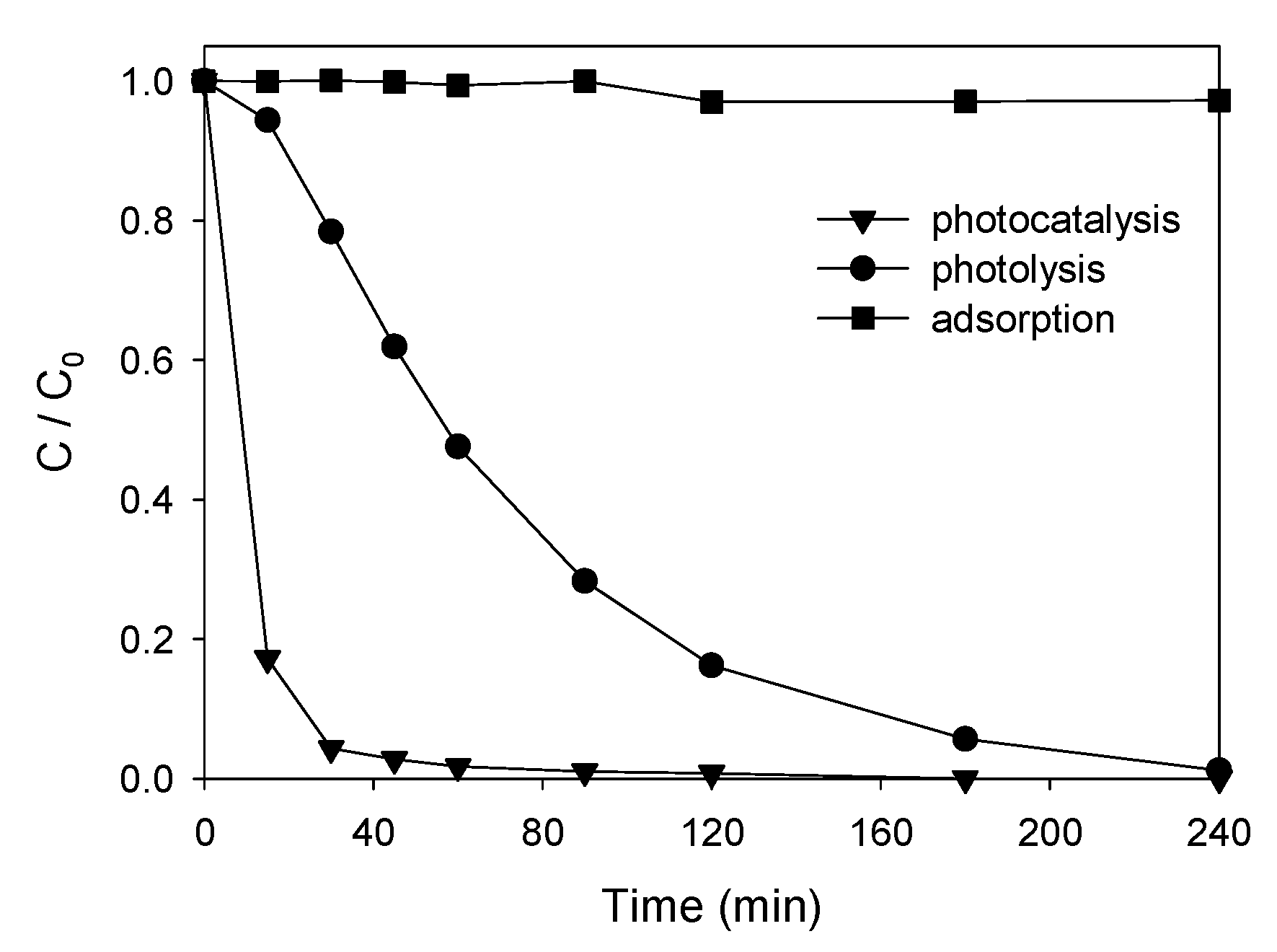
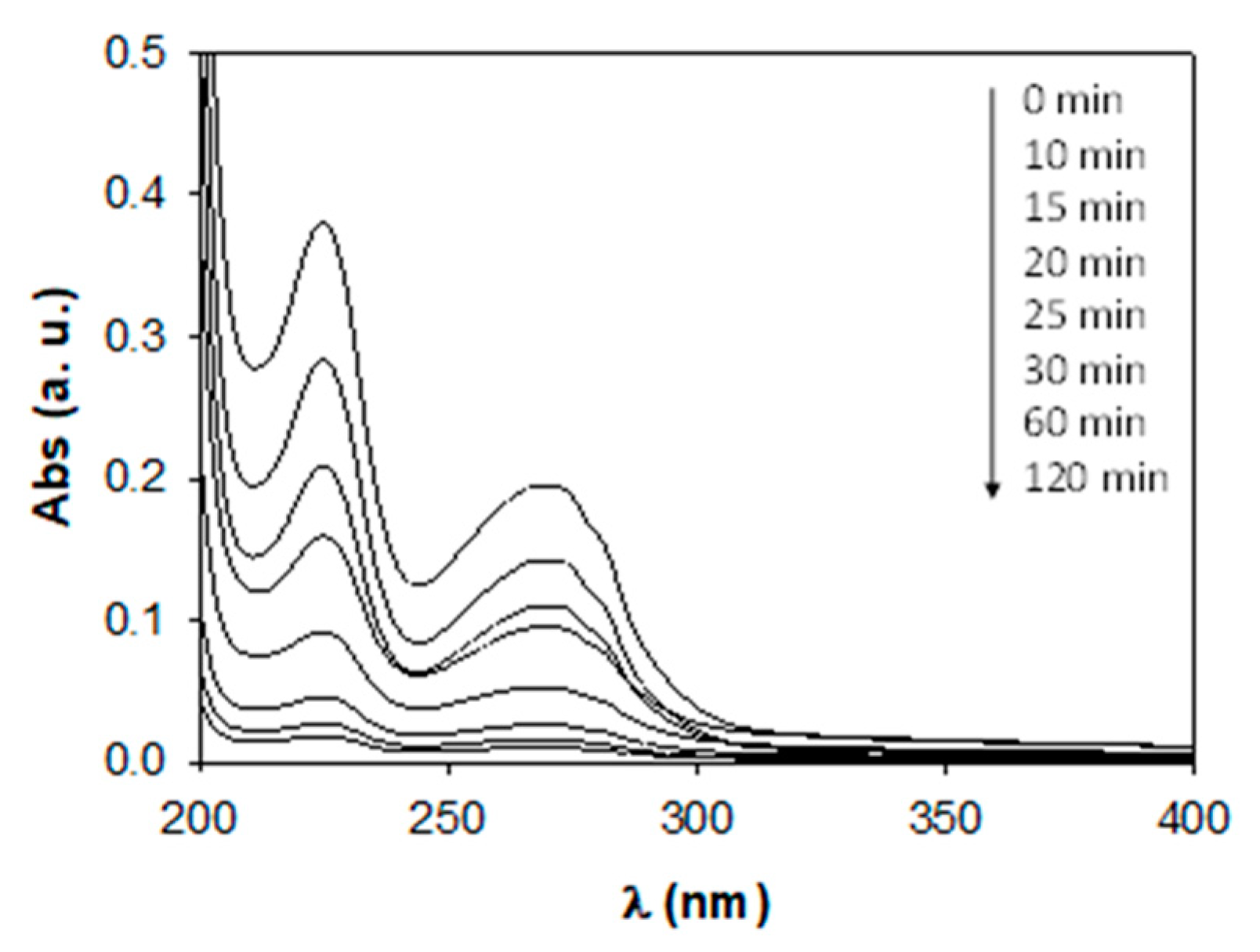
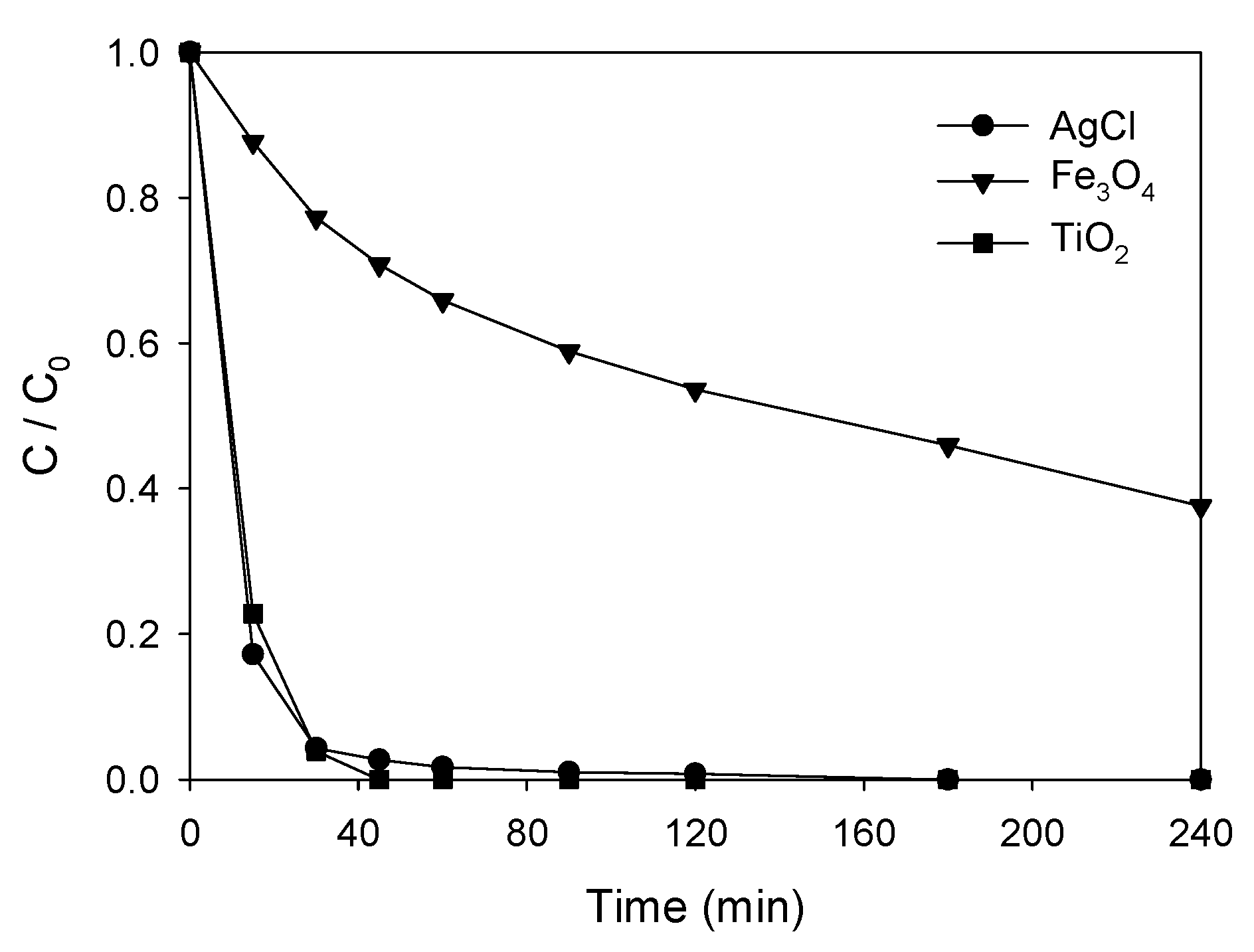
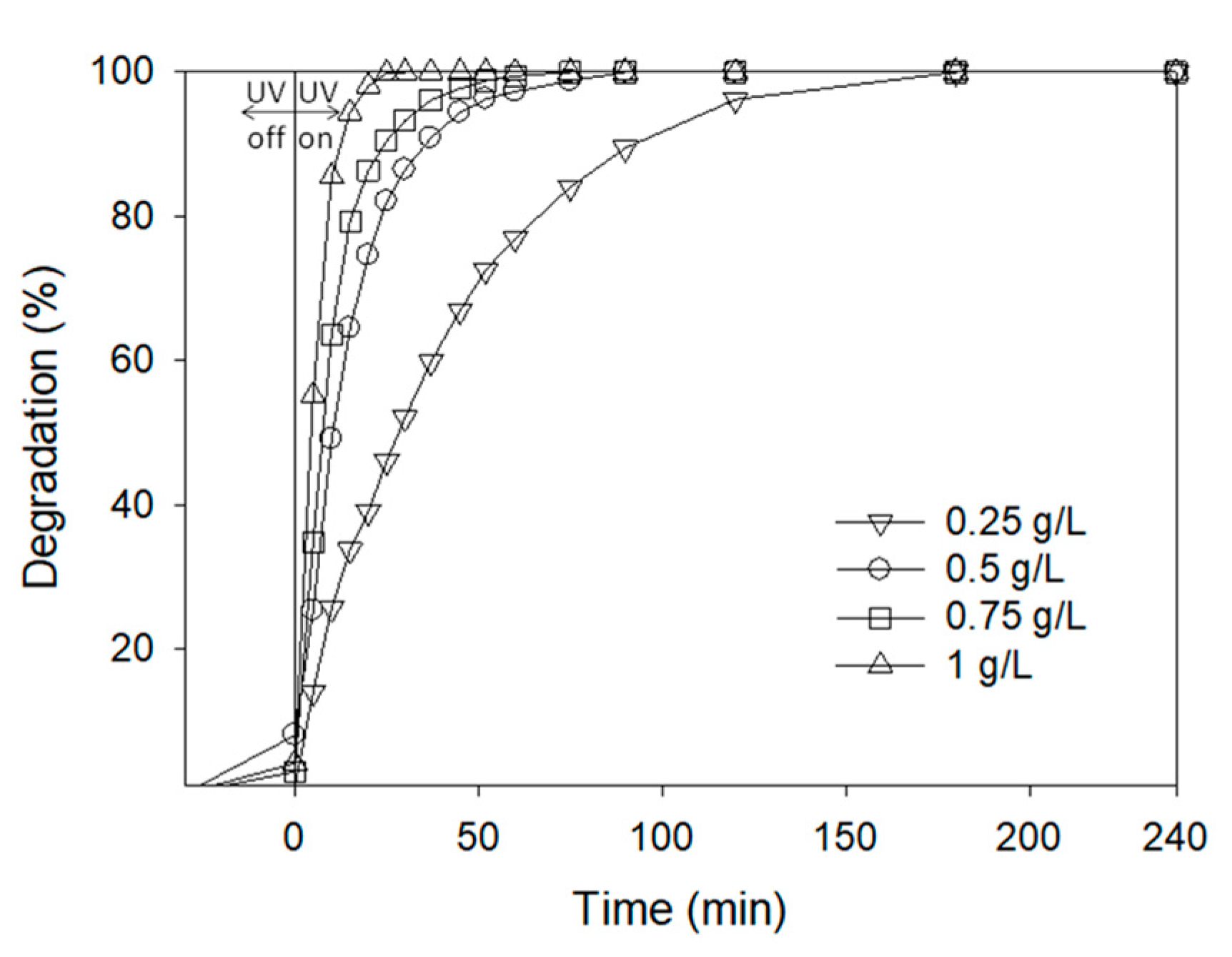

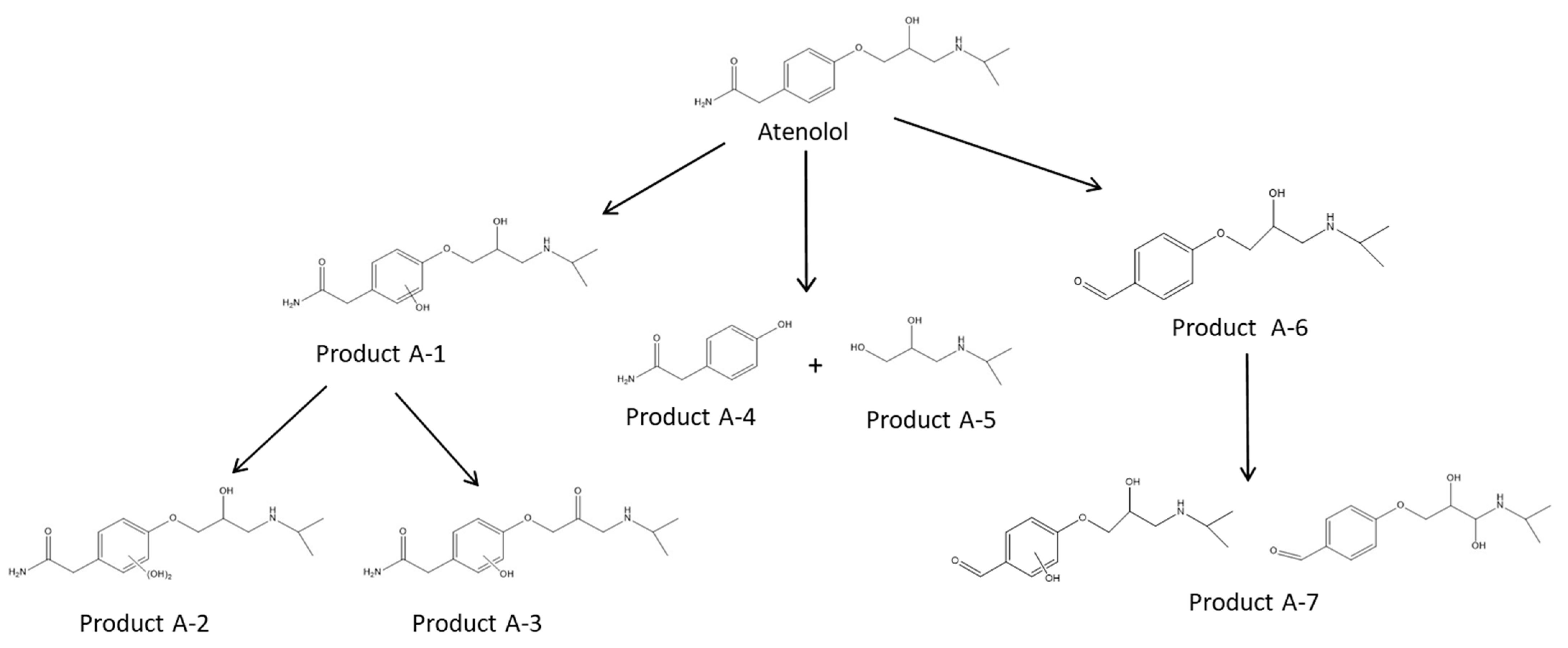
| Product | Experimental m/z | Proposed Formula | Mass Error (ppm) | Score |
|---|---|---|---|---|
| Atenolol | 267.1705 | C14H22O3N2 | −0.33 | 99.92 |
| A-1 | 283.1648 | C14H22O4N2 | 3.48 | 92.39 |
| A-2 | 299.1595 | C14H22O5N2 | 2.05 | 85.5 |
| A-3 | 281.1499 | C14H20O4N2 | −1 | 86.34 |
| A-4 | 152.0703 | C8H9O2N | 0.97 | 85.13 |
| A-5 | 134.1178 | C6H15O2N | −1.46 | 87.74 |
| A-6 | 238.1439 | C13H19O3N | 0.96 | 95.32 |
| A-7 | 254.1387 | C13H19O4N | 3.02 | 89.79 |
| Catalyst | Size (nm) | Degradation—30 min (%) |
|---|---|---|
| AgCl | <20 | 95.7 |
| TiO2 | <21 | 96.1 |
| Fe3O4 | <100 | 23.4 |
| Fe3O4@AgCl | <100 | 66.0 |
| Fe3O4@TiO2 | <100 | 43.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corchero, R.; Rodil, R.; Soto, A.; Rodil, E. Nanomaterial Synthesis in Ionic Liquids and Their Use on the Photocatalytic Degradation of Emerging Pollutants. Nanomaterials 2021, 11, 411. https://doi.org/10.3390/nano11020411
Corchero R, Rodil R, Soto A, Rodil E. Nanomaterial Synthesis in Ionic Liquids and Their Use on the Photocatalytic Degradation of Emerging Pollutants. Nanomaterials. 2021; 11(2):411. https://doi.org/10.3390/nano11020411
Chicago/Turabian StyleCorchero, Raquel, Rosario Rodil, Ana Soto, and Eva Rodil. 2021. "Nanomaterial Synthesis in Ionic Liquids and Their Use on the Photocatalytic Degradation of Emerging Pollutants" Nanomaterials 11, no. 2: 411. https://doi.org/10.3390/nano11020411
APA StyleCorchero, R., Rodil, R., Soto, A., & Rodil, E. (2021). Nanomaterial Synthesis in Ionic Liquids and Their Use on the Photocatalytic Degradation of Emerging Pollutants. Nanomaterials, 11(2), 411. https://doi.org/10.3390/nano11020411