Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
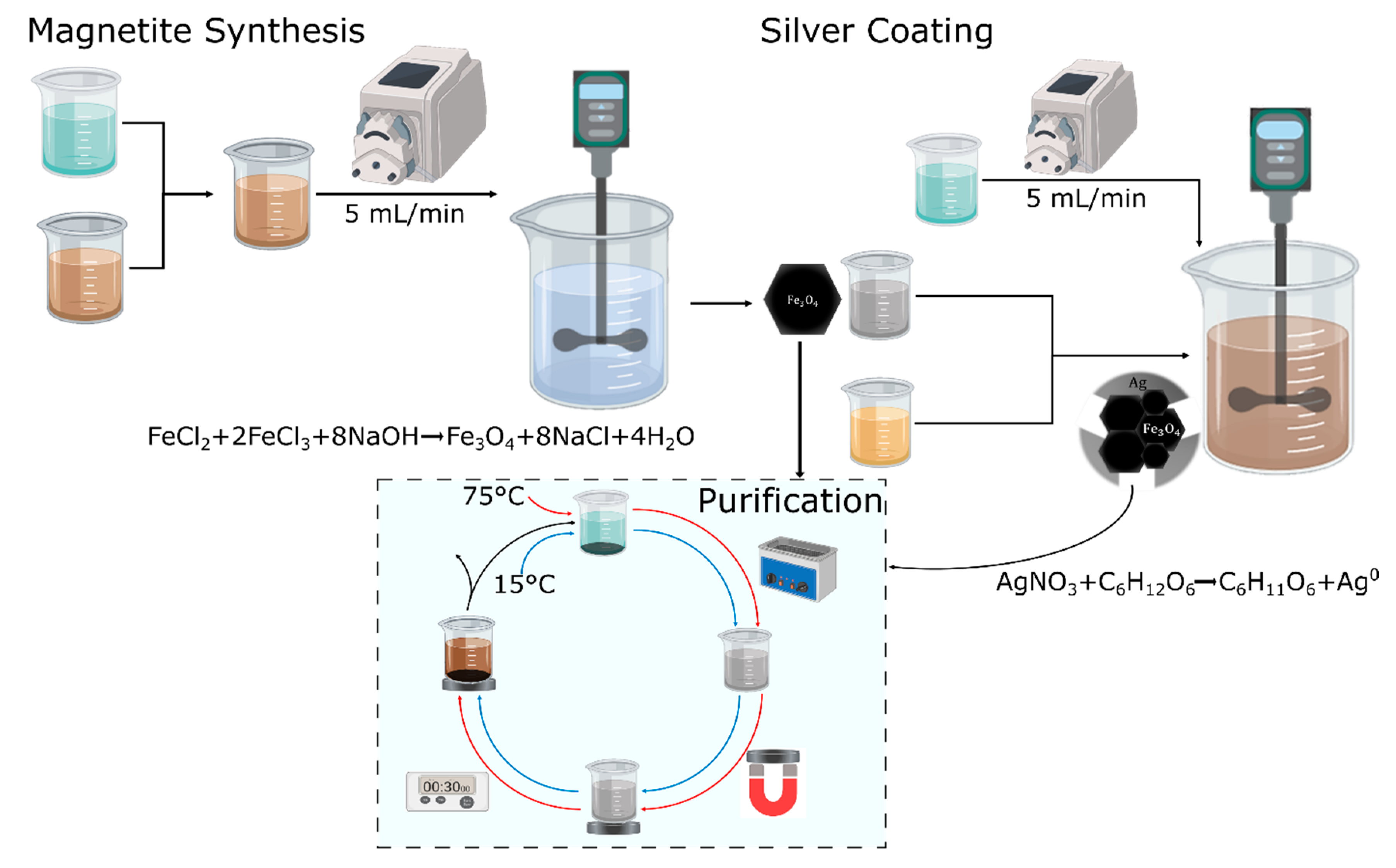
2.1. Synthesis and Characterization of the Magnetite Core
2.2. Silver Shell Formation
2.3. Magnetite/Silver Purification and Physical Characterization
2.4. Magnetite/Silver Surface Chemistry
2.5. Biocompatibility
3. Results and Discussion
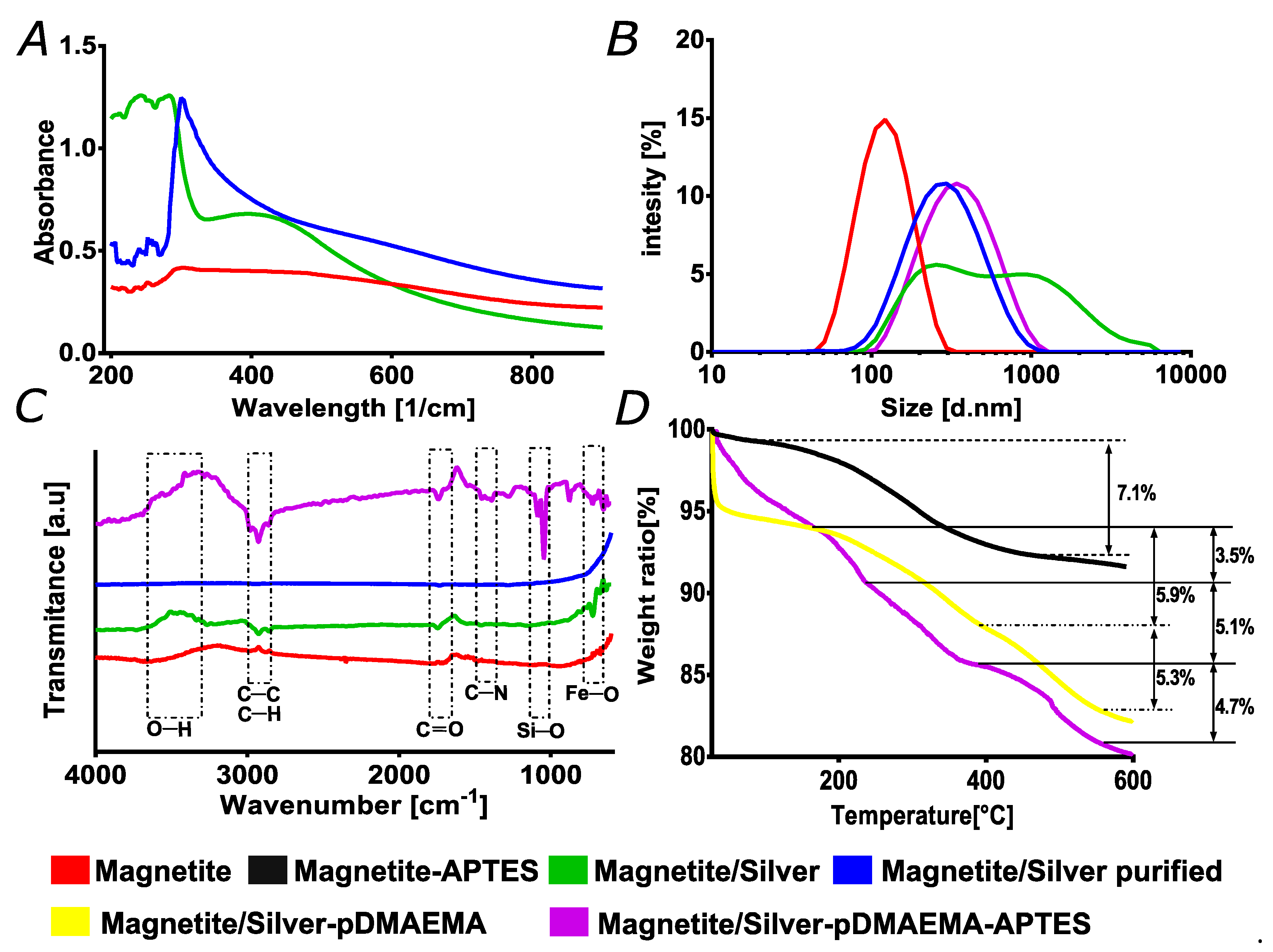
3.1. Morphology and Elemental Composition
3.2. Magnetite/Silver Characterization
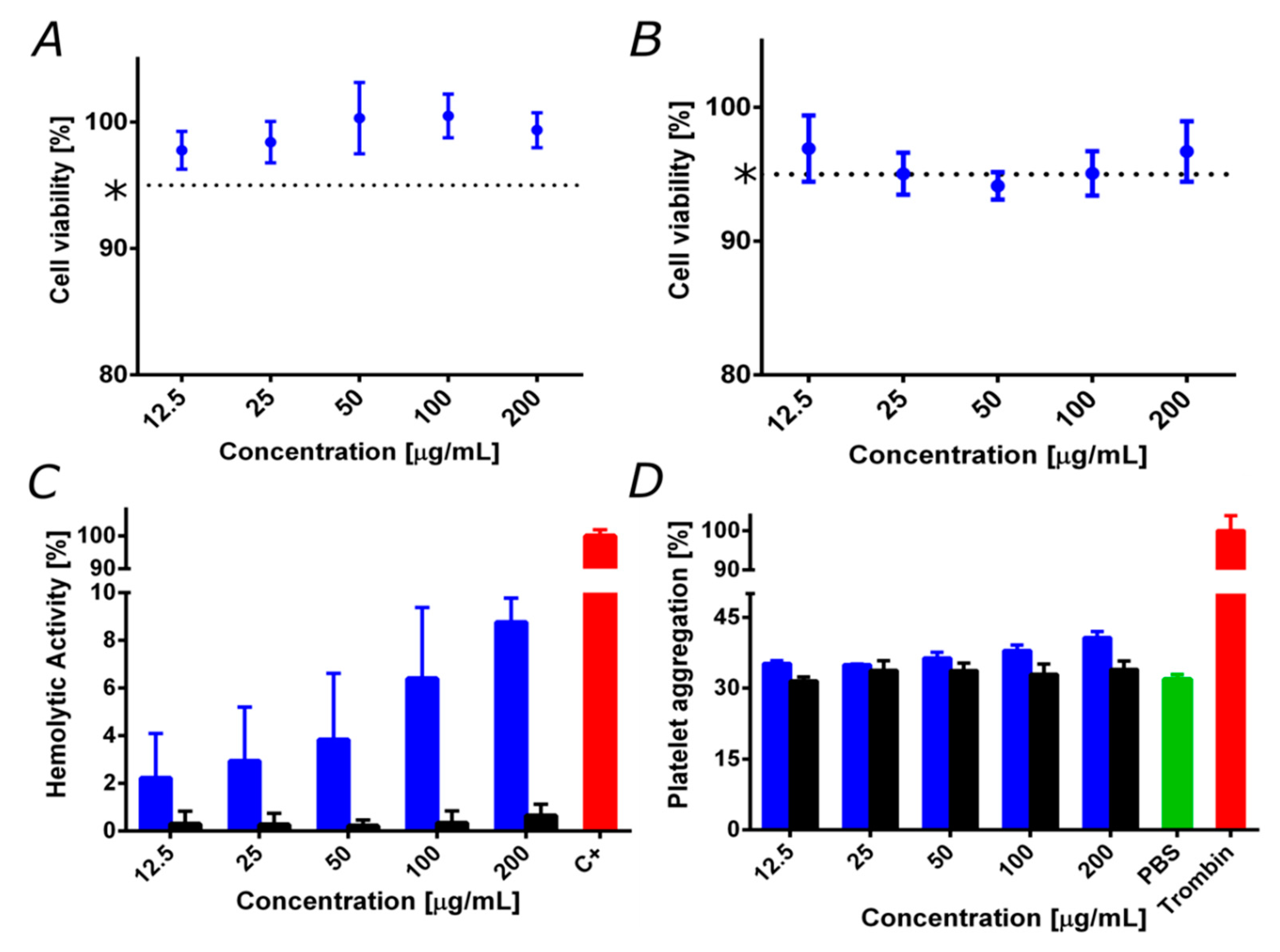
3.3. Biocompatibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Varadan, V.K.; Chen, L.; Xie, J. Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors and Nanosystems, 1st ed.; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale Metal—Organic Frameworks for Therapeutic, Imaging, and Sensing Applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef] [PubMed]
- Medina-Cruz, D.; Saleh, B.; Vernet-Crua, A.; Nieto-Argüello, A.; Lomelí-Marroquín, D.; Vélez-Escamilla, L.Y.; Cholula-Díaz, J.L.; García-Martín, J.M.; Webster, T. Bimetallic nanoparticles for biomedical applications: A review. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 397–434. ISBN 9783030344719. [Google Scholar]
- Srinoi, P.; Chen, Y.T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tsukahara, Y.; Yamada, K.; Sakata, T.; Wada, Y. Nucleation and growth of magnetic Ni?Co (Core?Shell) nanoparticles in a one-pot reaction under microwave irradiation. Chem. Mater. 2011, 23, 75–84. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Oladipo, A.O.; Msagati, T.A.M.; Lebelo, S.L.; Meddows-Taylor, S.; More, G.K. Novel silver-platinum bimetallic nanoalloy synthesized from Vernonia mespilifolia extract: Antioxidant, antimicrobial, and cytotoxic activities. Arab. J. Chem. 2020, 13, 6639–6648. [Google Scholar] [CrossRef]
- Ma, W.F.; Zhang, Y.; Li, L.L.; You, L.J.; Zhang, P.; Zhang, Y.T.; Li, J.M.; Yu, M.; Guo, J.; Lu, H.J.; et al. Tailor-made magnetic Fe3O4@mTiO2 microspheres with a tunable mesoporous anatase shell for highly selective and effective enrichment of phosphopeptides. ACS Nano 2012, 6, 3179–3188. [Google Scholar] [CrossRef]
- Poon, Z.; Chen, S.; Engler, A.C.; Lee, H.; Atas, E.; von Maltzahn, G.; Bhatia, S.N.; Hammond, P.T. Ligand-Clustered “Patchy” Nanoparticles for Modulated Cellular Uptake and In Vivo Tumor Targeting. Angew. Chem. 2010, 122, 7424–7428. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Sharifi, I. Biosynthesis of bimetallic and core-shell nanoparticles: Their biomedical applications—A review. IET Nanobiotechnol. 2018, 12, 879–887. [Google Scholar] [CrossRef]
- Geor malar, C.; Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on surface modification of nanocarriers to overcome diffusion limitations: An enzyme immobilization aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar] [CrossRef]
- Du, J.; Reilly, R.K.O. Anisotropic particles with patchy, multicompartment and Janus architectures: Preparation and application. Chem. Soc. Rev. 2011, 40, 2402–2416. [Google Scholar] [CrossRef]
- Li, Y.; Bolinger, J.; Yu, Y.; Glass, Z.; Shi, N.; Yang, L.; Wang, M.; Xu, Q. Intracellular delivery and biodistribution study of CRISPR/Cas9 ribonucleoprotein loaded bioreducible lipidoid nanoparticles. Biomater. Sci. 2019, 7, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Fukuda, Y.; Sasaki, D.; Maruyama, M.; Harashima, H. Development of a nanoparticle that releases nucleic acids in response to a mitochondrial environment. Mitochondrion 2020, 52, 67–74. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 32019. [Google Scholar]
- Bianchi, E.; Capone, B.; Coluzza, I.; Rovigatti, L.; Van Oostrum, P.D.J. Limiting the valence: Advancements and new perspectives on patchy colloids, soft functionalized nanoparticles and biomolecules. Phys. Chem. Chem. Phys. 2017, 19, 19847–19868. [Google Scholar] [CrossRef] [PubMed]
- Isaacoff, B.P.; Brown, K.A. Progress in Top-Down Control of Bottom-Up Assembly. Nano Lett. 2017, 17, 6508–6510. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.J.; Finnegan, J.R.; Manners, I. Self-assembly of “patchy” nanoparticles: A versatile approach to functional hierarchical materials. Chem. Sci. 2015, 6, 3663–3673. [Google Scholar] [CrossRef]
- Pawar, A.B.; Kretzschmar, I. Fabrication, assembly, and application of patchy particles. Macromol. Rapid Commun. 2010, 31, 150–168. [Google Scholar] [CrossRef]
- Ravelo-Acuña, D.; Fuentes-García, J.A.; Yee-Madeira, H.T.; Diaz-Cano, A.I.; Goya, G.F.; Santoyo-Salazar, J. Sonochemical magnetite encapsulation in silica at low irradiation power. Mater. Lett. 2019, 250, 103–107. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Tsai, T.H.; Thiagarajan, S.; Chen, S.M. Ionic liquid assisted one step green synthesis of Au-Ag bimetallic nanoparticles. J. Appl. Electrochem. 2010, 40, 493–497. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, F.; Liu, Z.; Ma, J.; Yang, J. Expression and characterization of cecropinXJ, a bioactive antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in Escherichia coli. Exp. Ther. Med. 2013, 5, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Deplanche, K.; Merroun, M.L.; Casadesus, M.; Tran, D.T.; Mikheenko, I.P.; Bennett, J.A.; Zhu, J.; Jones, I.P.; Attard, G.A.; Wood, J.; et al. Microbial synthesis of core/shell gold/palladium nanoparticles for applications in green chemistry. J. R. Soc. Interface 2012, 9, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fernández, D.; Liz-Marzán, L.M. Metallic Janus and patchy particles. Part. Part. Syst. Charact. 2013, 30, 46–60. [Google Scholar] [CrossRef]
- Li, J.L.; Tian, B.; Li, T.; Dai, S.; Weng, Y.L.; Lu, J.J.; Xu, X.L.; Jin, Y.; Pang, R.J.; Hua, Y.J. Biosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using protein extracts of deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411–1424. [Google Scholar] [CrossRef]
- Garza-Navarro, M.; Torres-Castro, A.; González, V.; Ortiz, U.; De la Rosa, E. Magnetite and magnetite/silver core/shell nanoparticles with diluted magnet-like behavior. J. Solid State Chem. 2010, 183, 99–104. [Google Scholar] [CrossRef]
- Philip, D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1078–1081. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, A.L.K.; Tao, Y.; Shan, D.; Ting, K.E.; Yin, X.J. Synthesis and characterization of iron oxide nanoparticles and applications in the removal of heavy metals from industrial wastewater. Int. J. Photoenergy 2012, 2012, 608298. [Google Scholar] [CrossRef]
- Su, H.; Tian, Q.; Hurd Price, C.A.; Xu, L.; Qian, K.; Liu, J. Nanoporous core@shell particles: Design, preparation, applications in bioadsorption and biocatalysis. Nano Today 2020, 31, 100834. [Google Scholar] [CrossRef]
- Dai, L.; Song, L.; Huang, Y.; Zhang, L.; Lu, X.; Zhang, J.; Chen, T. Bimetallic Au/Ag Core-Shell Superstructures with Tunable Surface Plasmon Resonance in the Near-Infrared Region and High Performance Surface-Enhanced Raman Scattering. Langmuir 2017, 33, 5378–5384. [Google Scholar] [CrossRef]
- Hu, Q.; Tay, L.L.; Noestheden, M.; Pezacki, J.P. Mammalian cell surface imaging with nitrile-functionalized nanoprobes: Biophysical characterization of aggregation and polarization anisotropy in SERS imaging. J. Am. Chem. Soc. 2007, 129, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-acosta, C.M.; Cifuentes, J.; Castellanos, M.C.; Moreno, R.J.; Muñoz-camargo, C.; Cruz, J.C.; Reyes, L.H. Ph-responsive, cell-penetrating, core/shell magnetite/silver nanoparticles for the delivery of plasmids: Preparation, characterization, and preliminary in vitro evaluation. Pharmaceutics 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, M.; Cifuentes, J.; Cruz, J.; Perez, J.; Suarez-Arnedo, A.; Muñoz-Camargo, C.; Serna, J.; Groot, H. Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities. Int. J. Nanomed. 2018, 13, 8087–8094. [Google Scholar] [CrossRef]
- Perez, J.; Cifuentes, J.; Cuellar, M.; Suarez-Arnedo, A.; Cruz, J.C.; Muñoz-Camargo, C. Cell-Penetrating And Antibacterial BUF-II Nanobioconjugates: Enhanced Potency Via Immobilization On Polyetheramine-Modified Magnetite Nanoparticles. Int. J. Nanomed. 2019, 14, 8483–8497. [Google Scholar] [CrossRef]
- Matahum, J.S.; Su, C.M.; Wang, W.J.; Lou, S.L.; Ger, T.R. Effect of Surface Charge on the Uptake of Magnetic Nanoparticles in Mouse Fibroblast Cells. IEEE Magn. Lett. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Garcia, J.G.; Cifuentes, J.; Castro, L.M.; Vargas, F.; Ostos, C.; Cardona-Gomez, G.P.; Hernandez, A.M.; Cruz, J.C. Multifunctional magnetite nanoparticles to enable delivery of siRNA for the potential treatment of Alzheimer’s. Drug Deliv. 2020, 27, 864–875. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Fan, X.X.; Xie, R.; Zhao, Q.; Li, X.Y.; Ju, X.J.; Wang, W.; Liu, Z.; Chu, L.Y. Dual pH-responsive smart gating membranes. J. Memb. Sci. 2018, 555, 20–29. [Google Scholar] [CrossRef]
- You, Y.Z.; Manickam, D.S.; Zhou, Q.H.; Oupický, D. Reducible poly(2-dimethylaminoethyl methacrylate): Synthesis, cytotoxicity, and gene delivery activity. J. Control. Release 2007, 122, 217–225. [Google Scholar] [CrossRef]
- Majewski, A.P.; Schallon, A.; Jérôme, V.; Freitag, R.; Müller, A.H.E.; Schmalz, H. Dual-responsive magnetic core-shell nanoparticles for nonviral gene delivery and cell separation. Biomacromolecules 2012, 13, 857–866. [Google Scholar] [CrossRef]
- Liu, X.; Ni, P.; He, J.; Zhang, M. Synthesis and micellization of pH/temperature-responsive double-hydrophilic diblock copolymers polyphosphoester- block -poly[2-(dimethylamino)ethyl methacrylate] prepared via ROP and ATRP. Macromolecules 2010, 43, 4771–4781. [Google Scholar] [CrossRef]
- Ghutepatil, P.R.; Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. APTES (3-aminopropyltriethoxy silane) functionalized MnFe2O4 nanoparticles: A potential material for magnetic fluid hyperthermia. Chem. Pap. 2019, 73, 2189–2197. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Baez-Cruz, R.; Montoya, L.F.; Medinam, C.; Pérez-Tijerina, E.; Salazar, F.; Rojas, D.; Melendrez, M.F. Estimation of the surface interaction mechanism of ZnO nanoparticles modified with organosilane groups by Raman Spectroscopy. Ceram. Int. 2017, 43, 11838–11847. [Google Scholar] [CrossRef]
- Meyer, F.; Minoia, A.; Raquez, J.M.; Spasova, M.; Lazzaroni, R.; Dubois, P. Poly(amino-methacrylate) as versatile agent for carbon nanotube dispersion: An experimental, theoretical and application study. J. Mater. Chem. 2010, 20, 6873–6880. [Google Scholar] [CrossRef]
- Sperling, L.E.; Reis, K.P.; Pranke, P.; Wendorff, J.H. Advantages and challenges offered by biofunctional core-shell fiber systems for tissue engineering and drug delivery. Drug Discov. Today 2016, 21, 1243–1256. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Yuan, X.; Xie, J.; Leong, D.T. The influence of lysosomal stability of silver nanomaterials on their toxicity to human cells. Biomaterials 2014, 35, 6707–6715. [Google Scholar] [CrossRef]
- Sanyasi, S.; Majhi, R.K.; Kumar, S.; Mishra, M.; Ghosh, A.; Suar, M.; Satyam, P.V.; Mohapatra, H.; Goswami, C.; Goswami, L. Polysaccharide-capped silver Nanoparticles inhibit biofilm formation and eliminate multi-drug-resistant bacteria by disrupting bacterial cytoskeleton with reduced cytotoxicity towards mammalian cells. Sci. Rep. 2016, 6, 24929. [Google Scholar] [CrossRef]
- Choi, J.; Reipa, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical Characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 2011, 123, 133–143. [Google Scholar] [CrossRef]
- Jun, E.A.; Lim, K.M.; Kim, K.; Bae, O.N.; Noh, J.Y.; Chung, K.H.; Chung, J.H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology 2011, 5, 157–167. [Google Scholar] [CrossRef]
- Kumar, R.; Mondal, K.; Panda, P.K.; Kaushik, A.; Abolhassani, R.; Ahuja, R.; Rubahn, H.-G.; Mishra, Y.K. Core-Shell Nanostructures: Perspectives towards Drug Delivery Applications. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Acosta, C.M.; Cifuentes, J.; Cruz, J.C.; Reyes, L.H. Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility. Nanomaterials 2020, 10, 1857. https://doi.org/10.3390/nano10091857
Ramírez-Acosta CM, Cifuentes J, Cruz JC, Reyes LH. Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility. Nanomaterials. 2020; 10(9):1857. https://doi.org/10.3390/nano10091857
Chicago/Turabian StyleRamírez-Acosta, Carlos M., Javier Cifuentes, Juan C. Cruz, and Luis H. Reyes. 2020. "Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility" Nanomaterials 10, no. 9: 1857. https://doi.org/10.3390/nano10091857
APA StyleRamírez-Acosta, C. M., Cifuentes, J., Cruz, J. C., & Reyes, L. H. (2020). Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility. Nanomaterials, 10(9), 1857. https://doi.org/10.3390/nano10091857






