Rational Design of an Ion-Imprinted Polymer for Aqueous Methylmercury Sorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
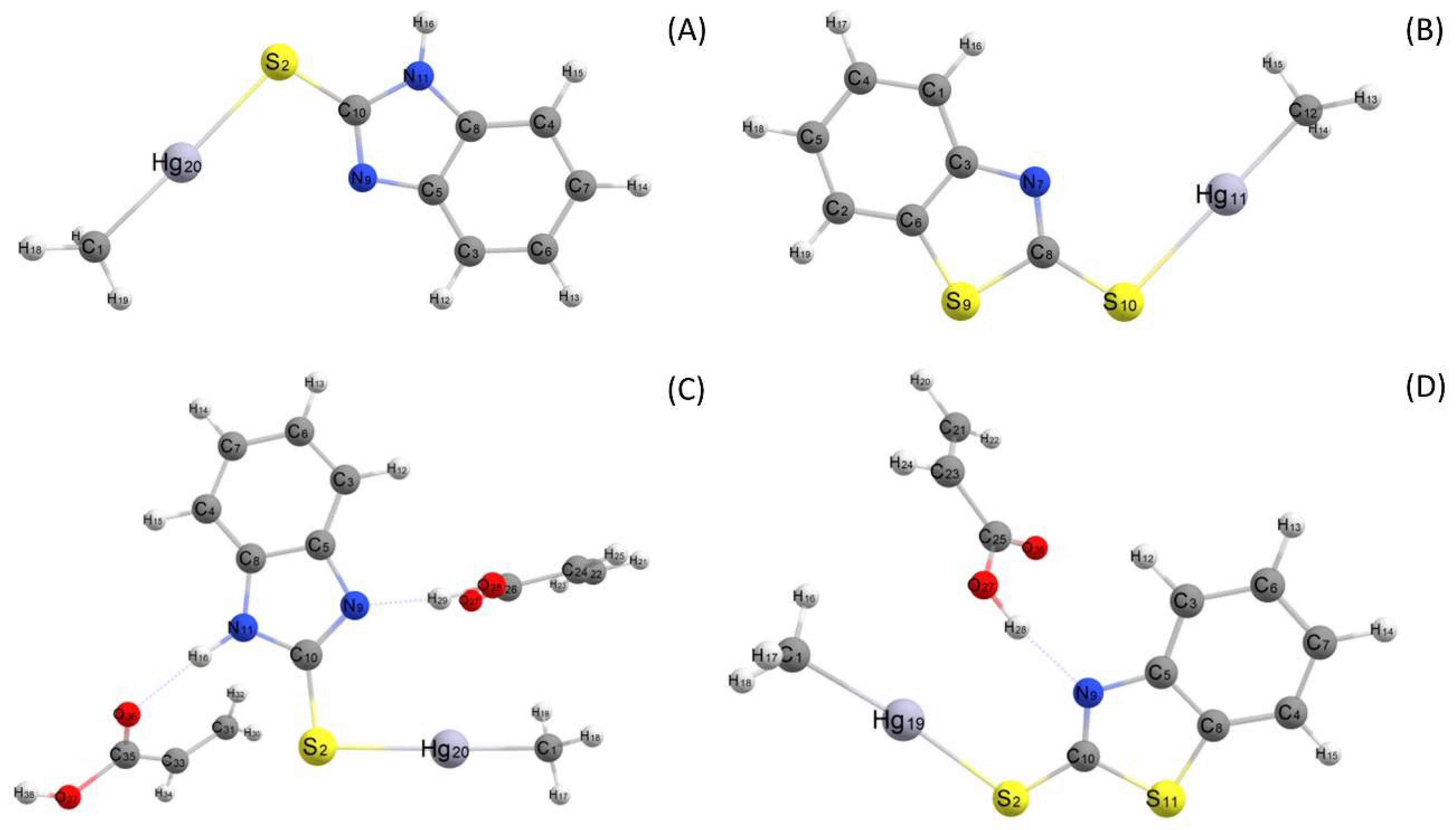
2.2. Density Functional Method
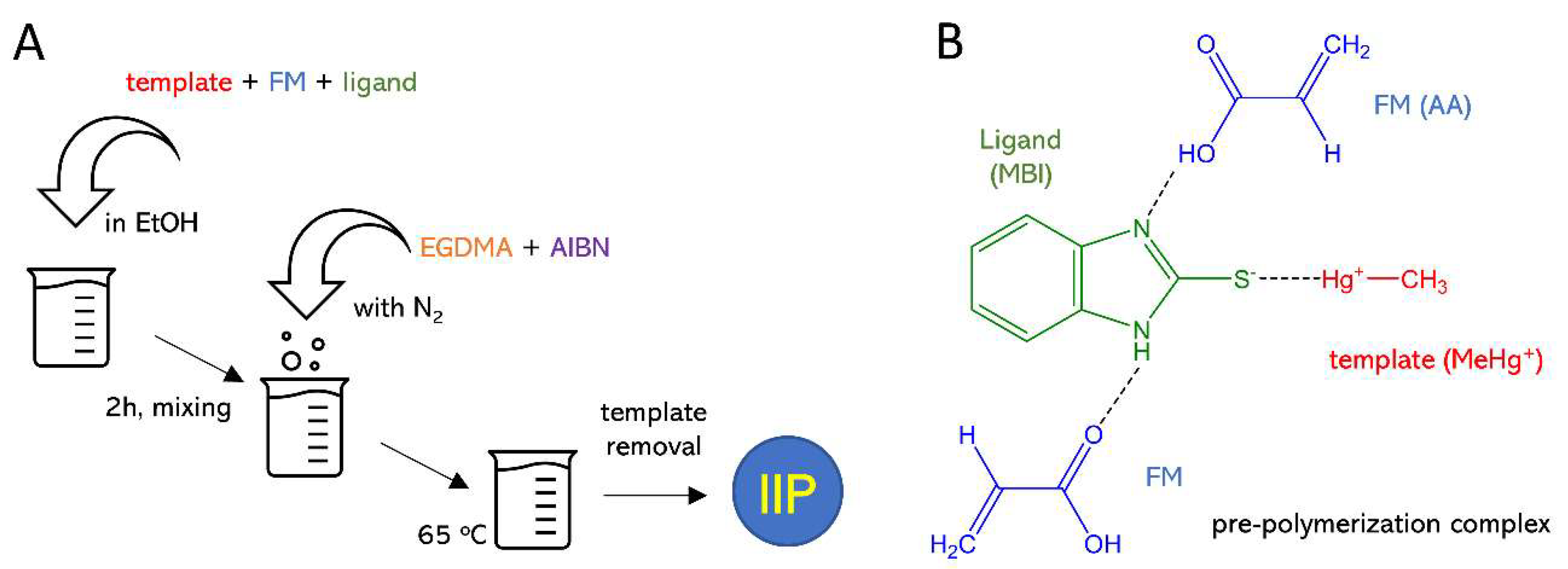
2.3. Ion-Imprinted Polymer (IIP) Synthesis
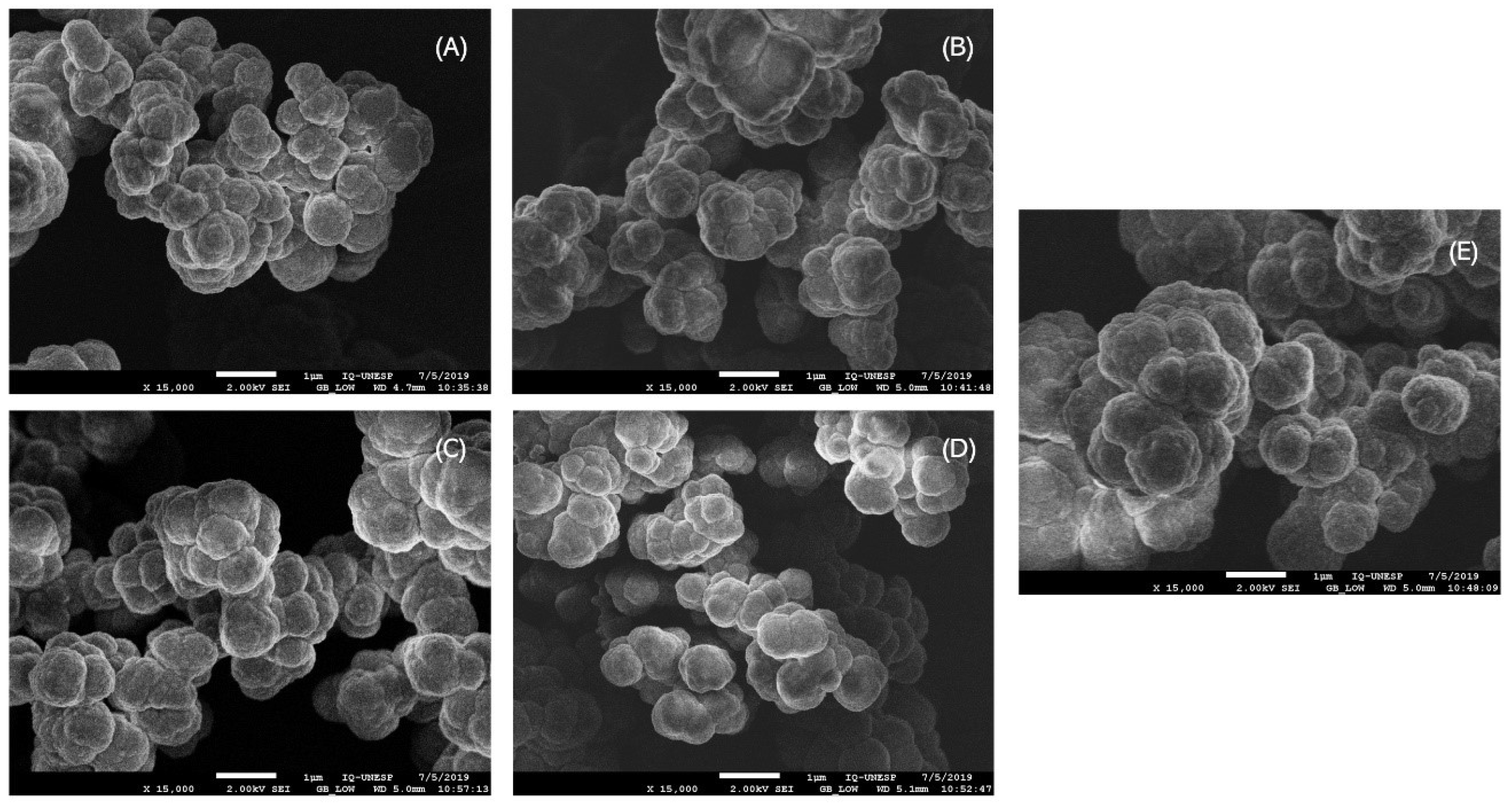
2.4. Polymer Characterization
2.5. Sorption Studies
2.6. Selectivity Studies and Water Analysis
3. Results and Discussion
3.1. Theoretical Selection of the Functional Monomer and Solvent
3.2. Characterization of the Synthesized Polymers
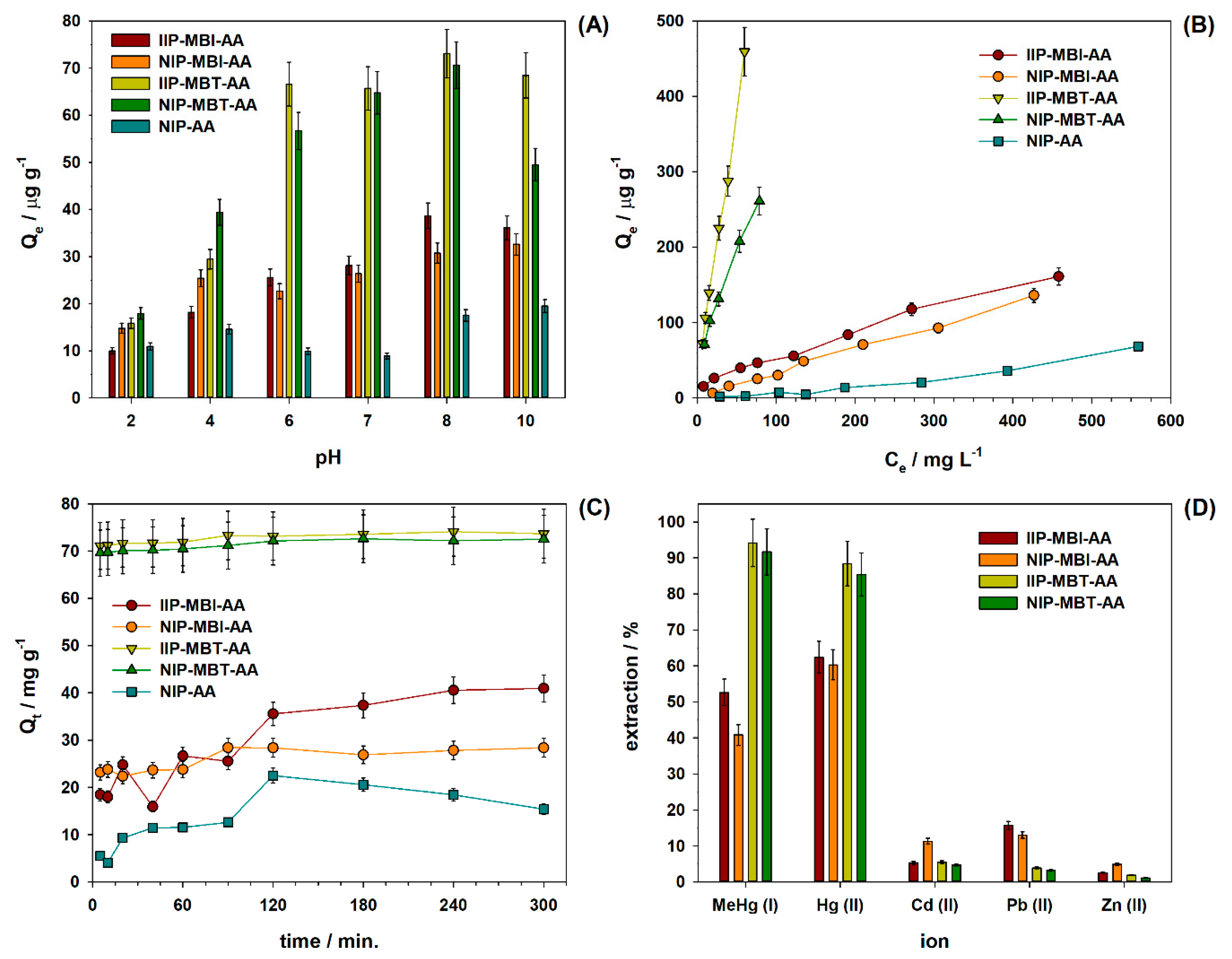
3.3. Adsorption Studies
3.4. Application in Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiener, J.; Krabbenhoft, D.; Heinz, G.; Scheuhammer, A. Ecotoxicology of mercury. In Handbook of Ecotoxicology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Hong, Y.-S.; Kim, Y.-M.; Lee, K.-E. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Steuerwald, U.; Weihe, P.; Jørgensen, P.J.; Bjerve, K.; Brock, J.; Heinzow, B.; Budtz-Jørgensen, E.; Grandjean, P. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J. Pediatr. 2000, 136, 599–605. [Google Scholar] [CrossRef]
- Chen, C.Y.; Serrell, N.; Evers, D.C.; Fleishman, B.J.; Lambert, K.F.; Weiss, J.; Mason, R.P.; Bank, M.S. Meeting report: Methylmercury in marine ecosystems—From sources to seafood consumers. Environ. Health Perspect. 2008, 116, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, J.R.D.; Betancourt, O.; Miranda, M.R.; Barriga, R.; Cueva, E.; Betancourt, S. Long-range effect of cyanide on mercury methylation in a gold mining area in southern Ecuador. Sci. Total Environ. 2011, 409, 5026–5033. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Total and methyl mercury distribution in water, sediment, plankton and fish along the Tapajós River basin in the Brazilian Amazon. Chemosphere 2019, 235, 690–700. [Google Scholar] [CrossRef]
- Vacchina, V.; Séby, F.; Chekri, R.; Verdeil, J.; Dumont, J.; Hulin, M.; Sirot, V.; Volatier, J.-L.; Serreau, R.; Rousseau, A.; et al. Optimization and validation of the methods for the total mercury and methylmercury determination in breast milk. Talanta 2017, 167, 404–410. [Google Scholar] [CrossRef]
- Covaci, E.; Senila, M.; Ponta, M.; Darvasi, E.; Petreus, D.; Frentiu, M.; Frentiu, T. Methylmercury determination in seafood by photochemical vapor generation capacitively coupled plasma microtorch optical emission spectrometry. Talanta 2017, 170, 464–472. [Google Scholar] [CrossRef]
- Qin, C.; Chen, M.; Yan, H.; Shang, L.; Yao, H.; Li, P.; Feng, X. Compound specific stable isotope determination of methylmercury in contaminated soil. Sci. Total Environ. 2018, 644, 406–412. [Google Scholar] [CrossRef]
- de Paiva, E.L.; Milani, R.F.; Boer, B.S.; Quintaes, K.D.; Morgano, M.A. Methylmercury in fish species used in preparing sashimi: A case study in Brazil. Food Control 2017, 80, 104–112. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Gai, L.; Gong, Y.; Guan, H.; He, R.; Lyu, H. Different approaches for preparing a novel thiol-functionalized graphene oxide/Fe-Mn and its application for aqueous methylmercury removal. Chem. Eng. J. 2017, 319, 229–239. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef]
- Fu, X.-C.; Wu, J.; Nie, L.; Xie, C.-G.; Liu, J.-H.; Huang, X.-J. Electropolymerized surface ion imprinting films on a gold nanoparticles/single-wall carbon nanotube nanohybrids modified glassy carbon electrode for electrochemical detection of trace mercury(II) in water. Anal. Chim. Acta 2012, 720, 29–37. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef]
- Benachio, I.; Lobato, A.; Gonçalves, L.M. Employing molecularly imprinted polymers in the development of electroanalytical methodologies for antibiotic determination. J. Mol. Recognit. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.M. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Zhou, Z.; Kong, D.; Zhu, H.; Wang, N.; Wang, Z.; Wang, Q.; Liu, W.; Li, Q.; Zhang, W.; Ren, Z. Preparation and adsorption characteristics of an ion-imprinted polymer for fast removal of Ni(II) ions from aqueous solution. J. Hazard. Mater. 2018, 341, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Dahaghin, Z.; Mousavi, H.Z.; Sajjadi, S.M. A novel magnetic ion imprinted polymer as a selective magnetic solid phase for separation of trace lead(II) ions from agricultural products, and optimization using a Box–Behnken design. Food Chem. 2017, 237, 275–281. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Novel ion imprinted polymer electrochemical sensor for the selective detection of lead(II). Food Chem. 2020, 303, 125374. [Google Scholar] [CrossRef]
- R. Ganjali, M.; Alizadeh, T.; Larijani, B.; Aghazadeh, M.; Pourbasheer, E.; Norouzi, P. Biomimetic electrochemical sensors based on imprinted polymers for determination of mercury ion. Curr. Anal. Chem. 2016, 13, 62–69. [Google Scholar] [CrossRef]
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. A critical review on the synthesis and application of ion-imprinted polymers for selective preconcentration, speciation, removal and determination of trace and essential metals from different matrices. Crit. Rev. Anal. Chem. 2020, 1–13. [Google Scholar] [CrossRef]
- Branger, C.; Meouche, W.; Margaillan, A. Recent advances on ion-imprinted polymers. React. Funct. Polym. 2013, 73, 859–875. [Google Scholar] [CrossRef]
- Biju, V.; Gladis, J.M.; Rao, T.P. Ion imprinted polymer particles: Synthesis, characterization and dysprosium ion uptake properties suitable for analytical applications. Anal. Chim. Acta 2003, 478, 43–51. [Google Scholar] [CrossRef]
- Jinadasa, K.K.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. New adsorbents based on imprinted polymers and composite nanomaterials for arsenic and mercury screening/speciation: A review. Microchem. J. 2020, 156, 104886. [Google Scholar] [CrossRef]
- Khodadadian, M.; Ahmadi, F. Computer-assisted design and synthesis of molecularly imprinted polymers for selective extraction of acetazolamide from human plasma prior to its voltammetric determination. Talanta 2010, 81, 1446–1453. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, Z.; Cheng, X.; Wu, W.; Wu, Y.; Xu, L.; Fu, F. A novel ion imprinted SiO 2 microsphere for the specific and rapid extraction and pre-concentration of ultra-trace methyl mercury. RSC Adv. 2016, 6, 40100–40105. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, X.; Yu, X.; Wu, W.; Xu, L.; Fu, F. Ion-imprinted magnetic nanoparticles for specific separation and concentration of ultra-trace methyl mercury from aqueous sample. J. Chromatogr. A 2017, 1496, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, T.; Dakova, I.; Balashev, K.; Karadjova, I. Polymeric ion-imprinted nanoparticles for mercury speciation in surface waters. Microchem. J. 2014, 113, 42–47. [Google Scholar] [CrossRef]
- Rodríguez-Reino, M.P.; Rodríguez-Fernández, R.; Peña-Vázquez, E.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Mercury speciation in seawater by liquid chromatography-inductively coupled plasma-mass spectrometry following solid phase extraction pre-concentration by using an ionic imprinted polymer based on methyl-mercury–phenobarbital interaction. J. Chromatogr. A 2015, 1391, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fernández, R.; Peña-Vázquez, E.; Bermejo-Barrera, P. Synthesis of an imprinted polymer for the determination of methylmercury in marine products. Talanta 2015, 144, 636–641. [Google Scholar] [CrossRef]
- Jinadasa, K.K.; Herbello-Hermelo, P.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Mercury speciation in edible seaweed by liquid chromatography—Inductively coupled plasma mass spectrometry after ionic imprinted polymer-solid phase extraction. Talanta 2020, 121841. [Google Scholar] [CrossRef]
- De Barros, L.A.; Pereira, L.A.; Custódio, R.; Rath, S. A novel computational approach for development of highly selective fenitrothion imprinted polymer: Theoretical predictions and experimental validations. J. Braz. Chem. Soc. 2014, 25, 619–628. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.P.; Delerue-Matos, C.; Braga, A.A.C.; Gonçalves, L.M.; et al. Electrochemical sensing of ecstasy with electropolymerized molecularly imprinted poly(o-phenylenediamine) polymer on the surface of disposable screen-printed carbon electrodes. Sens. Actuators B Chem. 2019, 290, 378–386. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Mounssef, B.; Carvalho, F.; Rodrigues, C.M.P.; Braga, A.A.C.; Aldous, L.; Gonçalves, L.M.; Quinaz, M.B. Methylone screening with electropolymerized molecularly imprinted polymer on screen-printed electrodes. Sens. Actuators B Chem. 2020, 316, 128133. [Google Scholar] [CrossRef]
- Khan, S.; Hussain, S.; Wong, A.; Foguel, M.V.; Gonçalves, L.M.; Pividori Gurgo, M.I.; Taboada Sotomayor, M.d.P. Synthesis and characterization of magnetic-molecularly imprinted polymers for the HPLC-UV analysis of ametryn. React. Funct. Polym. 2018, 122, 175–182. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc: Wallingford, CT, USA, 2009. [Google Scholar]
- Singh, I.; El-Emam, A.A.; Pathak, S.K.; Srivastava, R.; Shukla, V.K.; Prasad, O.; Sinha, L. Experimental and theoretical DFT (B3LYP, X3LYP, CAM-B3LYP and M06-2X) study on electronic structure, spectral features, hydrogen bonding and solvent effects of 4-methylthiadiazole-5-carboxylic acid. Mol. Simul. 2019, 45, 1029–1043. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Rabenstein, D.L. The aqueous solution chemistry of methylmercury and its complexes. Acc. Chem. Res. 1978, 11, 100–107. [Google Scholar] [CrossRef]
- Bravo, J.; Casas, J.S.; Castano, M.V.; Gayoso, M.; Mascarenhas, Y.P.; Sanchez, A.; Santos, C.d.O.P.; Sordo, J. Methyl- and phenylmercury(II) derivatives of 2-mercaptobenzothiazole. Crystal structure of (2-benzothiazolylthio)methylmercury(II). Inorg. Chem. 1985, 24, 3435–3438. [Google Scholar] [CrossRef]
- Fu, X.-C.; Chen, X.; Guo, Z.; Xie, C.-G.; Kong, L.-T.; Liu, J.-H.; Huang, X.-J. Stripping voltammetric detection of mercury(II) based on a surface ion imprinting strategy in electropolymerized microporous poly(2-mercaptobenzothiazole) films modified glassy carbon electrode. Anal. Chim. Acta 2011, 685, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Doneux, T.; Buess-Herman, C.; Lipkowski, J. Electrochemical and FTIR characterization of the self-assembled monolayer of 2-mercaptobenzimidazole on Au(111). J. Electroanal. Chem. 2004, 564, 65–75. [Google Scholar] [CrossRef]
- Bigotto, A. Vibrational spectra of benzimidazol-2-thione. Spectrosc. Lett. 1991, 24, 69–80. [Google Scholar] [CrossRef]
- Luliński, P.; Kalny, P.; Giebułtowicz, J.; Maciejewska, D.; Wroczyński, P. Synthesis and characterization of cadmium(II)-imprinted poly(1-allyl-2-thiourea-co-ethylene glycol dimethacrylate) particles for selective separation. Polym. Bull. 2014, 71, 1727–1741. [Google Scholar] [CrossRef]
- Woźnica, M.; Luliński, P. Design of selective molecularly imprinted sorbent for the optimized solid-phase extraction of S-pramipexole from the model multicomponent sample of human urine. J. Sep. Sci. 2019, 42, 1412–1422. [Google Scholar] [CrossRef]
- Marć, M.; Wieczorek, P.P. The preparation and evaluation of core-shell magnetic dummy-template molecularly imprinted polymers for preliminary recognition of the low-mass polybrominated diphenyl ethers from aqueous solutions. Sci. Total Environ. 2020, 724, 138151. [Google Scholar] [CrossRef]
- Magalhães, P.J.; Vieira, J.S.; Gonçalves, L.M.; Pacheco, J.G.; Guido, L.F.; Barros, A.A. Isolation of phenolic compounds from hop extracts using polyvinylpolypyrrolidone: Characterization by high-performance liquid chromatography–diode array detection–electrospray tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3258–3268. [Google Scholar] [CrossRef]
- Khairi, N.; Yusof, N.; Abdullah, A.; Mohammad, F. Removal of toxic mercury from petroleum oil by newly synthesized molecularly-imprinted polymer. Int. J. Mol. Sci. 2015, 16, 10562–10577. [Google Scholar] [CrossRef]
- Adauto, A.; Khan, S.; Augusto da Silva, M.; Gomes Neto, J.A.; Picasso, G.; Sotomayor, M.d.P.T. Synthesis, characterization and application of a novel ion hybrid imprinted polymer to adsorb Cd(II) in different samples. Environ. Res. 2020, 187, 109669. [Google Scholar] [CrossRef]
- Luna Quinto, M.; Khan, S.; Picasso, G.; Taboada Sotomayor, M.D.P. Synthesis, characterization, and evaluation of a selective molecularly imprinted polymer for quantification of the textile dye acid violet 19 in real water samples. J. Hazard. Mater. 2020, 384, 121374. [Google Scholar] [CrossRef]
| Complex | Binding Energy/kcal mol−1 | |||||
|---|---|---|---|---|---|---|
| Water | EtOH | DMSO | DMF | Acetonitrile | Vacuum | |
| MeHg+–MBI | −10.5 | −15.4 | −12.2 | −13.1 | −13.3 | −168.5 |
| MeHg+–MBT | −7.8 | −12.0 | −9.2 | −10.0 | −10.2 | −161.5 |
| Material | BET Surface Area/m2 g−1 | Pore Size/nm |
|---|---|---|
| IIP–MBI–AA | 11 | 9.5 |
| NIP–MBI–AA | 6.8 | 21 |
| IIP–MBT–AA | 5.3 | 17 |
| NIP–MBT–AA | 5.5 | 11 |
| NIP–AA | 3.0 | 12 |
| Material | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm/μg g−1 | b/L μg−1 | R2 | Kf/μg g−1 | n−1/L μg−1 | R2 | |
| IIP–MBI–AA | 217.4 | 0.004 | 0.76 | 4.281 | 0.572 | 0.98 |
| IIP–MBT–AA | 1250 | 0.009 | 0.84 | 13.02 | 0.866 | 0.99 |
| Pseudo-First-Order | ||||
| Material | Qexp/μg g−1 | Qthe/μg g−1 | K1/min−1 × 10−3 | R2 |
| IIP–MBI–AA | 40.93 | 33.23 | 15.43 | 0.886 |
| NIP–MBI–AA | 28.42 | 8.72 | 15.89 | 0.816 |
| NIP–AA | 22.52 | 14.54 | 4.606 | 0.582 |
| IIP–MBT–AA | 74.13 | 2.75 | 7.369 | 0.853 |
| NIP–MBT–AA | 72.62 | 3.40 | 11.28 | 0.916 |
| Pseudo-Second-Order | ||||
| Material | Qexp/μg g−1 | Qthe/μg g−1 | K2/mg g−1 min−1 × 10−3 | R2 |
| IIP–MBI–AA | 40.93 | 44.44 | 0.678 | 0.958 |
| NIP–MBI–AA | 28.42 | 28.57 | 7.239 | 0.998 |
| NIP–AA | 22.52 | 18.48 | 2.766 | 0.941 |
| IIP–MBT–AA | 74.13 | 74.07 | 15.85 | 0.999 |
| NIP–MBT–AA | 72.62 | 72.46 | 14.21 | 0.999 |
| Sample | MeHg+ Added/µg L−1 | Found in IIP a/µg L−1 | Recovery/% |
|---|---|---|---|
| 30.1 | 25.5 ± 0.4 | 84.8 | |
| river water | 60.4 | 53.7 ± 0.4 | 88.8 |
| 114.5 | 103 ± 5 | 89.6 | |
| 22.4 | 21 ± 2 | 93.7 | |
| tap water | 58.2 | 55 ± 2 | 95.2 |
| 113.5 | 101 ± 2 | 88.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesa, R.L.M.; Villa, J.E.L.; Khan, S.; Peixoto, R.R.A.; Morgano, M.A.; Gonçalves, L.M.; Sotomayor, M.D.P.T.; Picasso, G. Rational Design of an Ion-Imprinted Polymer for Aqueous Methylmercury Sorption. Nanomaterials 2020, 10, 2541. https://doi.org/10.3390/nano10122541
Mesa RLM, Villa JEL, Khan S, Peixoto RRA, Morgano MA, Gonçalves LM, Sotomayor MDPT, Picasso G. Rational Design of an Ion-Imprinted Polymer for Aqueous Methylmercury Sorption. Nanomaterials. 2020; 10(12):2541. https://doi.org/10.3390/nano10122541
Chicago/Turabian StyleMesa, Ruddy L. M., Javier E. L. Villa, Sabir Khan, Rafaella R. Alves Peixoto, Marcelo A. Morgano, Luís Moreira Gonçalves, Maria D. P. T. Sotomayor, and Gino Picasso. 2020. "Rational Design of an Ion-Imprinted Polymer for Aqueous Methylmercury Sorption" Nanomaterials 10, no. 12: 2541. https://doi.org/10.3390/nano10122541
APA StyleMesa, R. L. M., Villa, J. E. L., Khan, S., Peixoto, R. R. A., Morgano, M. A., Gonçalves, L. M., Sotomayor, M. D. P. T., & Picasso, G. (2020). Rational Design of an Ion-Imprinted Polymer for Aqueous Methylmercury Sorption. Nanomaterials, 10(12), 2541. https://doi.org/10.3390/nano10122541









