3,4-Methylenedioxypyrovalerone (MDPV) Sensing Based on Electropolymerized Molecularly Imprinted Polymers on Silver Nanoparticles and Carboxylated Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Equipment
2.3. Preparation of SPCE-MWCNT-AgNP-MIP
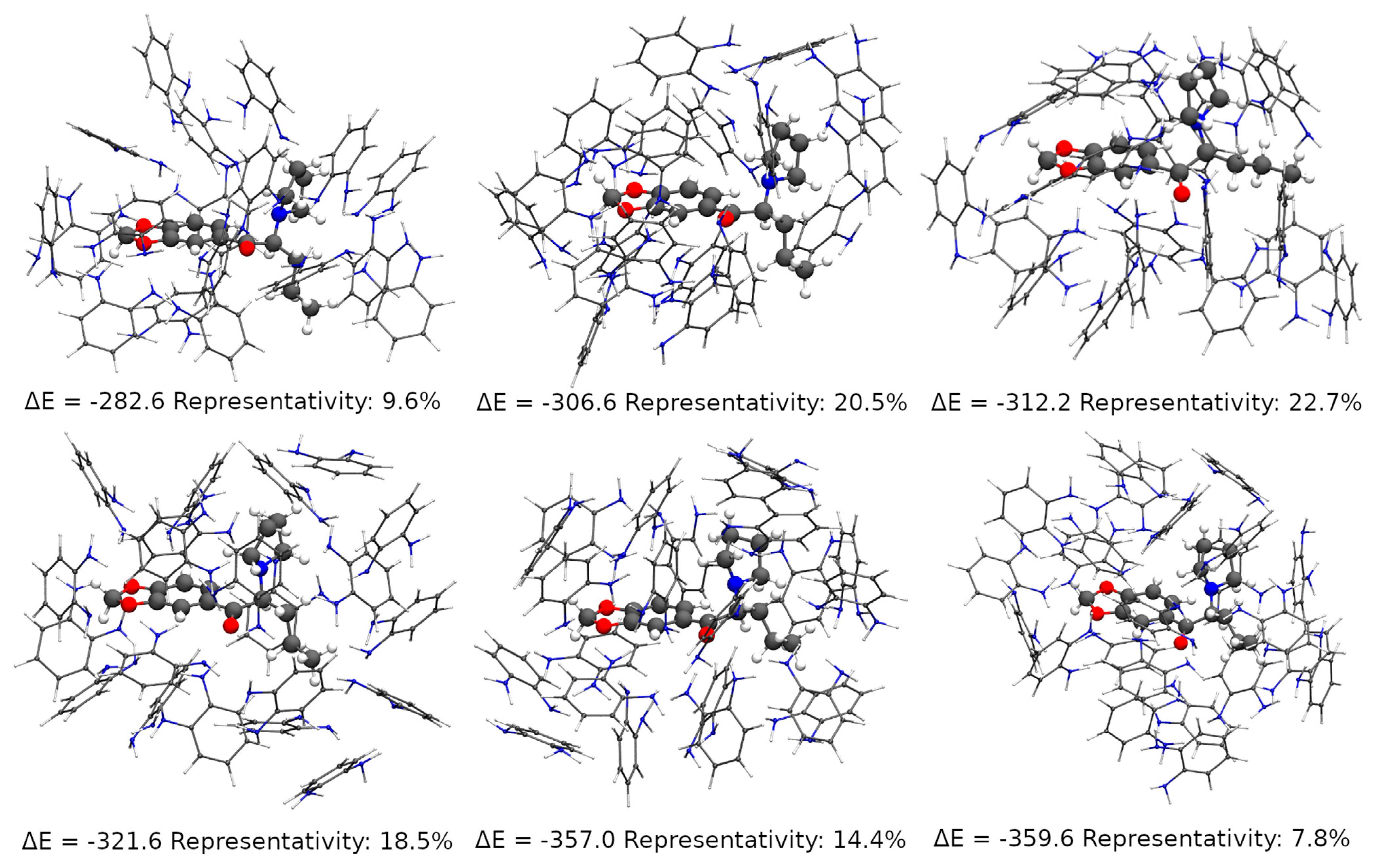
2.4. Theoretical Studies
3. Results and Discussion
3.1. Characterization of the Electrodes during the Modification Process
3.1.1. Electrochemical Evaluation
3.1.2. Morphological Study
3.2. Theoretical Studies
3.3. Influence of the Experimental Conditions
3.4. Analytical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Desharnais, B.; Dazé, Y.; Huppertz, L.M.; Mireault, P.; Skinner, C.D. A case of fatal idiosyncratic reaction to the designer drug 3,4-methylenedioxypyrovalerone (MDPV) and review of the literature. Forensic Sci. Med. Pathol. 2017, 13, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Olives, T.; Orozco, B.; Stellpflug, S. Bath salts: The ivory wave of trouble. West. J. Emerg. Med. 2012, 13, 58–62. [Google Scholar] [CrossRef]
- Colon-Perez, L.M.; Tran, K.; Thompson, K.; Pace, M.C.; Blum, K.; Goldberger, B.A.; Gold, M.S.; Bruijnzeel, A.W.; Setlow, B.; Febo, M. The psychoactive designer drug and bath salt constituent MDPV causes widespread disruption of brain functional connectivity. Neuropsychopharmacology 2016, 41, 2352–2365. [Google Scholar] [CrossRef] [Green Version]
- Giannotti, G.; Canazza, I.; Caffino, L.; Bilel, S.; Ossato, A.; Fumagalli, F.; Marti, M. The cathinones MDPV and α-PVP elicit different behavioral and molecular effects following acute exposure. Neurotox. Res. 2017, 32, 594–602. [Google Scholar] [CrossRef]
- Kesha, K.; Boggs, C.L.; Ripple, M.G.; Allan, C.H.; Levine, B.; Jufer-Phipps, R.; Doyon, S.; Chi, P.; Fowler, D.R. Methylenedioxypyrovalerone (“bath salts”), related death: Case eeport and review of the literature. J. Forensic Sci. 2013, 58, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Liveri, K.; Constantinou, M.A.; Afxentiou, M.; Kanari, P. A fatal intoxication related to MDPV and pentedrone combined with antipsychotic and antidepressant substances in Cyprus. Forensic Sci. Int. 2016, 265, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Watterson, L.R.; Kufahl, P.R.; Nemirovsky, N.E.; Sewalia, K.; Grabenauer, M.; Thomas, B.F.; Marusich, J.A.; Wegner, S.; Olive, M.F. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict. Biol. 2014, 19, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Diestelmann, M.; Zangl, A.; Herrle, I.; Koch, E.; Graw, M.; Paul, L.D. MDPV in forensic routine cases: Psychotic and aggressive behavior in relation to plasma concentrations. Forensic Sci. Int. 2018, 283, 72–84. [Google Scholar] [CrossRef]
- Kohler, R.J.; Perrine, S.A.; Baker, L.E. Repeated exposure to 3,4-methylenedioxypyrovalerone and cocaine produces locomotor sensitization with minimal effects on brain monoamines. Neuropharmacology 2018, 134, 22–27. [Google Scholar] [CrossRef]
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef]
- Agency, W. Anti-Doping the World Anti-Doping Code. Available online: www.wada-ama.org/sites/default/files/wada_2020_english_prohibited_list_0.pdf (accessed on 1 September 2020).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). MDPV-EMCDDA–Europol Joint Report on a New Psychoactive Substance: MDPV (3,4-Methylenedioxypyrovalerone); Publications Office of the European Union: Lisbon, Portugal, 2014; ISBN 9789291686803. [Google Scholar]
- Erowid MDPV Effects. Available online: https://www.erowid.org/chemicals/mdpv/mdpv_effects.shtml (accessed on 15 May 2020).
- Soussan, C.; Kjellgren, A. The users of novel psychoactive substances: Online survey about their characteristics, attitudes and motivations. Int. J. Drug Policy 2016, 32, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonowicz, J.L.; Metzger, A.K.; Ramanujam, S.L. Paranoid psychosis induced by consumption of methylenedioxypyrovalerone: Two cases. Gen. Hosp. Psychiatry 2011. [Google Scholar] [CrossRef] [PubMed]
- Benturquia, N.; Chevillard, L.; Poiré, C.; Roussel, O.; Cohier, C.; Declèves, X.; Laplanche, J.-L.; Etheve-Quelquejeu, M.; Chen, H.; Mégarbane, B. Is the 3,4-methylendioxypyrovalerone/mephedrone combination responsible for enhanced stimulant effects? A rat study with investigation of the effect/concentration relationships. Psychopharmacology (Berl) 2019, 236, 891–901. [Google Scholar] [CrossRef]
- Bertol, E.; Mari, F.; Boscolo Berto, R.; Mannaioni, G.; Vaiano, F.; Favretto, D. A mixed MDPV and benzodiazepine intoxication in a chronic drug abuser: Determination of MDPV metabolites by LC–HRMS and discussion of the case. Forensic Sci. Int. 2014, 243, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Galindo, K.I.; Mesmin, M.P.; Sulima, A.; Rice, K.C.; Collins, G.T. Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 2018, 134, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Colon-Perez, L.M.; Pino, J.A.; Saha, K.; Pompilus, M.; Kaplitz, S.; Choudhury, N.; Jagnarine, D.A.; Geste, J.R.; Levin, B.A.; Wilks, I.; et al. Functional connectivity, behavioral and dopaminergic alterations 24 hours following acute exposure to synthetic bath salt drug methylenedioxypyrovalerone. Neuropharmacology 2018, 137, 178–193. [Google Scholar] [CrossRef]
- Allen, S.A.; Tran, L.H.; Oakes, H.V.; Brown, R.W.; Pond, B.B. Dopaminergic effects of major bath salt constituents 3,4-methylenedioxypyrovalerone (MDPV), mephedrone, and methylone are enhanced following co-exposure. Neurotox. Res. 2019, 36, 132–143. [Google Scholar] [CrossRef]
- Horsley, R.R.; Lhotkova, E.; Hajkova, K.; Feriancikova, B.; Himl, M.; Kuchar, M.; Páleníček, T. Behavioural, pharmacokinetic, metabolic, and hyperthermic profile of 3,4-Methylenedioxypyrovalerone (MDPV) in the Wistar rat. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Aftab, A.; Shah, M.; Nayar, J. Physical and psychological effects of the new legal high ‘Ivory Wave’: A case report. Br. J. Med. Pract. 2010, 3, a343. [Google Scholar]
- Colucci, P.; Mancini, G.F.; Santori, A.; Zwergel, C.; Mai, A.; Trezza, V.; Roozendaal, B.; Campolongo, P. Amphetamine and the smart drug 3,4-methylenedioxypyrovalerone (MDPV) induce generalization of fear memory in rats. Front. Mol. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- World Health Organisation. 3,4-Methylenedioxypyrovalerone (MDPV); World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Strano-Rossi, S.; Cadwallader, A.B.; de la Torre, X.; Botrè, F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MPDV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2706–2714. [Google Scholar] [CrossRef] [PubMed]
- Marinetti, L.J.; Antonides, H.M. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: Method development, drug distribution and interpretation of results. J. Anal. Toxicol. 2013, 37, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bade, R.; Bijlsma, L.; Sancho, J.V.; Baz-Lomba, J.A.; Castiglioni, S.; Castrignanò, E.; Causanilles, A.; Gracia-Lor, E.; Kasprzyk-Hordern, B.; Kinyua, J.; et al. Liquid chromatography-tandem mass spectrometry determination of synthetic cathinones and phenethylamines in influent wastewater of eight European cities. Chemosphere 2017, 168, 1032–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez, M.; Pozo, Ó.J.; Sancho, J.V.; Orengo, T.; Haro, G.; Hernández, F. Analytical strategy to investigate 3,4-methylenedioxypyrovalerone (MDPV) metabolites in consumers’ urine by high-resolution mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 151–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.R.; Keasling, R.; Brown, S.D.; Pond, B.B. Quantification of synthetic cathinones in rat brain using HILIC–ESI-MS/MS. J. Anal. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fornal, E. Identification of substituted cathinones: 3,4-Methylenedioxy derivatives by high performance liquid chromatography–quadrupole time of flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 81–82, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Ippisch, J.; Herrmann, C.; Guber, S.; Schultis, W. Analysis of new designer drugs and common drugs of abuse in urine by a combined targeted and untargeted LC-HR-QTOFMS approach. Anal. Bioanal. Chem. 2014, 406, 4425–4441. [Google Scholar] [CrossRef]
- Anizan, S.; Ellefsen, K.; Concheiro, M.; Suzuki, M.; Rice, K.C.; Baumann, M.H.; Huestis, M.A. 3,4-methylenedioxypyrovalerone (MDPV) and metabolites quantification in human and rat plasma by liquid chromatography–high resolution mass spectrometry. Anal. Chim. Acta 2014, 827, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Mardal, M.; Meyer, M.R. Studies on the microbial biotransformation of the novel psychoactive substance methylenedioxypyrovalerone (MDPV) in wastewater by means of liquid chromatography-high resolution mass spectrometry/mass spectrometry. Sci. Total Environ. 2014, 493, 588–595. [Google Scholar] [CrossRef]
- Wang, C.C.; Hartmann-Fischbach, P.; Krueger, T.R.; Wells, T.L.; Feineman, A.R.; Compton, J.C. Rapid and sensitive analysis of 3,4-methylenedioxypyrovalerone in equine plasma using liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2012, 36, 327–333. [Google Scholar] [CrossRef] [Green Version]
- van Nuijs, A.L.N.; Gheorghe, A.; Jorens, P.G.; Maudens, K.; Neels, H.; Covaci, A. Optimization, validation, and the application of liquid chromatography-tandem mass spectrometry for the analysis of new drugs of abuse in wastewater. Drug Test. Anal. 2014, 6, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, P.; Gil, D.; Skulska, A.; Tokarczyk, B. Analysis of MDPV in blood-determination and interpretation. J. Anal. Toxicol. 2013, 37, 308–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Castro, A.; Lendoiro, E.; Fernández-Vega, H.; Steinmeyer, S.; López-Rivadulla, M.; Cruz, A. Liquid chromatography tandem mass spectrometry determination of selected synthetic cathinones and two piperazines in oral fluid. Cross reactivity study with an on-site immunoassay device. J. Chromatogr. A 2014, 1374, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Du, P.; Schuster, F.; Maurer, H.H. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. J. Mass Spectrom. 2010, 45, 1426–1442. [Google Scholar] [CrossRef] [PubMed]
- Ojanperä, I.A.; Heikman, P.K.; Rasanen, I.J. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography–mass spectrometry. Ther. Drug Monit. 2011, 1. [Google Scholar] [CrossRef]
- Abiedalla, Y.F.H.; Abdel-Hay, K.; DeRuiter, J.; Clark, C.R. Synthesis and GC–MS analysis of a series of homologs and regioisomers of 3,4-methylenedioxypyrovalerone (MDPV). Forensic Sci. Int. 2012, 223, 189–197. [Google Scholar] [CrossRef]
- Leffler, A.M.; Smith, P.B.; de Armas, A.; Dorman, F.L. The analytical investigation of synthetic street drugs containing cathinone analogs. Forensic Sci. Int. 2014, 234, 50–56. [Google Scholar] [CrossRef]
- Hong, W.-Y.; Ko, Y.-C.; Lin, M.-C.; Wang, P.-Y.; Chen, Y.-P.; Chiueh, L.-C.; Shih, D.Y.-C.; Chou, H.-K.; Cheng, H.-F. Determination of synthetic cathinones in urine using gas chromatography–mass spectrometry techniques. J. Anal. Toxicol. 2015, bkv108. [Google Scholar] [CrossRef] [Green Version]
- Abiedalla, Y.F.H.; Abdel-Hay, K.; DeRuiter, J.; Clark, C.R. GC–MS, MS/MS and GC–IR analysis of a series of methylenedioxyphenyl-aminoketones: Precursors, ring regioisomers and side-chain homologs of 3,4-methylenedioxypyrovalerone. J. Chromatogr. Sci. 2017, 55, 99–108. [Google Scholar] [CrossRef]
- Wyman, J.F.; Lavins, E.S.; Engelhart, D.; Armstrong, E.J.; Snell, K.D.; Boggs, P.D.; Taylor, S.M.; Norris, R.N.; Miller, F.P. Postmortem tissue distribution of MDPV following lethal intoxication by “bath salts”. J. Anal. Toxicol. 2013, 37, 182–185. [Google Scholar] [CrossRef] [Green Version]
- de las Nieves Peiró, M.; Armenta, S.; Garrigues, S.; de la Guardia, M. Determination of 3,4-methylenedioxypyrovalerone (MDPV) in oral and nasal fluids by ion mobility spectrometry. Anal. Bioanal. Chem. 2016, 408, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Baciu, T.; Borrull, F.; Calull, M.; Aguilar, C. Enantioselective determination of cathinone derivatives in human hair by capillary electrophoresis combined in-line with solid-phase extraction. Electrophoresis 2016, 37, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.; Horan, E.; Fox, J.; O’Donnell, C.; Byrne, H.J.; McDermott, S.; Power, J.; Kavanagh, P. Discrimination of cathinone regioisomers, sold as ‘legal highs’, by Raman spectroscopy. Drug Test. Anal. 2014, 6, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Strano Rossi, S.; Odoardi, S.; Gregori, A.; Peluso, G.; Ripani, L.; Ortar, G.; Serpelloni, G.; Romolo, F.S. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun. Mass Spectrom. 2014, 28, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, A.A.; Poteshin, S.S.; Chernyshev, D.M.; Karpov, A.V.; Tuzkov, Y.B.; Kyzmin, V.V.; Sysoev, A.A. Analysis of new synthetic drugs by ion mobility time-of-flight mass spectrometry. Eur. J. Mass Spectrom. 2014, 20, 185–192. [Google Scholar] [CrossRef]
- Ellefsen, K.N.; Anizan, S.; Castaneto, M.S.; Desrosiers, N.A.; Martin, T.M.; Klette, K.L.; Huestis, M.A. Validation of the only commercially available immunoassay for synthetic cathinones in urine: Randox drugs of abuse V biochip array technology. Drug Test. Anal. 2014, 6, 728–738. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. Recent advances in membrane-aided extraction and separation for analytical purposes. Sep. Purif. Rev. 2017, 46, 179–194. [Google Scholar] [CrossRef]
- Gonçalves, L.M. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly imprinted polymers: Antibody mimics for bioimaging and therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Yarman, A.; Kurbanoglu, S.; Zebger, I.; Scheller, F.W. Simple and robust: The claims of protein sensing by molecularly imprinted polymers. Sens. Actuators B Chem. 2020, 129369. [Google Scholar] [CrossRef]
- Gajda, M.; Rybakiewicz, R.; Cieplak, M.; Żołek, T.; Maciejewska, D.; Gilant, E.; Rudzki, P.J.; Grab, K.; Kutner, A.; Borowicz, P.; et al. Low-oxidation-potential thiophene-carbazole monomers for electro-oxidative molecular imprinting: Selective chemosensing of aripiprazole. Biosens. Bioelectron. 2020, 169, 112589. [Google Scholar] [CrossRef] [PubMed]
- Grothe, R.A.; Lobato, A.; Mounssef, B., Jr.; Tasic, N.; Maldaner, A.O.; Braga, A.A.C.; Aldous, L.; Paixao, T.R.L.C.; Gonçalves, L.M. Electroanalytical profiling of cocaine samples by means of an electropolymerized molecularly imprinted polymer using benzocaine as the template molecule. Analyst 2021. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.S.; Lobato, A.; Quinaz, M.B.; Gonçalves, L.M. Electropolymerized Molecularly Imprinted Polymers in Sensing Applications. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Cheng, J.; Li, Y.; Zhong, J.; Lu, Z.; Wang, G.; Sun, M.; Jiang, Y.; Zou, P.; Wang, X.; Zhao, Q.; et al. Molecularly imprinted electrochemical sensor based on biomass carbon decorated with MOF-derived Cr2O3 and silver nanoparticles for selective and sensitive detection of nitrofurazone. Chem. Eng. J. 2020, 398, 125664. [Google Scholar] [CrossRef]
- Lima, C.D.; Couto, R.A.S.; Arantes, L.C.; Marinho, P.A.; Pimentel, D.M.; Quinaz, M.B.; da Silva, R.A.B.; Richter, E.M.; Barbosa, S.L.; dos Santos, W.T.P. Electrochemical detection of the synthetic cathinone 3,4-methylenedioxypyrovalerone using carbon screen-printed electrodes: A fast, simple and sensitive screening method for forensic samples. Electrochim. Acta 2020, 354, 136728. [Google Scholar] [CrossRef]
- Alexandridou, A.; Mouskeftara, T.; Raikos, N.; Gika, H.G. GC-MS analysis of underivatised new psychoactive substances in whole blood and urine. J. Chromatogr. B 2020, 1156, 122308. [Google Scholar] [CrossRef]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M.; Benavente, F. Enantiodetermination of R,S-3,4-methylenedioxypyrovalerone in urine samples by high pressure in-line solid-phase extraction capillary electrophoresis-mass spectrometry. Talanta 2021, 225, 121994. [Google Scholar] [CrossRef]
- Araújo, A.M.; Bastos, M.L.; Carvalho, F.; Pinho, P.G.; Carvalho, M. Effect of temperature on 3,4-methylenedioxypyrovalerone (MDPV)-induced metabolome disruption in primary mouse hepatic cells. Toxicology 2020, 441, 152503. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A. In Progress in Chemometrics Research; Pomerantsev, A.L., Ed.; Nova Science Publishers: New York, NY, USA, 2005. [Google Scholar]
- Cezar, H.M.; Canuto, S.; Coutinho, K. DICE: A Monte Carlo code for molecular simulation including the configurational bias Monte Carlo method. J. Chem. Inf. Model. 2020, 60, 3472–3488. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl. Acad. Sci. 2005, 102, 6665–6670. [Google Scholar] [CrossRef] [Green Version]
- Dodda, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodda, L.S.; Vilseck, J.Z.; Tirado-Rives, J.; Jorgensen, W.L. 1.14*CM1A-LBCC: Localized bond-charge corrected CM1A charges for condensed-phase simulations. J. Phys. Chem. B 2017, 121, 3864–3870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01 2009; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for sll spd-block elements ( Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory, 1st ed.; Clarendon Press: Oxford, UK, 1994; ISBN 9780198558651. [Google Scholar]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Valeyev, E. Libint—A Library for the Evaluation of Molecular Integrals of Many-Body Operators over Gaussian Functions. Available online: libint.valeyev.net/ (accessed on 1 December 2020).
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved marching tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Mounssef, B.; Carvalho, F.; Rodrigues, C.M.P.; Braga, A.A.C.; Aldous, L.; Gonçalves, L.M.; Quinaz, M.B. Methylone screening with electropolymerized molecularly imprinted polymer on screen-printed electrodes. Sens. Actuators B Chem. 2020, 316, 128133. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.P.; Delerue-Matos, C.; Braga, A.A.C.; Moreira Gonçalves, L.; et al. Electrochemical sensing of ecstasy with electropolymerized molecularly imprinted poly(o-phenylenediamine) polymer on the surface of disposable screen-printed carbon electrodes. Sens. Actuators B Chem. 2019, 290. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Rebelo, P.; Cagide, F.; Gonçalves, L.M.; Borges, F.; Rodrigues, J.A.; Delerue-Matos, C. Electrochemical sensing of the thyroid hormone thyronamine (T0AM) via molecular imprinted polymers (MIPs). Talanta 2019, 194. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, H.; Wang, Z.; Wu, G.; Lu, X. A novel amperometric sensor for salicylic acid based on molecularly imprinted polymer-modified electrodes. Polym. Plast. Technol. Eng. 2009, 48, 639–645. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zhao, F.; Zhang, W.; Ye, Z. Molecularly imprinted polymer-based sensors for atrazine detection by electropolymerization of o-phenylenediamine. RSC Adv. 2015, 5, 56534–56540. [Google Scholar] [CrossRef]
- Zhang, H. Molecularly imprinted sensor based on o-phenylenediamine for electrochemical detection of sulfamethoxazole. Int. J. Electrochem. Sci. 2019, 11630–11640. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Zhang, X.; Shang, P.; Ding, S.; Zha, W.; Xu, W. Electrochemical determination of thrombin with molecularly imprinted polymers and multiwalled carbon nanotubes. Can. J. Chem. 2017, 95, 799–805. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Chen, H.; Chen, Y.; Huang, G. Disposable electrochemical ascorbic acid sensor based on molecularly imprinted poly(o-phenylenediamine)-modified dual channel screen-printed electrode for orange juice analysis. Food Anal. Methods 2014, 7, 1557–1563. [Google Scholar] [CrossRef]
- Beluomini, M.A.; Karimian, N.; Stradiotto, N.R.; Ugo, P. Tailor-made 3D-nanoelectrode ensembles modified with molecularly imprinted poly(o-phenylenediamine) for the sensitive detection of L-arabitol. Sens. Actuators B Chem. 2019, 284, 250–257. [Google Scholar] [CrossRef]
- Karimian, N.; Stortini, A.M.; Moretto, L.M.; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for trace analysis of perfluorooctanesulfonate in water based on a molecularly imprinted poly(o-phenylenediamine) polymer. ACS Sens. 2018, 3, 1291–1298. [Google Scholar] [CrossRef]
- Ramos, R.M.; Pacheco, J.G.; Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A.; Barros, A.A. Determination of free and total diacetyl in wine by HPLC-UV using gas-diffusion microextraction and pre-column derivatization. Food Control 2012, 24. [Google Scholar] [CrossRef]
- Montenegro, P.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A.; Barros, A.A. Single determination of α-ketoglutaric acid and pyruvic acid in beer by HPLC with UV detection. Anal. Methods 2011, 3. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A.; Barros, A.A. Gas-diffusion microextraction. J. Sep. Sci. 2010, 33, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Losito, I.; Palmisano, F.; Zambonin, P.G. o-phenylenediamine electropolymerization by cyclic voltammetry combined with electrospray ionization-ion trap mass spectrometry. Anal. Chem. 2003, 75, 4988–4995. [Google Scholar] [CrossRef]
- Ramos, R.M.; Gonçalves, L.M.; Vyskočil, V.; Rodrigues, J.A. Voltammetric determination of trace amounts of diacetyl at a mercury meniscus modified silver solid amalgam electrode following gas-diffusion microextraction. Talanta 2017, 169. [Google Scholar] [CrossRef] [PubMed]
- Baibarac, M.; Baltog, I.; Scocioreanu, M.; Ballesteros, B.; Mevellec, J.Y.; Lefrant, S. One-dimensional composites based on single walled carbon nanotubes and poly(o-phenylenediamine). Synth. Met. 2011, 161, 2344–2354. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Khaliel, A.B.; Aboud, A.A.; Mohamed, S.M. Chemical polymerization kinetics of poly-o-phenylenediamine and characterization of the obtained polymer in squeous hydrochloric acid solution using K2Cr2O7 as oxidizing agent. Int. J. Polym. Sci. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bilal, S.; Holze, R. Electrochemical copolymerization of o-toluidine and o-phenylenediamine. J. Electroanal. Chem. 2006, 592, 1–13. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, X.; Zhao, D. Computer-aided design of molecularly imprinted polymers for simultaneous detection of clenbuterol and its metabolites. Polymers 2018, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Gu, L.; Zhang, M.; Li, Z.; Yang, W.; Tang, X.; Xie, G. Computer-aided design of molecularly imprinted polymers for recognition of atrazine. Comput. Theor. Chem. 2017, 1121, 29–34. [Google Scholar] [CrossRef]
- Sobiech, M.; Żołek, T.; Luliński, P.; Maciejewska, D. A computational exploration of imprinted polymer affinity based on voriconazole metabolites. Analyst 2014, 139, 1779. [Google Scholar] [CrossRef]
- Bagdžiūnas, G. Theoretical design of molecularly imprinted polymers based on polyaniline and polypyrrole for detection of tryptophan. Mol. Syst. Des. Eng. 2020. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, S.; Seibert, J.; Ostermeir, K.; Hansen, A.; Göller, A.H.; Grimme, S. Quantum chemical calculation of molecular and periodic peptide and protein structures. J. Phys. Chem. B 2020, 124, 3636–3646. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Pal, S. Quantum mechanical studies on dioxin-imprinted polymer precursor composites: Fundamental insights to enhance the binding strength and selectivity of biomarkers. J. Mol. Recognit. 2018, 31, e2736. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Becke, A.D. A multicenter numerical integration scheme for polyatomic molecules. J. Chem. Phys. 1988, 88, 2547–2553. [Google Scholar] [CrossRef]
- Baumann, M.H.; Bukhari, M.O.; Lehner, K.R.; Anizan, S.; Rice, K.C.; Concheiro, M.; Huestis, M.A. Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. In Neuropharmacology of New Psychoactive Substances (NPS); Baumann, M.H., Glennon, R.A., Wiley, J.L., Eds.; Springer: New York, NY, USA, 2016; pp. 93–117. ISBN 1866-3370. [Google Scholar]
- Terracina, J.J.; Sharfstein, S.T.; Bergkvist, M. In silico characterization of enantioselective molecularly imprinted binding sites. J. Mol. Recognit. 2018, 31, e2612. [Google Scholar] [CrossRef]
- Woźnica, M.; Sobiech, M.; Pałka, N.; Luliński, P. Monitoring the role of enantiomers in the surface modification and adsorption process of polymers imprinted by chiral molecules: Theory and practice. J. Mater. Sci. 2020, 55, 10626–10642. [Google Scholar] [CrossRef]





| Technique | Extraction | LOD/ng mL−1 | LOQ/ng mL−1 | Matrix | Reference |
|---|---|---|---|---|---|
| SWV | none | 5 × 102 | 2 × 103 | buffer | this work |
| AdSDPV | none | 2 × 102 | 5 × 102 | seized samples | [60] |
| LC-MS | LLE | 2 | 10 | seized samples | [25] |
| LC-MS | SPE | 2 × 10−4 | 1 × 10−3 | wastewater | [27] |
| LC-MS | SPE | 1 | 5 | rat brain tissue | [29] |
| LC-MS | SALLE | 2 | 4 | urine | [31] |
| LC-MS | PHPP | 0.1 | 0.25 | plasma | [32] |
| LC-MS | LLE | 2 × 10−3 | 5 × 10−3 | equine plasma | [34] |
| LC-MS | SPE | - | 5 × 10−3 | wastewater | [35] |
| LC-MS | LLE | 0.5 | 5 | blood | [36] |
| LC-MS | SPE | 3 × 10−2 | 0.5 | saliva | [37] |
| GC-MS | LLE | 7 | 2 × 101 | blood, urine | [61] |
| GC-MS | LLE and derivatization | - | 2 × 101 | urine | [39] |
| GC-MS | SPE and derivatization | 2 × 101 | 5 × 101 | urine | [42] |
| GC-MS, LC-MS | SPE | 2 × 103 | - | hair, kidney, liver, bile | [44] |
| IMS | LLME | 2 × 101 | 7 × 101 | oral and nasal fluid | [45] |
| CE-UV | PLE-SPE | 1 × 105 | 4 × 105 | hair | [46] |
| CE-MS | SPE | 1 × 101 | 3 × 101 | urine | [62] |
| IM-MS | - | 1 × 104 | - | standards | [49] |
| immunoassay | none | 0.2 | - | urine | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, R.A.S.; Coelho, C.; Mounssef, B., Jr.; Morais, S.F.d.A.; Lima, C.D.; dos Santos, W.T.P.; Carvalho, F.; Rodrigues, C.M.P.; Braga, A.A.C.; Gonçalves, L.M.; et al. 3,4-Methylenedioxypyrovalerone (MDPV) Sensing Based on Electropolymerized Molecularly Imprinted Polymers on Silver Nanoparticles and Carboxylated Multi-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 353. https://doi.org/10.3390/nano11020353
Couto RAS, Coelho C, Mounssef B Jr., Morais SFdA, Lima CD, dos Santos WTP, Carvalho F, Rodrigues CMP, Braga AAC, Gonçalves LM, et al. 3,4-Methylenedioxypyrovalerone (MDPV) Sensing Based on Electropolymerized Molecularly Imprinted Polymers on Silver Nanoparticles and Carboxylated Multi-Walled Carbon Nanotubes. Nanomaterials. 2021; 11(2):353. https://doi.org/10.3390/nano11020353
Chicago/Turabian StyleCouto, Rosa A. S., Constantino Coelho, Bassim Mounssef, Jr., Sara F. de A. Morais, Camila D. Lima, Wallans T. P. dos Santos, Félix Carvalho, Cecília M. P. Rodrigues, Ataualpa A. C. Braga, Luís Moreira Gonçalves, and et al. 2021. "3,4-Methylenedioxypyrovalerone (MDPV) Sensing Based on Electropolymerized Molecularly Imprinted Polymers on Silver Nanoparticles and Carboxylated Multi-Walled Carbon Nanotubes" Nanomaterials 11, no. 2: 353. https://doi.org/10.3390/nano11020353
APA StyleCouto, R. A. S., Coelho, C., Mounssef, B., Jr., Morais, S. F. d. A., Lima, C. D., dos Santos, W. T. P., Carvalho, F., Rodrigues, C. M. P., Braga, A. A. C., Gonçalves, L. M., & Quinaz, M. B. (2021). 3,4-Methylenedioxypyrovalerone (MDPV) Sensing Based on Electropolymerized Molecularly Imprinted Polymers on Silver Nanoparticles and Carboxylated Multi-Walled Carbon Nanotubes. Nanomaterials, 11(2), 353. https://doi.org/10.3390/nano11020353









