Characterization of Local Structures of Confined Imidazolium Ionic Liquids in PVdF-co-HFP Matrices by High Pressure Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
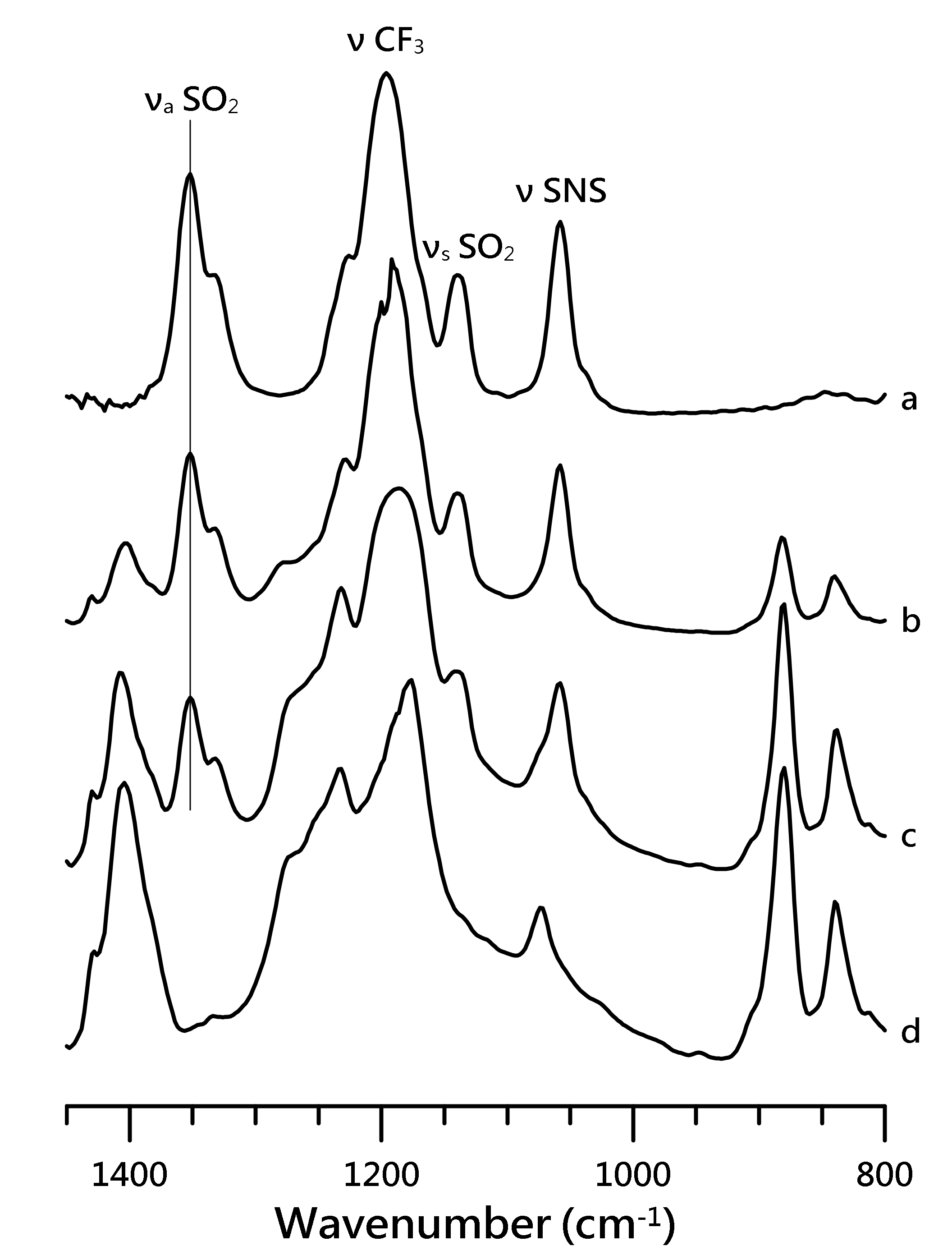
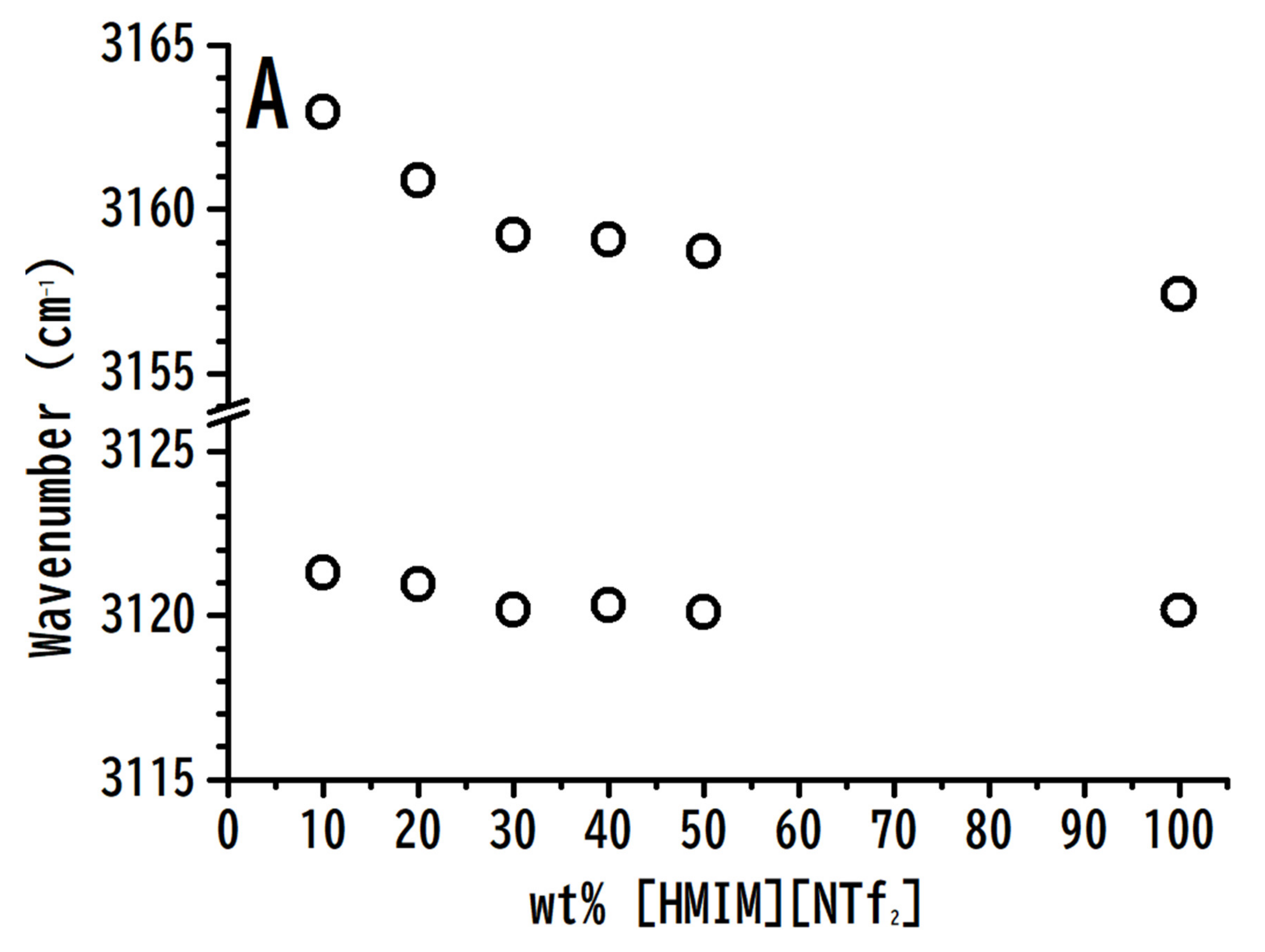
3.1. Ambient Pressure IR Study of [HMIM][NTf2]/PVdF-co-HEP System
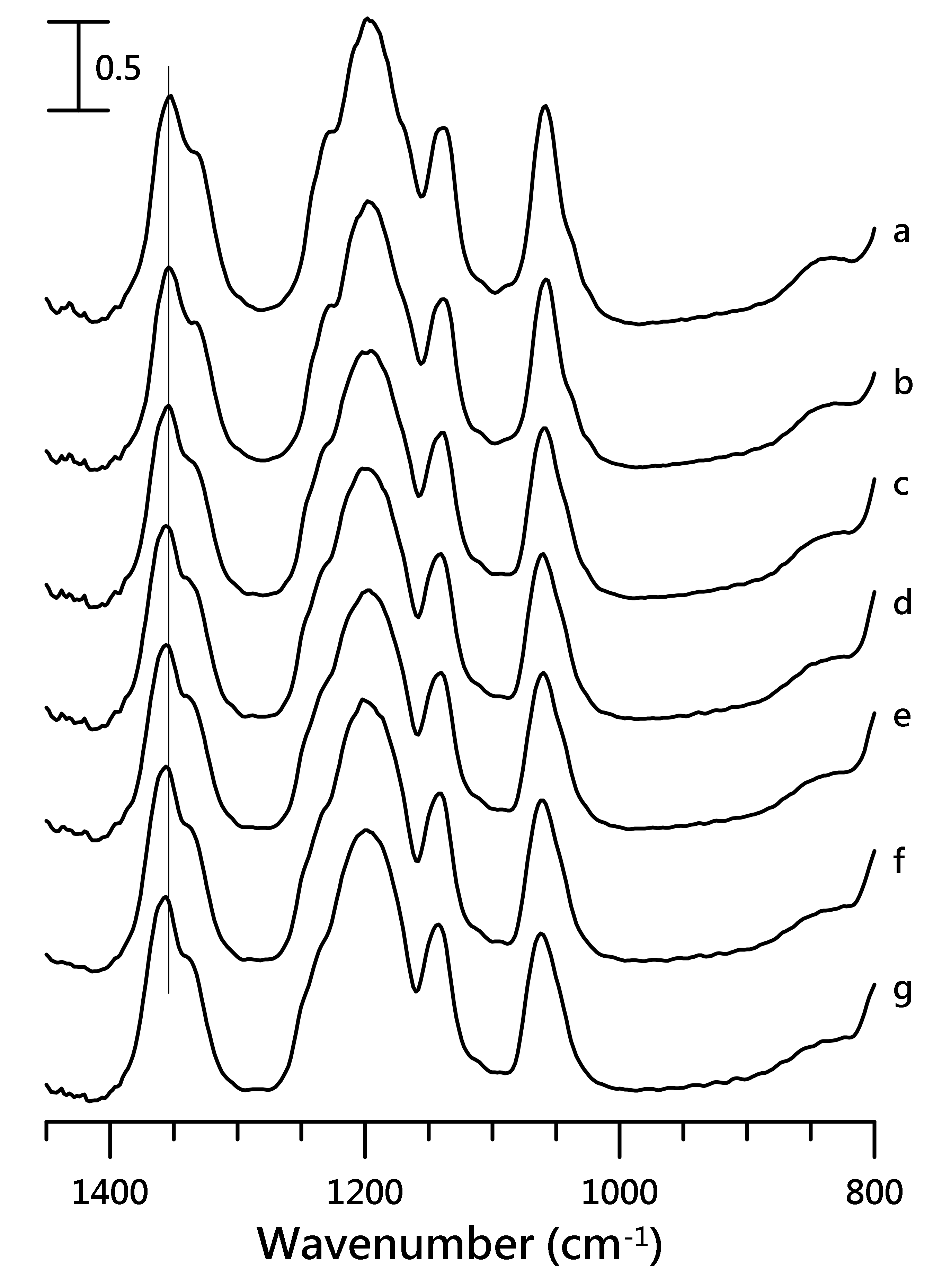
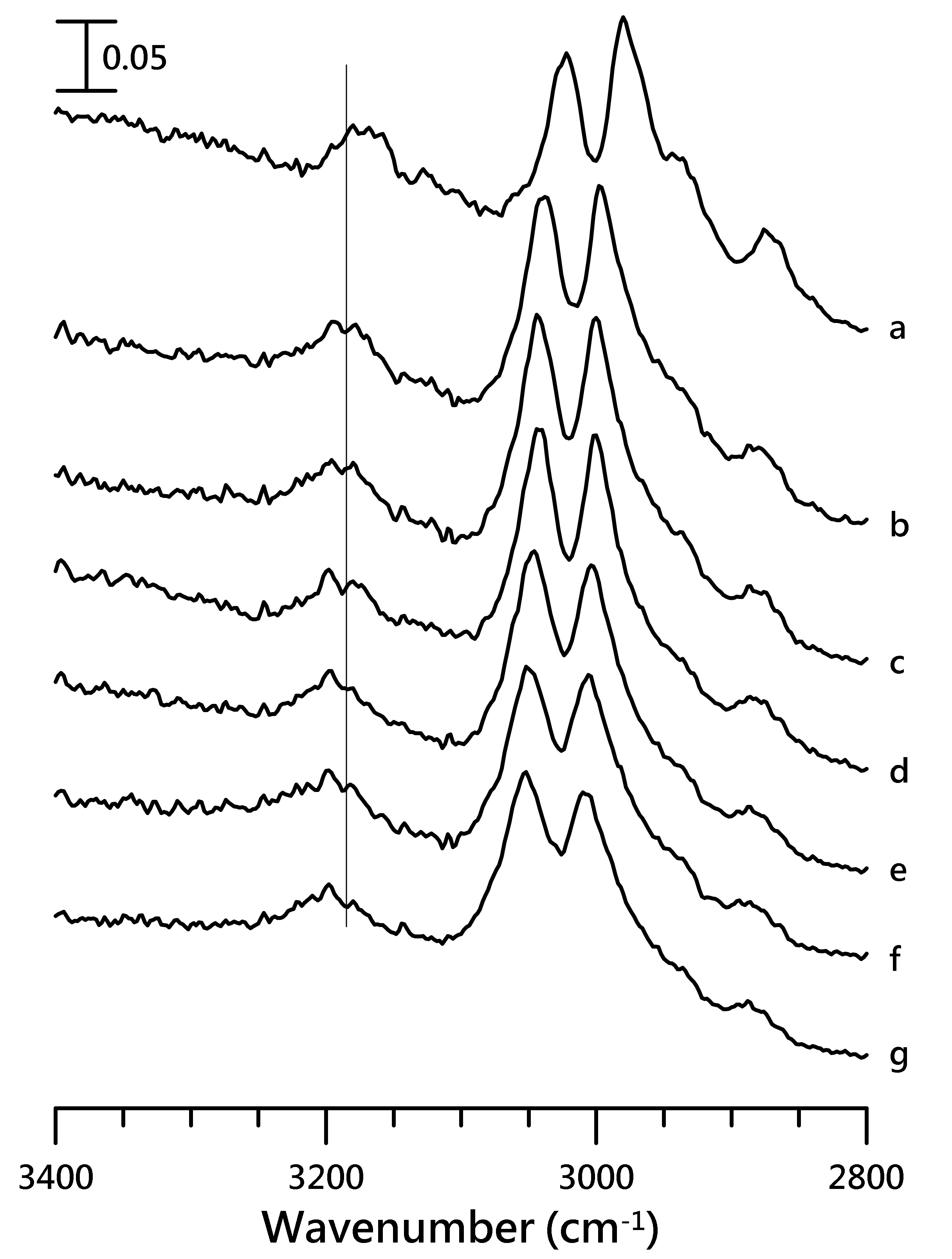
3.2. Pressure-Dependent IR Study of [HMIM][NTf2]/PVdF-co-HEP System
3.3. IR Study of [EMIM][NTf2]/PVdF-co-HEP System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Francis, C.F.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Mishra, K.; Hashmi, S. Nanofiller-incorporated porous polymer electrolyte for electrochemical energy storage devices. High Perform. Polym. 2018, 30, 957–970. [Google Scholar] [CrossRef]
- McGrath, L.M.; Jones, J.; Carey, E.; Rohan, J.F. Ionic Liquid Based Polymer Gel Electrolytes for Use with Germanium Thin Film Anodes in Lithium Ion Batteries. ChemistryOpen 2019, 8, 1429–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Dutta, B.; Deb, D.; Bhattacharya, S. Electroactive phase nucleation and isothermal crystallization kinetics in ionic liquid-functionalized ZnS nanoparticle-ingrained P (VDF-HFP) copolymer nanocomposites. J. Mater. Sci. 2019, 54, 2990–3008. [Google Scholar] [CrossRef]
- Tuhania, P.; Singh, P.K.; Bhattacharya, B.; Dhapola, P.S.; Yadav, S.; Shukla, P.; Gupta, M. PVDF-HFP and 1-ethyl-3-methylimidazolium thiocyanate–doped polymer electrolyte for efficient supercapacitors. High Perform. Polym. 2018, 30, 911–917. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xiao, C.; Liu, H.; Huang, Q.; Hao, J.; Fu, H. Poly (vinylidene fluoride-hexafluoropropylene) porous membrane with controllable structure and applications in efficient oil/water separation. Materials 2018, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Sasikumar, M.; Jagadeesan, A.; Raja, M.; Krishna, R.H.; Sivakumar, P. The effects of PVAc on surface morphological and electrochemical performance of P (VdF-HFP)-based blend solid polymer electrolytes for lithium ion-battery applications. Ionics 2019, 25, 2171–2181. [Google Scholar] [CrossRef]
- Correia, D.M.; Costa, C.M.; Lizundia, E.; Sabater i Serra, R.; Gómez-Tejedor, J.A.; Biosca, L.T.; Meseguer-Dueñas, J.M.; Gomez Ribelles, J.L.; Lanceros-Méndez, S. Influence of cation and anion type on the formation of the electroactive β-phase and thermal and dynamic mechanical properties of poly (vinylidene fluoride)/ionic liquids blends. J. Phys. Chem. C 2019, 123, 27917–27926. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Symmetric electric double-layer capacitor containing imidazolium ionic liquid-based solid polymer electrolyte: Effect of TiO2 and ZnO nanoparticles on electrochemical behavior. J. Appl. Polym. Sci. 2020, 137, 48757. [Google Scholar] [CrossRef]
- Luo, R.; Li, Q.; Du, B.; Zhou, S.; Chen, Y. Preparation and characterization of solid electrolyte doped with carbon nanotubes and its preliminary application in NO2 gas sensors. Front. Mater. 2019, 6, 113. [Google Scholar] [CrossRef]
- Thomas, M.; Rajiv, S. Dye-sensitized solar cells based on an electrospun polymer nanocomposite membrane as electrolyte. New J. Chem. 2019, 43, 4444–4454. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–367. [Google Scholar]
- Roth, C.; Chatzipapadopoulos, S.; Kerlé, D.; Friedriszik, F.; Lütgens, M.; Lochbrunner, S.; Kühn, O.; Ludwig, R. Hydrogen bonding in ionic liquids probed by linear and nonlinear vibrational spectroscopy. New J. Phys. 2012, 14, 105026. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [Green Version]
- Sosa, J.E.; Malheiro, C.; Ribeiro, R.P.; Castro, P.J.; Piñeiro, M.M.; Araújo, J.M.; Plantier, F.; Mota, J.P.; Pereiro, A.B. Adsorption of fluorinated greenhouse gases on activated carbons: Evaluation of their potential for gas separation. J. Chem. Technol. Biotechnol. 2020, 95, 1892–1905. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Luís, A.; Shimizu, K.; Araújo, J.M.; Carvalho, P.J.; Lopes-da-Silva, J.A.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Coutinho, J.A.; Freire, M.G.; Pereiro, A.B. Influence of nanosegregation on the surface tension of fluorinated ionic liquids. Langmuir 2016, 32, 6130–6139. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Bernardes, C.E.S.; Canongia Lopes, J.N. Structure and Aggregation in the 1-Alkyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide Ionic Liquid Homologous Series. J. Phys. Chem. B 2014, 118, 567–576. [Google Scholar] [CrossRef]
- Dutta, R.; Kundu, S.; Sarkar, N. Ionic liquid-induced aggregate formation and their applications. Biophys. Rev. 2018, 10, 861–871. [Google Scholar] [CrossRef]
- Burba, C.M.; Chang, H.-C. Temperature-and pressure-dependent infrared spectroscopy of 1-butyl-3-methylimidazolium trifluoromethanesulfonate: A dipolar coupling theory analysis. Spectrochim. Acta A 2018, 193, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.; Singh, R.; Chandra, S. Thermal stability, complexing behavior, and ionic transport of polymeric gel membranes based on polymer PVdF-HFP and ionic liquid, [BMIM][BF4]. J. Phys. Chem. B 2013, 117, 897–906. [Google Scholar]
- Liang, C.-L.; Mai, Z.-H.; Xie, Q.; Bao, R.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Induced formation of dominating polar phases of poly (vinylidene fluoride): Positive ion–CF2 dipole or negative ion–CH2 dipole interaction. J. Phys. Chem. B 2014, 118, 9104–9111. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Kundu, T. Density functional theory studies on PVDF/ionic liquid composite systems. J. Chem. Sci. 2018, 130, 115. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.C.; Clarizia, G.; Bernardo, P.; Bazzarelli, F.; Friess, K.; Randová, A.; Schauer, J.; Kubicka, D.; Kacirková, M.; Izak, P. Gas transport properties and pervaporation performance of fluoropolymer gel membranes based on pure and mixed ionic liquids. Sep. Purif. Technol. 2013, 109, 87–97. [Google Scholar] [CrossRef]
- Wang, T.-H.; Shen, M.-H.; Chang, H.-C. Pressure-Dependent Stability of Imidazolium-Based Ionic Liquid/DNA Materials Investigated by High-Pressure Infrared Spectroscopy. Materials 2019, 12, 4202. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-H.; Lin, E.-Y.; Chang, H.-C. Pressure-Dependent Confinement Effect of Ionic Liquids in Porous Silica. Nanomaterials 2019, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-H.; Hong, S.-Y.; Chang, H.-C. The validity of high pressure IR for detecting the interactions between β-cyclodextrin and imidazolium based ionic liquids. AIP Adv. 2019, 9, 075007. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-C.; Wang, T.-H.; Burba, C.M. Probing structures of interfacial 1-butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid on nano-aluminum oxide surfaces using high-pressure infrared spectroscopy. Appl. Sci. 2017, 7, 855. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-H.; Chang, H.-C.; Fu, Y.-P. Utilizing infrared spectroscopy to analyze the interfacial structures of ionic liquids/Al2O3 and ionic liquids/mica mixtures under high pressures. Nanomaterials 2019, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.; Moffatt, D.; Baudais, F. Crystalline quartz as an internal pressure calibrant for high-pressure infrared spectroscopy. Appl. Spectrosc. 1985, 39, 733–735. [Google Scholar] [CrossRef]
- Wong, P.; Moffatt, D. The uncoupled OH or OD stretch in water as an internal pressure gauge for high-pressure infrared spectroscopy of aqueous systems. Appl. Spectrosc. 1987, 41, 1070–1072. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Shrestha, S.; Siegel, A.; Varahramyan, K.; Agarwal, M. Cross-selectivity enhancement of poly (vinylidene fluoride-hexafluoropropylene)-based sensor arrays for detecting acetone and ethanol. Sensors 2017, 17, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanke, K.; Kaufmann, M.; Schwaab, G.; Havenith, M.; Wolke, C.T.; Gorlova, O.; Johnson, M.A.; Kar, B.P.; Sander, W.; Sanchez-Garcia, E. Understanding the ionic liquid [NC 4111][NTf 2] from individual building blocks: An IR-spectroscopic study. Phys. Chem. Chem. Phys. 2015, 17, 8518–8529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiri, A.; Borisenko, N.; Borodin, A.; Olschewski, M.; Endres, F. Characterisation of the solid electrolyte interface during lithiation/delithiation of germanium in an ionic liquid. Phys. Chem. Chem. Phys. 2016, 18, 5630–5637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.C.C.; Padua, A.A.H.; Costa Gomes, M.F. Glass transition of ionic liquids under high pressure. J. Chem. Phys. 2014, 140, 244514. [Google Scholar] [CrossRef]
- Faria, L.F.O.; Ribeiro, M.C.C. Phase transitions of triflate-based ionic liquids under high pressure. J. Phys. Chem. B 2015, 119, 14315–14322. [Google Scholar] [CrossRef]
- Freire, M.G.; Santos, L.M.N.B.F.; Fernandes, A.M.; Coutinho, J.A.P.; Marrucho, I.M. An overview of the mutual solubilities of water-imidazolium-based ionic liquids systems. Fluid Phase Equilib. 2007, 261, 449–454. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-H.; Wu, M.-S.; Chang, H.-C. Characterization of Local Structures of Confined Imidazolium Ionic Liquids in PVdF-co-HFP Matrices by High Pressure Infrared Spectroscopy. Nanomaterials 2020, 10, 1973. https://doi.org/10.3390/nano10101973
Wang T-H, Wu M-S, Chang H-C. Characterization of Local Structures of Confined Imidazolium Ionic Liquids in PVdF-co-HFP Matrices by High Pressure Infrared Spectroscopy. Nanomaterials. 2020; 10(10):1973. https://doi.org/10.3390/nano10101973
Chicago/Turabian StyleWang, Teng-Hui, Ming-Siou Wu, and Hai-Chou Chang. 2020. "Characterization of Local Structures of Confined Imidazolium Ionic Liquids in PVdF-co-HFP Matrices by High Pressure Infrared Spectroscopy" Nanomaterials 10, no. 10: 1973. https://doi.org/10.3390/nano10101973
APA StyleWang, T.-H., Wu, M.-S., & Chang, H.-C. (2020). Characterization of Local Structures of Confined Imidazolium Ionic Liquids in PVdF-co-HFP Matrices by High Pressure Infrared Spectroscopy. Nanomaterials, 10(10), 1973. https://doi.org/10.3390/nano10101973





