Bimetallic PdAu Catalysts within Hierarchically Porous Architectures for Aerobic Oxidation of Benzyl Alcohol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hierarchically Porous SAPO-5 Support Materials
2.3. Synthesis of PdAu Bimetallic Nanocatalyst within HP-SAPO-5 Architectures
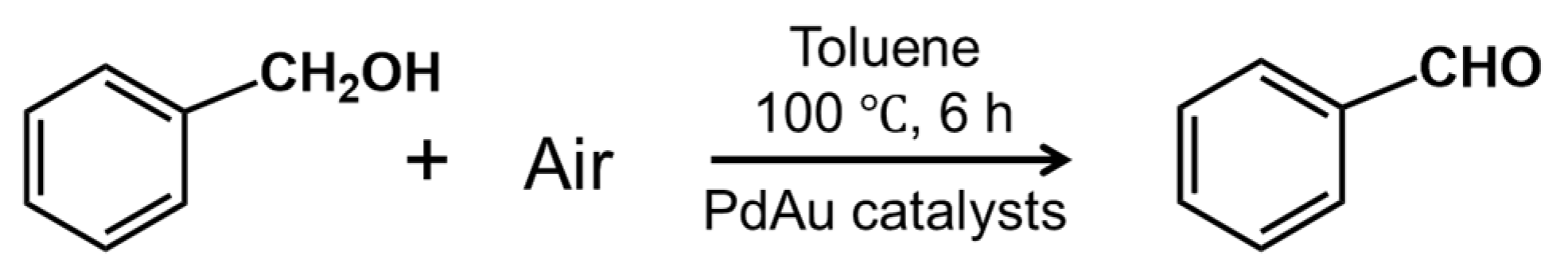
2.4. Catalytic Reaction
2.5. Characterisation
3. Results and Discussion
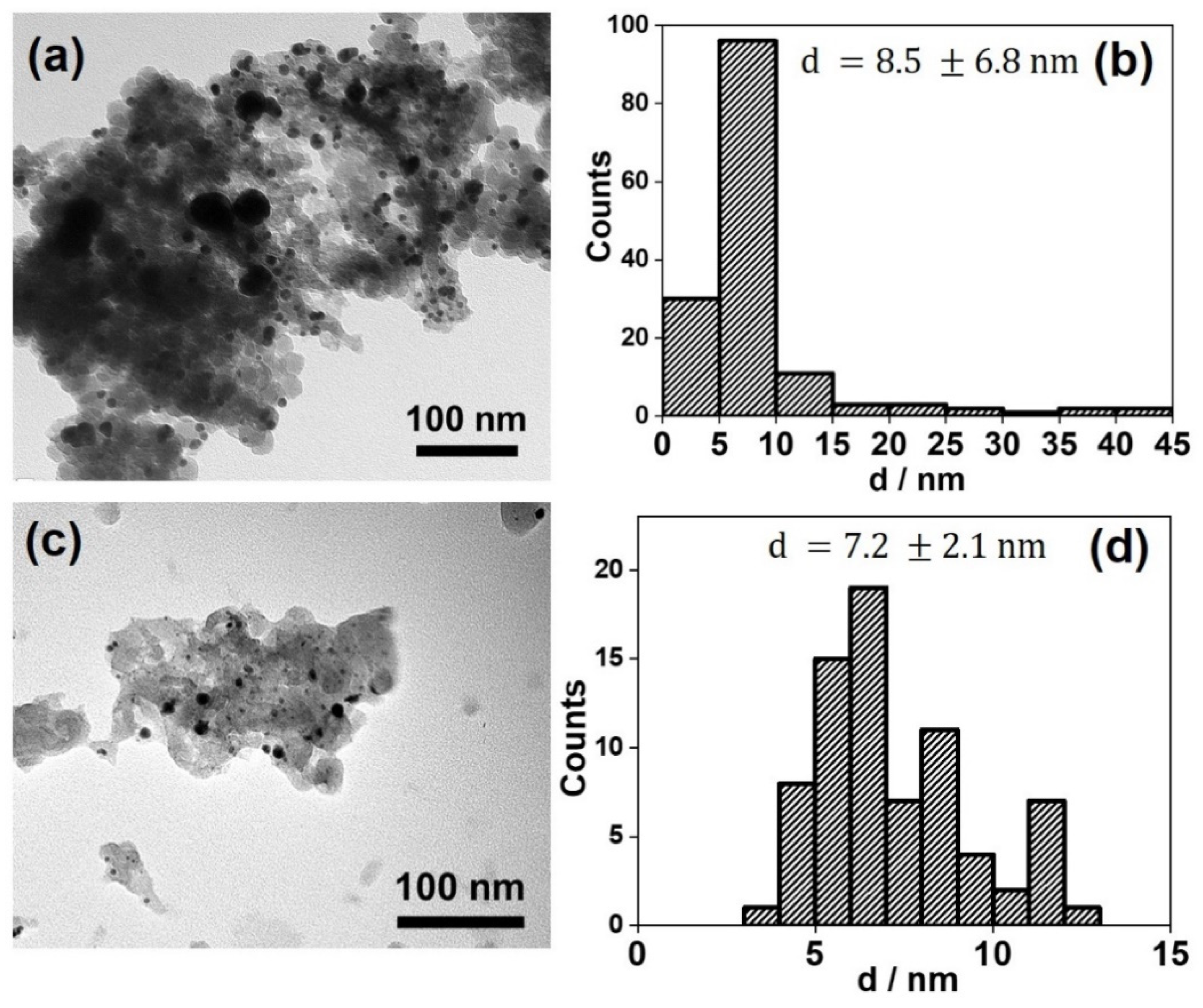
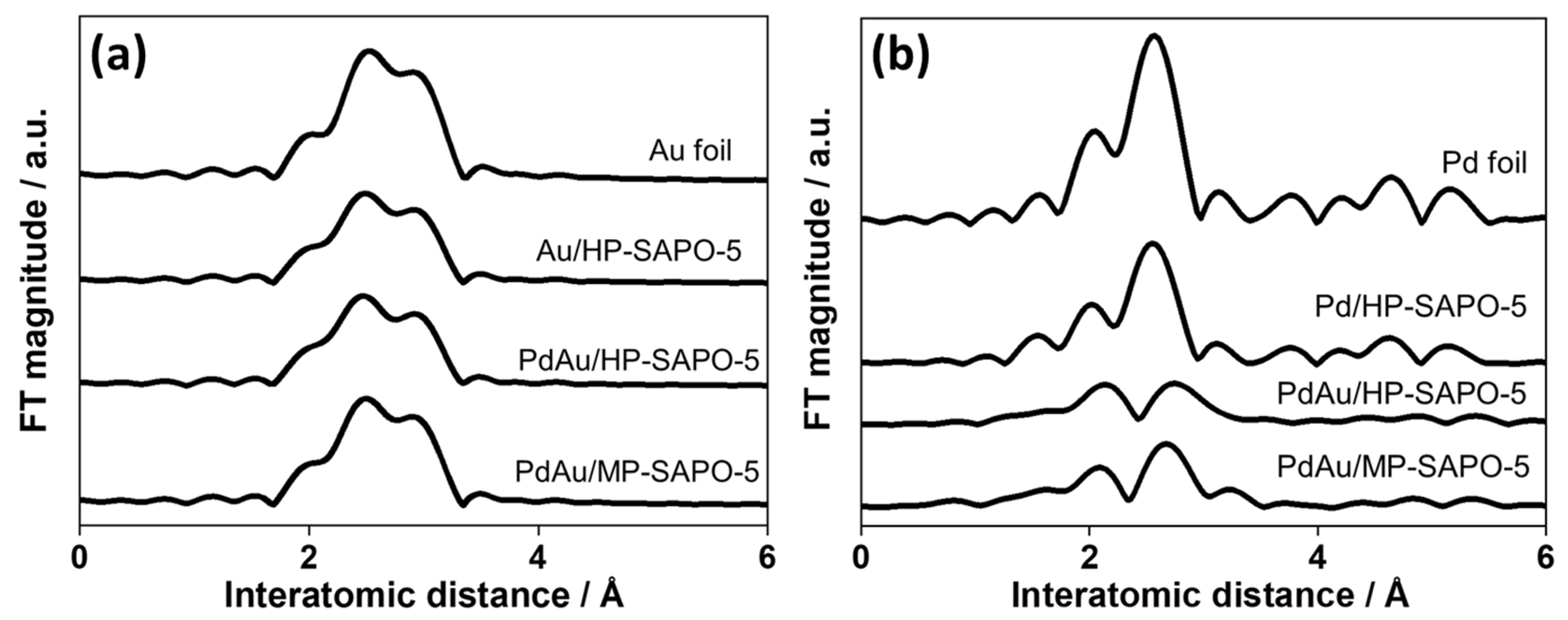
3.1. Structural Characterisation
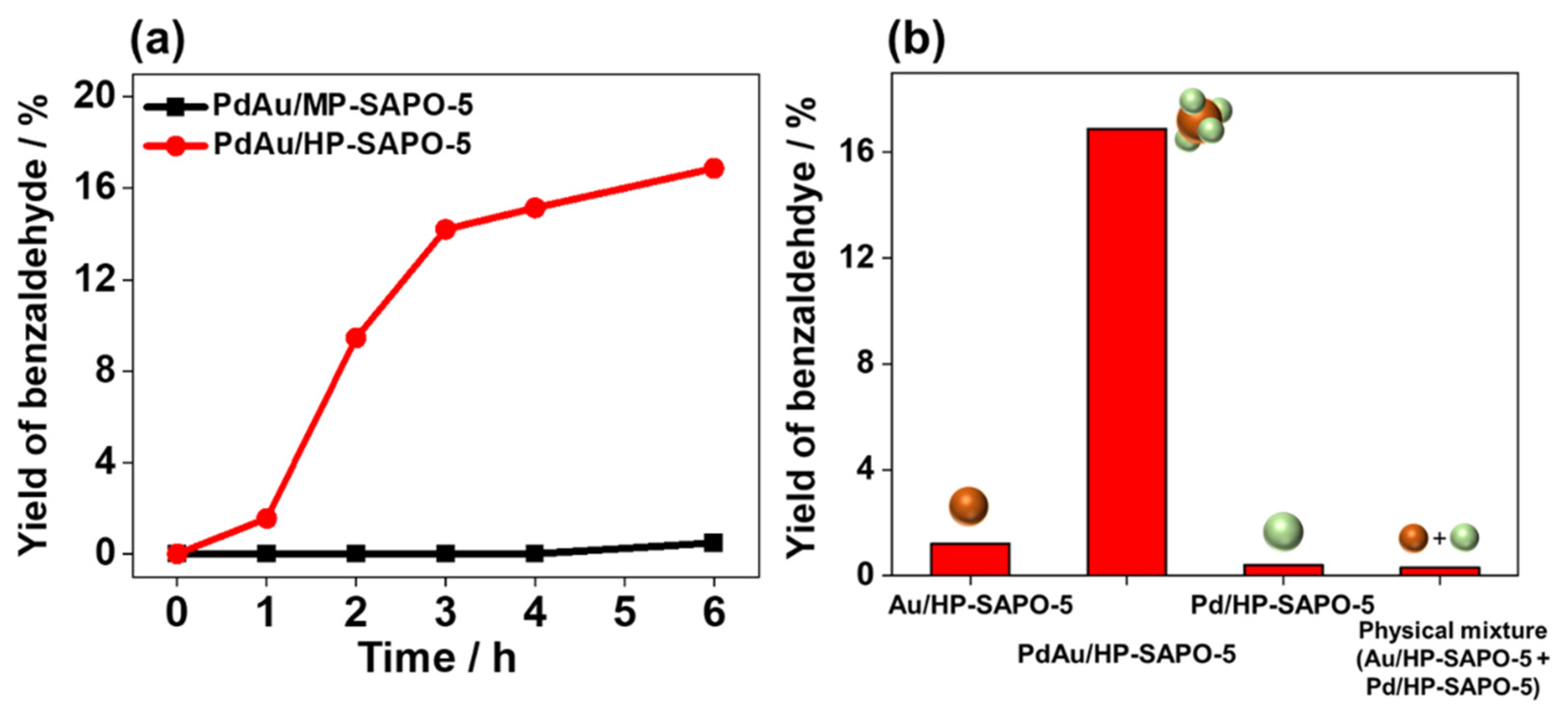
3.2. Catalysis of Benzyl Alcohol Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, A.; Tanihara, Y.; Kuwahara, Y.; Ohmichi, T.; Mori, K.; Nagase, T.; Yasuda, H.Y.; Calers, C.; Louis, C.; Yamashita, H. Skeletal Au prepared from Au-Zr amorphous alloys with controlled atomic compositions and arrangement for active oxidation of benzyl alcohol. J. Mater. Chem. A 2016, 4, 8458–8465. [Google Scholar] [CrossRef]
- Porta, F.; Prati, L. Selective oxidation of glycerol to sodium glycerate with gold-on-carbon catalyst: An insight into reaction selectivity. J. Catal. 2004, 224, 397–403. [Google Scholar] [CrossRef]
- Jo, S.; Verma, P.; Kuwahara, Y.; Mori, K.; Choi, W.; Yamashita, H. Enhanced hydrogen production from ammonia borane using controlled plasmonic performance of Au nanoparticles deposited on TiO2. J. Mater. Chem. A 2017, 5, 21883–21892. [Google Scholar] [CrossRef]
- Verma, P.; Mori, K.; Kuwahara, Y.; Cho, S.J.; Yamashita, H. Synthesis of plasmonic gold nanoparticles supported on morphology-controlled TiO2 for aerobic alcohol oxidation. Catal. Today 2020, 352, 255–261. [Google Scholar] [CrossRef]
- Liu, P.; Guan, Y.; Santen, R.A.V.; Li, C.; Hensen, E.J.M. Aerobic oxidation of alcohols over hydrotalcite-supported gold nanoparticles: The promotional effect of transition metal cations. Chem. Commun. 2011, 47, 11540–11542. [Google Scholar] [CrossRef]
- Siyo, B.; Schneider, M.; Radnik, J.; Pohl, M.M.; Langer, P.; Steinfeldt, N. Influence of support on the aerobic oxidation of HMF into FDCA over preformed Pd nanoparticle based materials. Appl. Catal. A Gen. 2014, 478, 107–116. [Google Scholar] [CrossRef]
- Wang, X.; Kawanami, H.; Islam, N.M.; Chattergee, M.; Yokoyama, T.; Ikushima, Y. Amphiphilic block copolymer-stabilized gold nanoparticles for aerobic oxidation of alcohols in aqueous solution. Chem. Commun. 2008, 4442–4444. [Google Scholar] [CrossRef]
- Della Pina, C.; Falletta, E.; Rossi, M. Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by bimetallic gold-copper catalyst. J. Catal. 2008, 260, 384–386. [Google Scholar] [CrossRef]
- Makwana, V.D.; Son, Y.C.; Howell, A.R.; Suib, S.L. The role of lattice oxygen in selective benzyl alcohol oxidation using OMS-2 catalyst: A kinetic and isotope-labeling study. J. Catal. 2002, 210, 46–52. [Google Scholar] [CrossRef]
- Li, C.J.; Xu, G.R.; Zhang, B.; Gong, J.R. High selectivity in visible-light-driven partial photocatalytic oxidation of benzyl alcohol into benzaldehyde over single-crystalline rutile TiO2 nanorods. Appl. Catal. B Environ. 2012, 115–116, 201–208. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Pereira, M.F.R.; Órfão, J.J.M. Glycerol oxidation with gold supported on carbon xerogels: Tuning selectivities by varying mesopore sizes. Appl. Catal. B Environ. 2012, 115–116, 1–6. [Google Scholar] [CrossRef]
- Yu, X.; Huo, Y.; Yang, J.; Chang, S.; Ma, Y.; Huang, W. Reduced graphene oxide supported Au nanoparticles as an efficient catalyst for aerobic oxidation of benzyl alcohol. Appl. Surf. Sci. 2013, 280, 450–455. [Google Scholar] [CrossRef]
- Ishida, T.; Nagaoka, M.; Akita, T.; Haruta, M. Deposition of gold clusters on porous coordination polymers by solid grinding and their catalytic activity in aerobic oxidation of alcohols. Chem. A Eur. J. 2008, 14, 8456–8460. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Rossetti, I.; Villa, A.; Prati, L. Benzyl alcohol oxidation on carbon-supported Pd nanoparticles: Elucidating the reaction mechanism. ChemCatChem 2014, 6, 3464–3473. [Google Scholar] [CrossRef]
- Savara, A.; Rossetti, I.; Chan-Thaw, C.E.; Prati, L.; Villa, A. Microkinetic Modeling of Benzyl Alcohol Oxidation on Carbon-Supported Palladium Nanoparticles. ChemCatChem 2016, 8, 2482–2491. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Sutton, J.E.; Wang, D.; Prati, L.; Villa, A. Molecular Origin of the Selectivity Differences between Palladium and Gold–Palladium in Benzyl Alcohol Oxidation: Different Oxygen Adsorption Properties. ChemCatChem 2017, 9, 253–257. [Google Scholar] [CrossRef]
- Lei, L.; Liu, H.; Wu, Z.; Qin, Z.; Wang, G.; Ma, J.; Luo, L.; Fan, W.; Wang, J. Aerobic Oxidation of Alcohols over Isolated Single Au Atoms Supported on CeO2 Nanorods: Catalysis of Interfacial [O-Ov-Ce-O-Au] Sites. ACS Appl. Nano Mater. 2019, 2, 5214–5223. [Google Scholar] [CrossRef]
- Estrada, M.; Costa, V.V.; Beloshapkin, S.; Fuentes, S.; Stoyanov, E.; Gusevskaya, E.V.; Simakov, A. Aerobic oxidation of benzyl alcohol in methanol solutions over Au nanoparticles: Mg(OH)2 vs MgO as the support. Appl. Catal. A Gen. 2014, 473, 96–103. [Google Scholar] [CrossRef]
- Delidovich, I.V.; Moroz, B.L.; Taran, O.P.; Gromov, N.V.; Pyrjaev, P.A.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Parmon, V.N. Aerobic selective oxidation of glucose to gluconate catalyzed by Au/Al2O3 and Au/C: Impact of the mass-transfer processes on the overall kinetics. Chem. Eng. J. 2013, 223, 921–931. [Google Scholar] [CrossRef]
- Mahdavi-Shakib, A.; Sempel, J.; Babb, L.; Oza, A.; Hoffman, M.; Whittaker, T.N.; Chandler, B.D.; Austin, R.N. Combining Benzyl Alcohol Oxidation Saturation Kinetics and Hammett Studies as Mechanistic Tools for Examining Supported Metal Catalysts. ACS Catal. 2020, 10, 10207–10215. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.K.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.X.; Lu, J. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective benzyl alcohol oxidation over pd catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef]
- Galvanin, F.; Sankar, M.; Cattaneo, S.; Bethell, D.; Dua, V.; Hutchings, G.J.; Gavriilidis, A. On the development of kinetic models for solvent-free benzyl alcohol oxidation over a gold-palladium catalyst. Chem. Eng. J. 2018, 342, 196–210. [Google Scholar] [CrossRef]
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Aerobic oxidation of alcohols in visible light on Pd-grafted Ti cluster. Tetrahedron 2017, 73, 5577–5580. [Google Scholar] [CrossRef]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles—Novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Hong, Y.; Jing, X.; Huang, J.; Sun, D.; Odoom-Wubah, T.; Yang, F.; Du, M.; Li, Q. Biosynthesized bimetallic Au-Pd nanoparticles supported on TiO2 for solvent-free oxidation of benzyl alcohol. ACS Sustain. Chem. Eng. 2014, 2, 1752–1759. [Google Scholar] [CrossRef]
- Yamazoe, S.; Koyasu, K.; Tsukuda, T. Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 2014, 47, 816–824. [Google Scholar] [CrossRef]
- Bracey, C.; Ellis, P.; Hutchings, G. Application of copper–gold alloys in catalysis: Current status and future perspectives. Chem. Soc. Rev. 2009, 38, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Pd/Ag and Pd/Au bimetallic nanocatalysts on mesoporous silica for plasmon-mediated enhanced catalytic activity under visible light irradiation. J. Mater. Chem. A 2016, 4, 10142–10150. [Google Scholar] [CrossRef]
- Yamashita, H.; Mori, K.; Kuwahara, Y.; Kamegawa, T.; Wen, M.; Verma, P.; Che, M. Single-site and nano-confined photocatalysts designed in porous materials for environmental uses and solar fuels. Chem. Soc. Rev. 2018, 47, 8072–8096. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Design of silver-based controlled nanostructures for plasmonic catalysis under visible light irradiation. Bull. Chem. Soc. Jpn. 2019, 92, 19–29. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Synthesis and characterization of a Pd/Ag bimetallic nanocatalyst on SBA-15 mesoporous silica as a plasmonic catalyst. J. Mater. Chem. A 2015, 3, 18889–18897. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Choi, M.; Srivastava, R.; Ryoo, R. Organosilane surfactant-directed synthesis of mesoporous aluminophosphates constructed with crystalline microporous frameworks. Chem. Commun. 2006, 4380–4382. [Google Scholar] [CrossRef]
- Newland, S.H.; Sinkler, W.; Mezza, T.; Bare, S.R.; Carravetta, M.; Haies, I.M.; Levy, A.; Keenan, S.; Raja, R. Expanding beyond the Micropore: Active-Site Engineering in Hierarchical Architectures for Beckmann Rearrangement. ACS Catal. 2015, 5, 6587–6593. [Google Scholar] [CrossRef]
- Newland, S.H.; Sinkler, W.; Mezza, T.; Bare, S.R.; Raja, R. Influence of dopant substitution mechanism on catalytic properties within hierarchical architectures. Proc. R. Soc. A Math. Phys. Eng. Sci. 2016, 472. [Google Scholar] [CrossRef]
- Potter, M.E.; Riley, L.N.; Oakley, A.E.; Mhembere, P.M.; Callison, J.; Raja, R. The influence of porosity on nanoparticle formation in hierarchical aluminophosphates. Beilstein J. Nanotechnol. 2019, 10, 1952–1957. [Google Scholar] [CrossRef]
- Chapman, S.; Carravetta, M.; Miletto, I.; Doherty, C.M.; Dixon, H.; Taylor, J.D.; Gianotti, E.; Yu, J.; Raja, R. Probing the Design Rationale of a High-Performing Faujasitic Zeotype Engineered to have Hierarchical Porosity and Moderated Acidity. Angew. Chemie 2020, 132, 19729–19737. [Google Scholar] [CrossRef]
- Potter, M.E.; Armstrong, L.M.; Carravetta, M.; Mezza, T.M.; Raja, R. Designing Multi-Dopant Species in Microporous Architectures to Probe Reaction Pathways in Solid-Acid Catalysis. Front. Chem. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.E.; Kezina, J.; Bounds, R.; Carravetta, M.; Mezza, T.M.; Raja, R. Investigating the role of framework topology and accessible active sites in silicoaluminophosphates for modulating acid-catalysis. Catal. Sci. Technol. 2018, 8, 5155–5164. [Google Scholar] [CrossRef]
- Gianotti, E.; Manzoli, M.; Potter, M.E.; Shetti, V.N.; Sun, D.; Paterson, J.; Mezza, T.M.; Levy, A.; Raja, R. Rationalising the role of solid-acid sites in the design of versatile single-site heterogeneous catalysts for targeted acid-catalysed transformations. Chem. Sci. 2014, 5, 1810–1819. [Google Scholar] [CrossRef]
- Potter, M.E.; Sun, D.; Gianotti, E.; Manzoli, M.; Raja, R. Investigating site-specific interactions and probing their role in modifying the acid-strength in framework architectures. Phys. Chem. Chem. Phys. 2013, 15, 13288–13295. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.D.; Boswell, E.M.; Captain, B.; Hungria, A.B.; Midgley, P.A.; Raja, R.; Thomas, J.M. Bimetallic Ru-Sn nanoparticle catalysts for the solvent-free selective hydrogenation of 1,5,9-cyclododecatriene to cyclododecene. Angew. Chemie Int. Ed. 2007, 46, 8182–8185. [Google Scholar] [CrossRef]
- Hungria, A.B.; Raja, R.; Adams, R.D.; Captain, B.; Thomas, J.M.; Midgley, P.A.; Golovko, V.; Johnson, B.F.G. Single-step conversion of dimethyl terephthalate into cyclohexanedimethanol with Ru5PtSn, a trimetallic nanoparticle catalyst. Angew. Chemie Int. Ed. 2006, 45, 4782–4785. [Google Scholar] [CrossRef]
- Jones, M.D.; Raja, R.; Thomas, J.M.; Johnson, B.F.G.; Lewis, D.W.; Rouzaud, J.; Harris, K.D.M. Enhancing the enantioselectivity of novel homogeneous organometallic hydrogenation catalysts. Angew. Chemie Int. Ed. 2003, 42, 4326–4331. [Google Scholar] [CrossRef]
- Raja, R.; Khimyak, T.; Thomas, J.M.; Hermans, S.; Johnson, B.F.G. Single-step, highly active, and highly selective nanoparticle catalysts for the hydrogenation of key organic compounds. Angew. Chemie Int. Ed. 2001, 40, 4638–4642. [Google Scholar] [CrossRef]
- Hermans, S.; Raja, R.; Thomas, J.M.; Johnson, B.F.G.; Sankar, G.; Gleeson, D. Solvent‐Free, Low‐Temperature, Selective Hydrogenation of Polyenes using a Bimetallic Nanoparticle Ru–Sn Catalyst. Angew. Chem. Int. Ed. Engl. 2001, 40, 1211–1215. [Google Scholar] [CrossRef]
- Thomas, J.M.; Johnson, B.F.G.; Raja, R.; Sankar, G.; Midgley, P.A. High-performance nanocatalysts for single-step hydrogenations. Acc. Chem. Res. 2003, 36, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Raja, R. Catalytic significance of organometallic compounds immobilized on mesoporous silica: Economically and environmentally important examples. J. Organomet. Chem. 2004, 689, 4110–4124. [Google Scholar] [CrossRef]
- Thomas, J.M.; Raja, R.; Lewis, D.W. Single-site heterogeneous catalysts. Angew. Chemie Int. Ed. 2005, 44, 6456–6482. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Huang, C.; Hao, Y.; Liu, F. Au/Pd core-shell nanoparticles for enhanced electrocatalytic activity and durability. Electrochem. Commun. 2012, 23, 133–136. [Google Scholar] [CrossRef]
- Carter, J.H.; Althahban, S.; Nowicka, E.; Freakley, S.J.; Morgan, D.J.; Shah, P.M.; Golunski, S.; Kiely, C.J.; Hutchings, G.J. Synergy and Anti-Synergy between Palladium and Gold in Nanoparticles Dispersed on a Reducible Support. ACS Catal. 2016, 6, 6623–6633. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Hou, W.; Dehm, N.A.; Scott, R.W.J. Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts. J. Catal. 2008, 253, 22–27. [Google Scholar] [CrossRef]
- Jover, J.; García-Ratés, M.; López, N. The Interplay between Homogeneous and Heterogeneous Phases of PdAu Catalysts for the Oxidation of Alcohols. ACS Catal. 2016, 6, 4135–4143. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Q.; Sun, Y.; Chen, S.; Wang, J.Q.; Wu, M.; Fu, W.; Tang, Y.; Duan, X.; Chen, D.; et al. Optimising surface d charge of AuPd nanoalloy catalysts for enhanced catalytic activity. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]


| Catalysts | BET Surface Area (m2g−1) | Total Pore Volume (cm3g−1) | Microporous Pore Volume (cm3g−1) | Mesoporous Pore Volume (cm3g−1) | BJH Mesopore Diameter (Å) |
|---|---|---|---|---|---|
| HP-SAPO-5 | 299 | 0.24 | 0.06 | 0.18 | 53 |
| Au/HP-SAPO-5 | 199 | 0.18 | 0.04 | 0.14 | 56 |
| Pd/HP-SAPO-5 | 195 | 0.18 | 0.04 | 0.14 | 55 |
| PdAu/HP-SAPO-5 | 194 | 0.17 | 0.04 | 0.13 | 56 |
| MP-SAPO-5 | 271 | 0.14 | 0.12 | 0.02 | -- |
| PdAu/MP-SAPO-5 | 36 | 0.03 | 0.02 | 0.01 | -- |
| Catalysts | ICP Experimental Metal Loading (Weight%) | ||||
|---|---|---|---|---|---|
| Au | Pd | Al | P | Si | |
| HP-SAPO-5 | -- | -- | 19.5 | 18.1 | 7.5 |
| Au/HP-SAPO-5 | 0.6 | -- | 19.6 | 19.9 | 9.6 |
| Pd/HP-SAPO-5 | -- | 0.4 | 19.3 | 18.0 | 7.6 |
| PdAu/HP-SAPO-5 | 0.7 | 0.1 | 19.2 | 17.3 | 8.7 |
| PdAu/MP-SAPO-5 | 0.6 | 0.2 | 19.6 | 19.7 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, P.; Potter, M.E.; Oakley, A.E.; Mhembere, P.M.; Raja, R. Bimetallic PdAu Catalysts within Hierarchically Porous Architectures for Aerobic Oxidation of Benzyl Alcohol. Nanomaterials 2021, 11, 350. https://doi.org/10.3390/nano11020350
Verma P, Potter ME, Oakley AE, Mhembere PM, Raja R. Bimetallic PdAu Catalysts within Hierarchically Porous Architectures for Aerobic Oxidation of Benzyl Alcohol. Nanomaterials. 2021; 11(2):350. https://doi.org/10.3390/nano11020350
Chicago/Turabian StyleVerma, Priyanka, Matthew E. Potter, Alice E. Oakley, Panashe M. Mhembere, and Robert Raja. 2021. "Bimetallic PdAu Catalysts within Hierarchically Porous Architectures for Aerobic Oxidation of Benzyl Alcohol" Nanomaterials 11, no. 2: 350. https://doi.org/10.3390/nano11020350
APA StyleVerma, P., Potter, M. E., Oakley, A. E., Mhembere, P. M., & Raja, R. (2021). Bimetallic PdAu Catalysts within Hierarchically Porous Architectures for Aerobic Oxidation of Benzyl Alcohol. Nanomaterials, 11(2), 350. https://doi.org/10.3390/nano11020350