A New Approach to the Formation of Nanosized Gold and Beryllium Films by Ion-Beam Sputtering Deposition
Abstract
1. Introduction
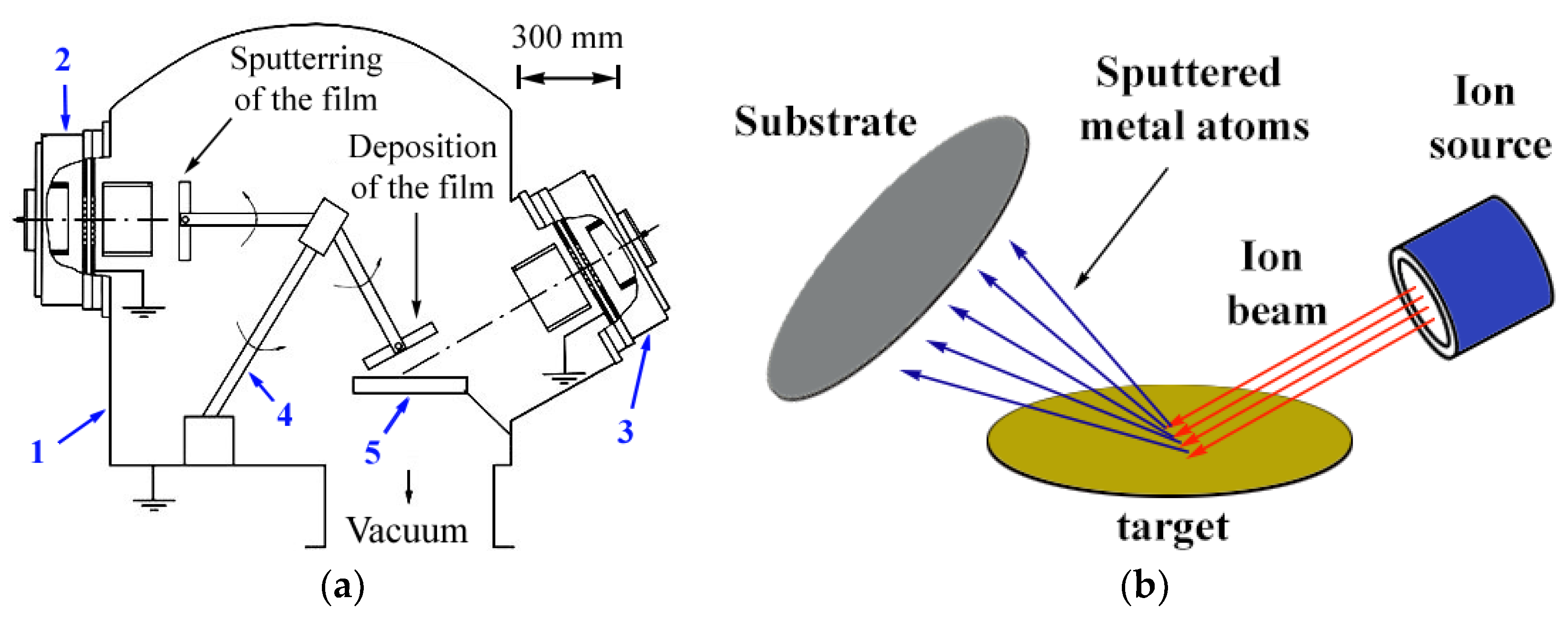
2. Materials and Methods
2.1. Preparation of Materials
2.2. Characterization Methods
3. Results and Discussion
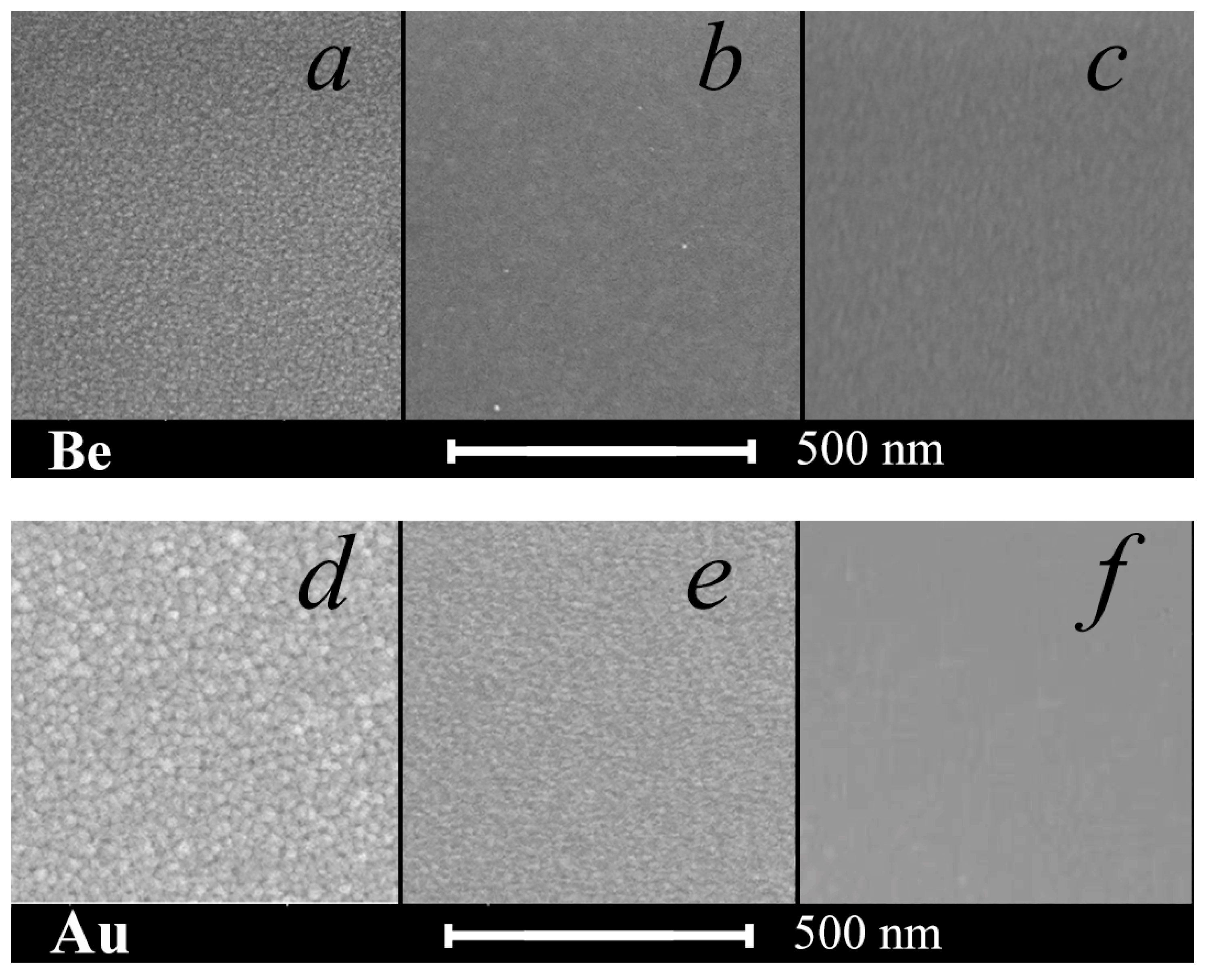
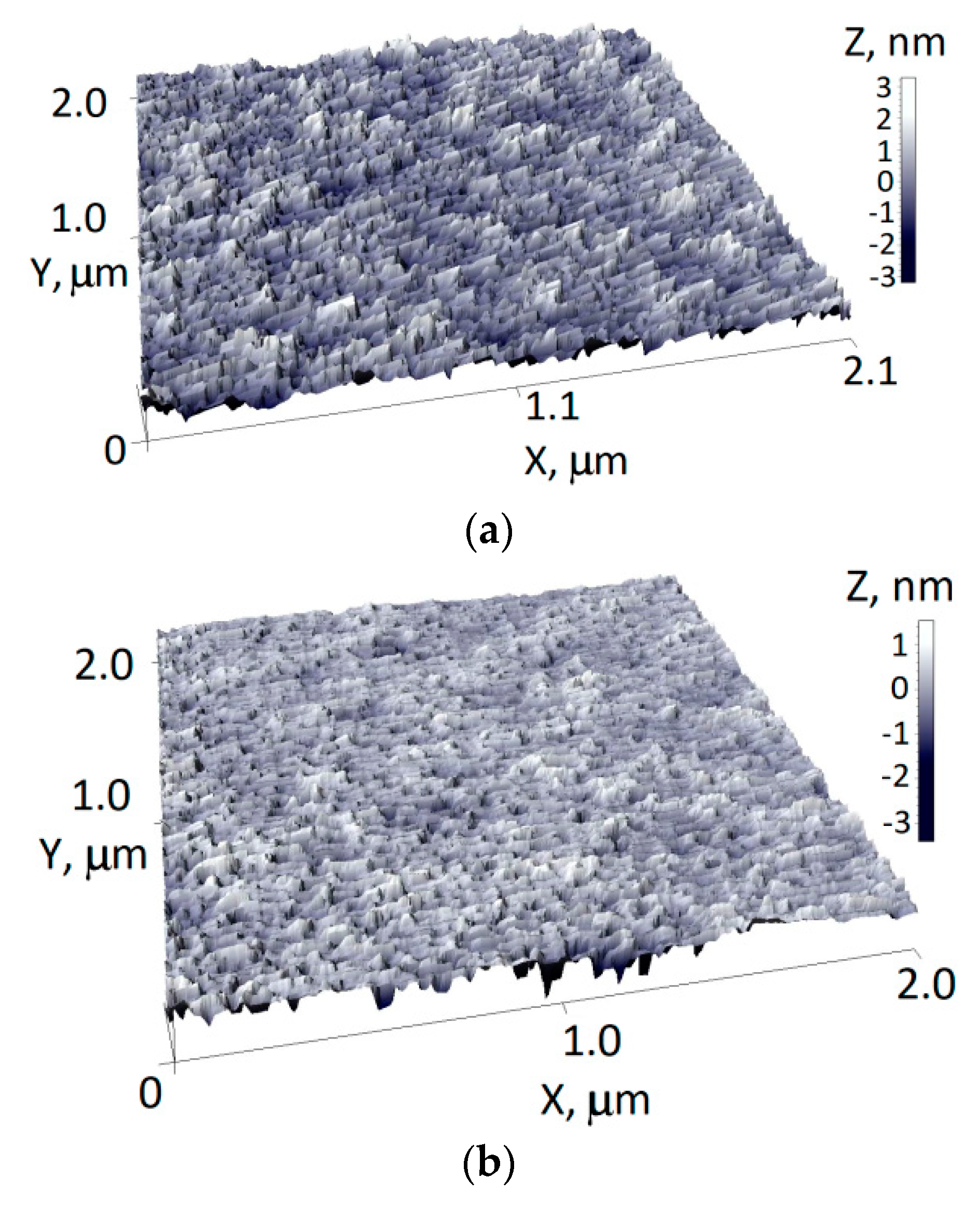
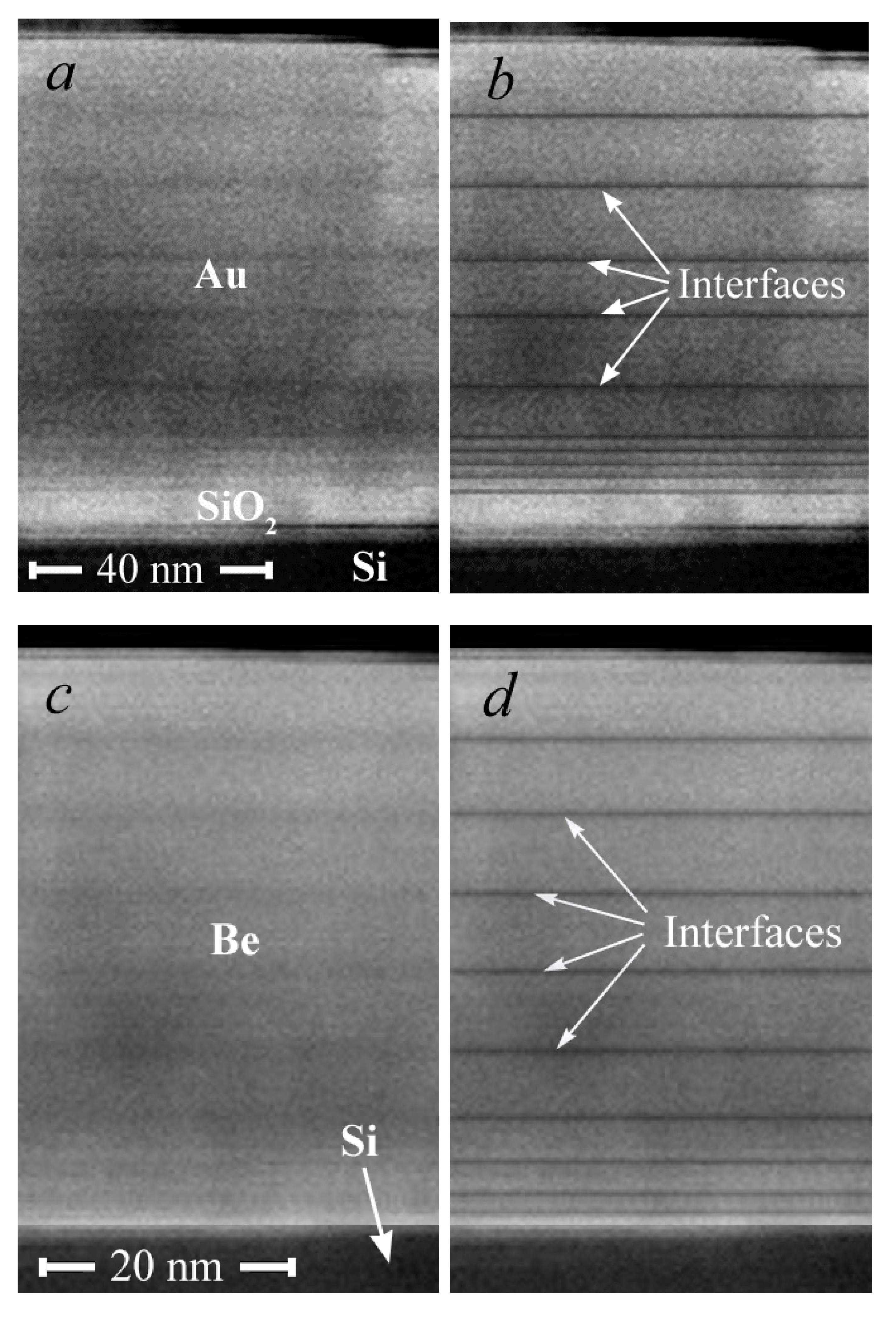
3.1. Surface Morphology and SEM
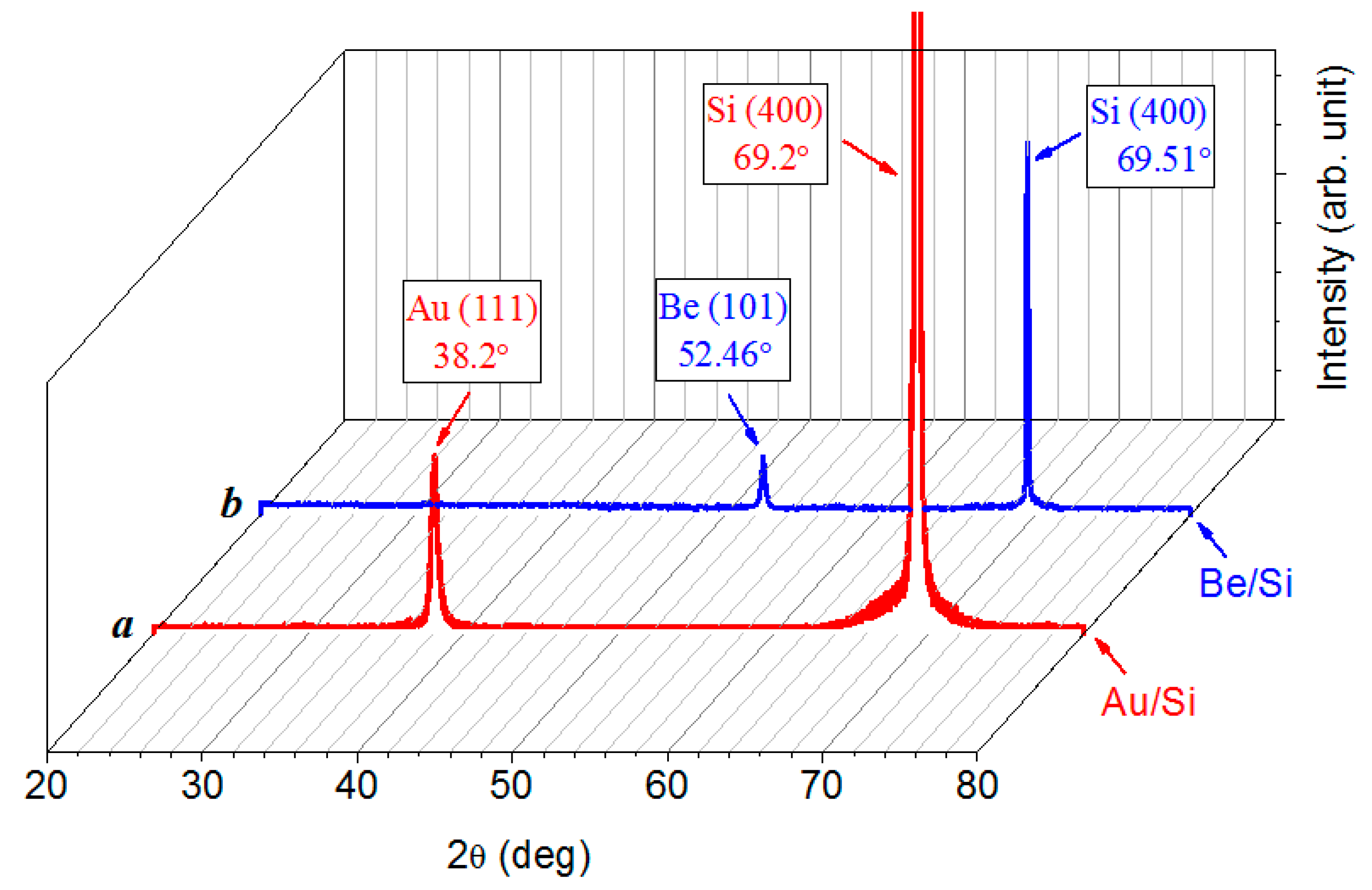
3.2. X-ray Diffraction
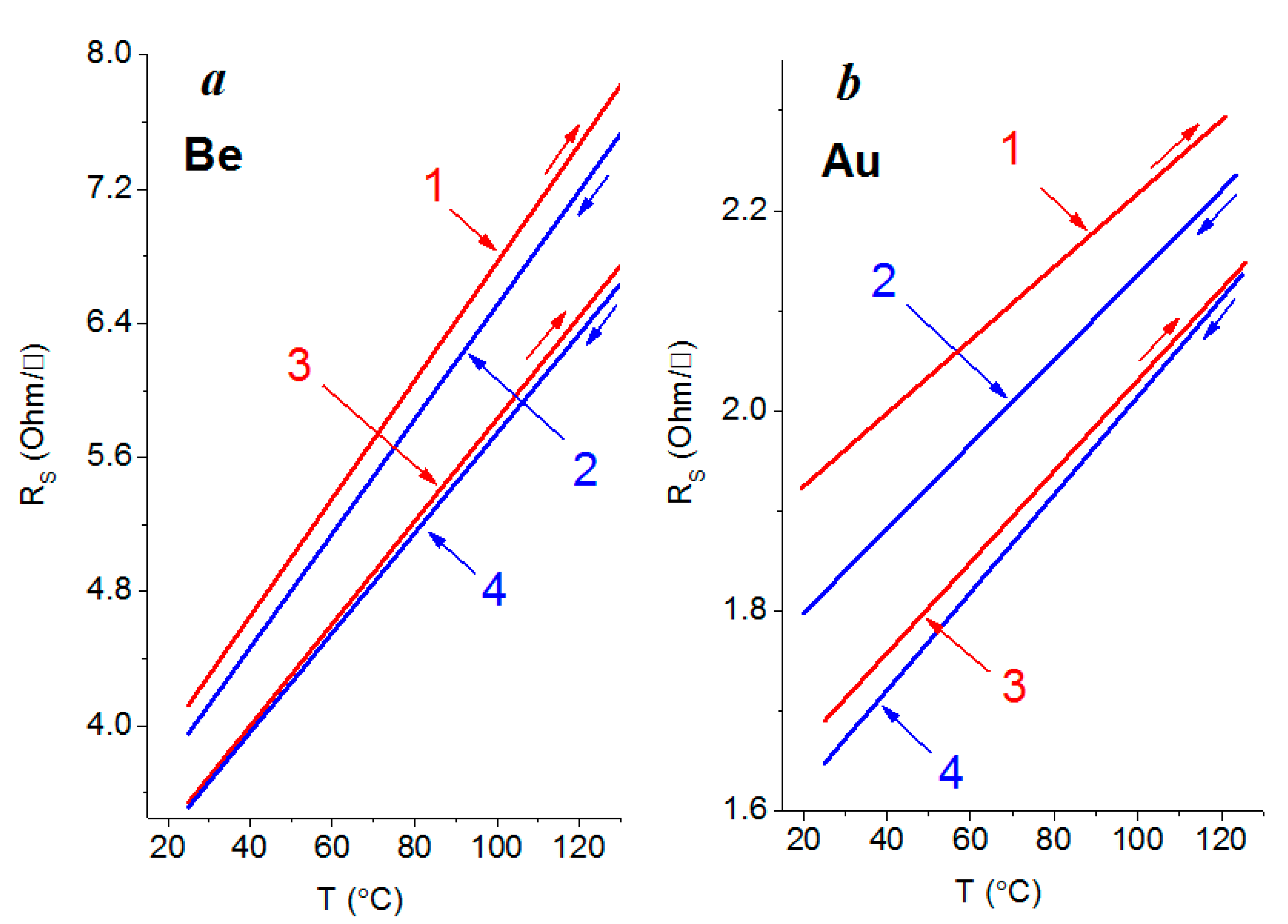
3.3. Electrical Properties
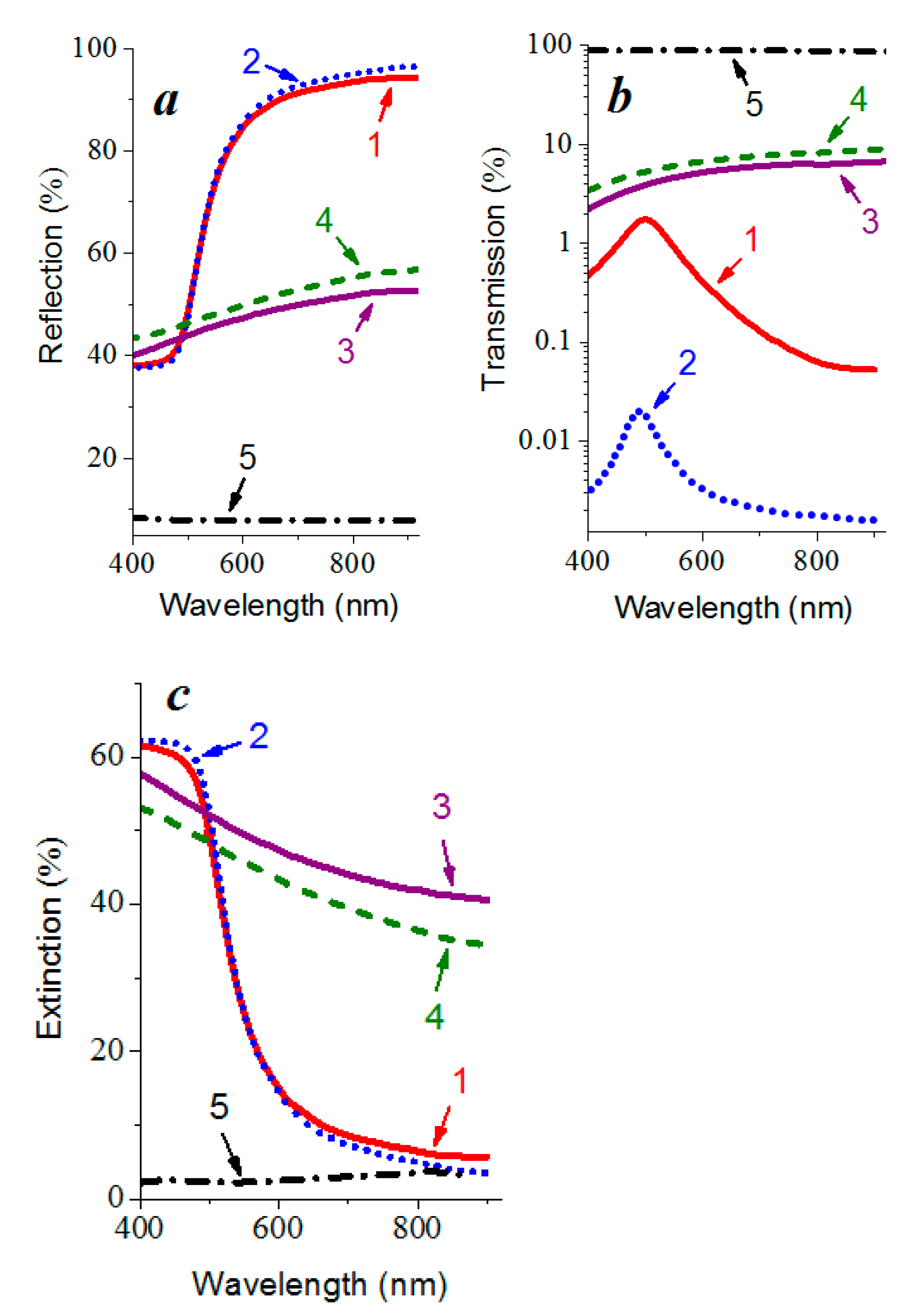
3.4. Optical Properties
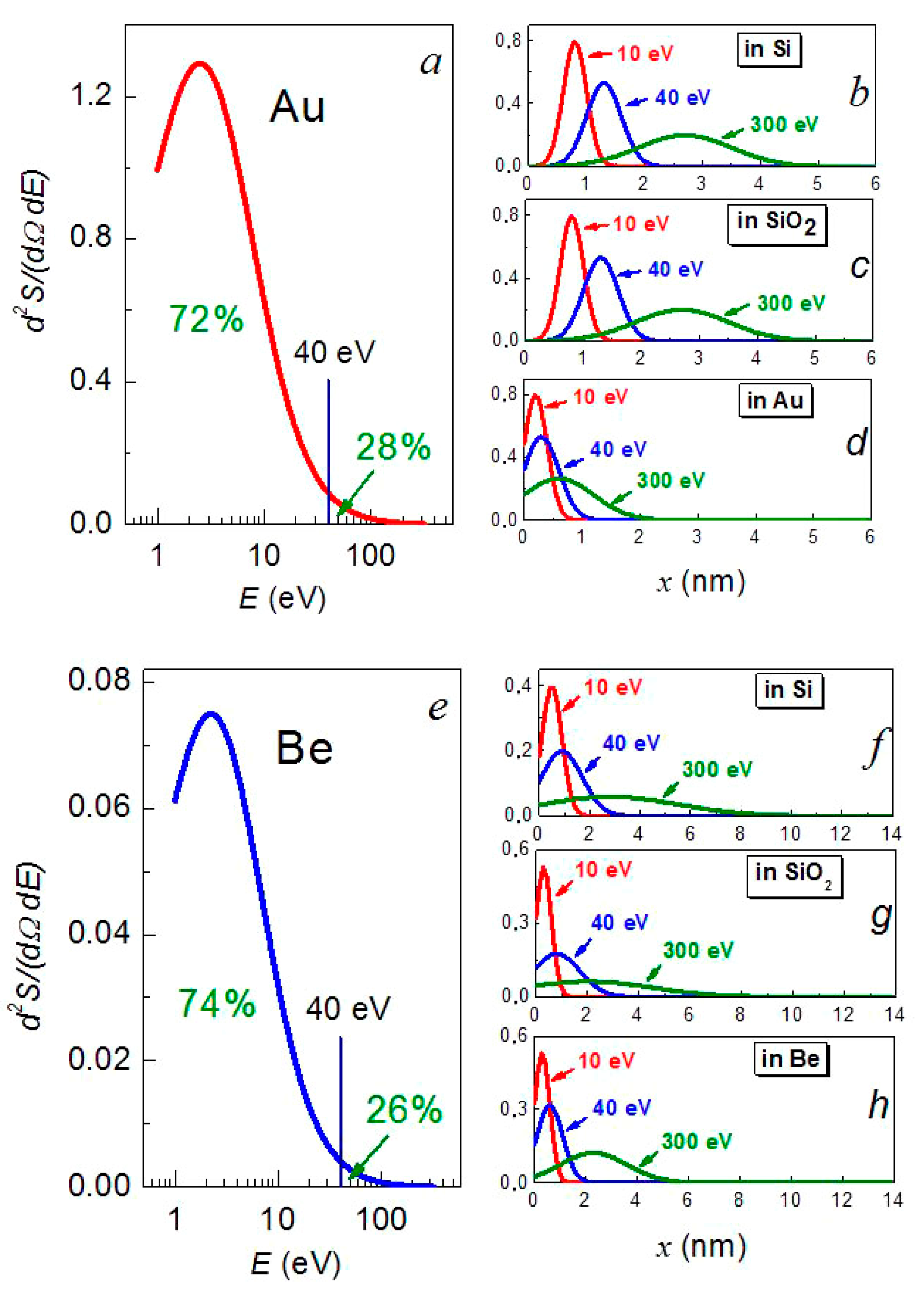
3.5. Plasmonic Properties
3.6. Formation Mechanism of Nanosized Metal Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stupakiewicz, A.; Pashkevich, M.; Maziewski, A.; Stognij, A.; Novitskii, N. Spin precession modulation in a magnetic bilayer. Appl. Phys. Lett. 2012, 101, 262406. [Google Scholar] [CrossRef]
- Pashkevich, M.; Stupakiewicz, A.; Kirilyuk, A.; Maziewski, A.; Stognij, A.; Novitskii, N.; Kimel, A.; Rasing, T. Tunable magnetic properties in ultrathin Co/garnet heterostructures. J. Appl. Phys. 2012, 111, 023913. [Google Scholar] [CrossRef]
- Stognii, A.I.; Pashkevich, M.V.; Novitskii, N.N.; Gribkov, B.A.; Mironov, V.L.; Ketsko, V.A.; Fettar, F.; Garad, H. Controlled Growth of Co nanofilms on Si(100) by ion-beam sputtering. Inorg. Mater. 2011, 47, 869–875. [Google Scholar] [CrossRef]
- Leandro, L.; Malureanu, R.; Rozlosnik, N.; Lavrinenko, A. Ultrathin, ultrasmooth gold layer on dielectrics without the use of additional metallic adhesion layers. ACS Appl. Mater. Interfaces 2015, 7, 5797–5802. [Google Scholar] [CrossRef]
- Kazlou, A.; Chekhov, A.L.; Stognij, A.I.; Razdolski, I.; Stupakiewicz, A. Surface plasmon-enhanced photomagnetic excitation of spin dynamics in Au/YIG:Co magneto-plasmonic crystals. ACS Photonics 2021, 8, 2197–2202. [Google Scholar] [CrossRef]
- Naydenov, P.N.; Chekhov, A.L.; Golikova, O.L.; Bespalov, A.V.; Geraskin, A.A.; Savin, S.S.; Murzina, T.V. Double-lattice magnetoplasmonic structures based on BIG and perforated gold films. Phys. Solid State 2019, 61, 1658–1664. [Google Scholar] [CrossRef]
- Stognij, A.I.; Novitsky, N.N.; Tushina, S.D.; Kalinnikov, S.V. Preparation of ultrathin gold films by oxygen-ion sputtering and their optical properties. J. Techn. Phys. 2003, 73, 745–748. [Google Scholar] [CrossRef]
- Sheu, J.K.; Su, Y.K.; Chi, G.C.; Koh, P.L.; Jou, M.J.; Chang, C.M.; Liu, C.C.; Hung, W.C. High-transparency Ni/Au ohmic contact to p-type GaN. Appl. Phys. Lett. 1999, 74, 2340–2342. [Google Scholar] [CrossRef]
- Yun, J. Ultrathin metal films for transparent electrodes of flexible optoelectronic devices. Adv. Funct. Mater. 2017, 27, 1606641. [Google Scholar] [CrossRef]
- Bi, Y.-G.; Feng, J.; Ji, J.-H.; Chen, Y.; Liu, Y.-S.; Li, Y.-F.; Liu, Y.-F.; Zhang, X.-L.; Sun, H.-B. Ultrathin and ultrasmooth Au films as transparent electrodes in ITO-free organic light-emitting devices. Nanoscale 2016, 8, 10010–10015. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: Bath, UK, 2007; p. 229. [Google Scholar]
- Sukham, J.; Takayama, O.; Lavrinenko, A.V.; Malureanu, R. High-quality ultrathin gold layers with an APTMS adhesion for optimal performance of surface plasmon polariton-based devices. ACS Appl. Mater. Interf. 2017, 9, 25049–25056. [Google Scholar] [CrossRef] [PubMed]
- Malureanu, R.; Lavrinenko, A. Ultra-thin films for plasmonics: A technology overview. Nanotechnol. Rev. 2015, 4, 259–275. [Google Scholar] [CrossRef]
- Yakubovsky, D.I.; Arsenin, A.V.; Stebunov, Y.V.; Fedyanin, D.Y.; Volkov, V.S. Optical constants and structural properties of thin gold films. Opt. Exp. 2017, 25, 25574–25587. [Google Scholar] [CrossRef] [PubMed]
- Bochenkov, V.; Baumberg, J.; Noginov, M.; Benz, F.; Aldewachi, H.; Schmid, S.; Podolskiy, V.; Aizpurua, J.; Lin, K.; Ebbesen, T.; et al. Applications of plasmonics: General discussion. Faraday Discuss. 2015, 178, 435–466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chekhov, A.L.; Krutyanskiy, V.L.; Shaimanov, A.N.; Stognij, A.I.; Murzina, T.V. Wide tunability of magnetoplasmonic crystals due to excitation of multiple waveguide and plasmon modes. Opt. Exp. 2014, 22, 17762–17768. [Google Scholar] [CrossRef] [PubMed]
- Chekhov, A.L.; Krutyanskiy, V.L.; Ketsko, V.A.; Stognij, A.I.; Murzina, T.V. High-quality Au/BIG/GGG magnetoplasmonic crystals fabricated by a combined ion-beam etching technique. Opt. Mater. Exp. 2015, 5, 1647–1652. [Google Scholar] [CrossRef]
- Svechnikov, M.; Chkhalo, N.; Lopatin, A.; Pleshkov, R.; Polkovnikov, V.; Salashchenko, N.; Schäfers, F.; Sertsu, M.G.; Sokolov, A.; Tsybin, N. Optical constants of sputtered beryllium thin films determined from photoabsorption measurements in the spectral range 20.4–250 eV. J. Synchrotron Rad. 2020, 27, 75–82. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, Q.; Sun, K.; Wang, L. Formation of ultrafine uniform gold nanoparticles by sputtering and redeposition. Appl. Phys. Lett. 2009, 94, 133107. [Google Scholar] [CrossRef]
- Bahamondes, S.; Donoso, S.; Henríquez, R.; Flores, M. Morphological and electrical study of gold ultrathin films on mica. Thin Solid Films 2013, 548, 646–649. [Google Scholar] [CrossRef]
- Lozovoy, K.A.; Korotaev, A.G.; Kokhanenko, A.P.; Dirko, V.V.; Voitsekhovskii, A.V. Kinetics of epitaxial formation of nanostructures by Frank–van der Merwe, Volmer–Weber and Stranski–Krastanow growth modes. Surf. Coatings Technol. 2020, 384, 125289. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Nucleation Theory and Growth of Nanostructures; Springer: Berlin, Germany, 2014; p. 601. [Google Scholar]
- Poate, J.M.; Tu, K.N.; Mayer, J.W. Thin Films—Interdiffusion and Reactions; The Electrochemical Society. Inc.: Princeton, NJ, USA, 1978; 578p. [Google Scholar]
- Stognij, A.I.; Koryakin, S.V.; Novitsky, N.N. Cobalt redistribution over the surface in inhomogeneous cobalt—Copper alloys films. J. Techn. Phys. 2003, 48, 496–502. [Google Scholar] [CrossRef]
- Stognij, A.I.; Novitsky, N.N.; Stukalov, O.M.; Demchenko, A.I.; Khitko, V.I. Inhomogeneous character of the initial stage of ion beam deposition of ultrathin gold films. Techn. Phys. Lett. 2004, 30, 256–258. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of sheet resistivities with the four-point probe. Bell Syst. Technol. J. 1958, 34, 711–718. [Google Scholar] [CrossRef]
- Chopra, K.L.; Das, S.R. Thin Film Solar Cells; Springer Science—Business Media: New York, NY, USA, 1983; 615p. [Google Scholar] [CrossRef]
- Gall, D. Electron mean free path in elemental metals. J. Appl. Phys. 2016, 119, 085101. [Google Scholar] [CrossRef]
- Fuchs, K. The conductivity of thin metallic films according to the electron theory of metals. Proc. Camb. Philos. Soc. 1938, 34, 100–108. [Google Scholar] [CrossRef]
- Sondheimer, E.H. The mean free paths of electrons in metals. Adv. Phys. 1952, 1, 1–42. [Google Scholar] [CrossRef]
- Mayadas, A.F.; Shatzkes, M.; Janak, J.F. Electrical resistivity model for polycrystalline films: The case of specular reflection at external surfaces. Appl. Phys. Lett. 1969, 14, 345–347. [Google Scholar] [CrossRef]
- Mayadas, A.F.; Shatzkes, M. Electrical-resistivity model for polycrystalline Ffilms: The Case of arbitrary reflection at external surfaces. Phys. Rev. B 1970, 1, 1382–1389. [Google Scholar] [CrossRef]
- Camacho, J.M.; Oliva, A.I. Surface and grain boundary contributions in the electrical resistivity of metallic nanofilms. Thin Solid Films 2006, 515, 1881–1885. [Google Scholar] [CrossRef]
- Schneider, M.A.; Wenderoth, M.; Heinrich, A.J.; Rosentreter, M.A.; Ulbrich, R.G. Current transport through single grain boundaries: A scanning tunneling potentiometry study. Appl. Phys. Lett. 1996, 69, 1327–1329. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Tatmyshevskiy, M.K.; Yakubovsky, D.I.; Kapitanova, O.O.; Solovey, V.R.; Vyshnevyy, A.A.; Ermolaev, G.A.; Klishin, Y.A.; Mironov, M.S.; Voronov, A.A.; Arsenin, A.V.; et al. Hybrid metal-dielectric-metal sandwiches for SERS applications. Nanomaterials 2021, 11, 3205. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Cond. Mater. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Anikin, K.; Rodyakina, E.; Veber, S.; Milekhin, A.; Latyshev, A.; Zahn, D.R.T. Localized surface plasmon resonance in gold nanocluster arrays on opaque substrates. Plasmonics 2019, 14, 1527–1537. [Google Scholar] [CrossRef]
- Ji, J.; Li, Z.; Sun, W.; Wang, H. Surface plasmon resonance tuning in gold film on silver nanospheres through optical absorption. Sens. Bio-Sens. Res. 2020, 30, 100374. [Google Scholar] [CrossRef]
- Dmitruk, N.L.; Korovin, A.V. Physical nature of anomalous optical transmission of thin absorptive corrugated films. JETP Lett. 2009, 89, 68–72. [Google Scholar] [CrossRef]
- Ranjgar, A.; Norouzi, R.; Zolanvari, A.; Sadeghi, H. Characterization and optical absorption properties of plasmonic nanostructured thin films. Armen. J. Phys. 2013, 6, 198–203. [Google Scholar]
- Gezgin, S.Y.; Kepceoğlu, A.; Gündoğdu, Y.; Zongo, S.; Zawadzka, A.; Kiliç, H.Ș.; Sahraoui, B. Effect of Ar gas pressure on LSPR property of Au nanoparticles: Comparison of experimental and theoretical studies. Nanomaterials 2020, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Axelevitch, A.; Apter, B.; Golan, G. Simulation and experimental investigation of optical transparency in gold island films. Opt. Exp. 2013, 21, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- Grytsenko, K.; Lozovski, V.; Strilchuk, G.; Schrader, S. Evaluation of the mechanism of the gold cluster growth during heating of the composite gold-polytetrafluoroethylene thin film. Nanomaterials 2012, 2, 366. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Mechanics, 3rd ed.; Butterworth-Heinemann: Nauka, Moscow, 1976; Volume 1, p. 197. [Google Scholar]
- Thompson, M.V. The velocity distribution of sputtered atoms. Nucl. Instr. Methods Phys. Res. B. 1986, 18, 411–429. [Google Scholar] [CrossRef]
- Falcone, G. Sputtering theory. Revista Nuovo Cinwnto 1990, 13, 1–52. [Google Scholar] [CrossRef]
- Stognij, A.I.; Meshcheryakov, V.F.; Novitsky, N.N.; Fettar, F.; Pashkevich, M.V. Magnetic properties of cobalt films at the initial stage of ion-beam deposition. Techn. Phys. Lett. 2009, 35, 528–531. [Google Scholar] [CrossRef]
- Biersack, J.P.; Eckstein, W. Sputtering studies with the monte carlo program TRIM.SP. Appl. Phys. A 1984, 34, 73–94. [Google Scholar] [CrossRef]
- Emsley, J. The Elements, 2nd ed.; Oxford University Press: Oxford, UK, 1998; 300p. [Google Scholar]
| Metal | Conditions of Deposition | λ0, (nm) | D, (nm) | R | α | ρGB/ρ0 |
|---|---|---|---|---|---|---|
| Au | single | 37.7 | 20 | 0.8 | 7.5 | 6.4 |
| 0.7 | 4.4 | 3.8 | ||||
| 0.6 | 2.8 | 2.6 | ||||
| Au | tenfold | 37.7 | 1 | 0.8 | 150.8 | 125.7 |
| 0.7 | 87.9 | 73.3 | ||||
| 0.6 | 56.6 | 47.1 | ||||
| Be | single | 48.0 | 10 | 0.6 | 7.2 | 6.1 |
| 0.5 | 4.8 | 4.1 | ||||
| Be | tenfold | 48.0 | 1 | 0.6 | 72 | 60 |
| 0.5 | 48 | 40 |
| Metal | M (g/mol) | U (eV) | γ | A | Eth (eV) | Emax (eV) |
|---|---|---|---|---|---|---|
| Be | 9.012 | 3.48 | 0.6 | 0.24 | 14.5 | 308 |
| Au | 196.967 | 3.92 | 0.56 | 0.246 | 15.91 | 316 |
| Energy of Sputtered Atoms (eV) | Penetration Depth (nm) | |||||
|---|---|---|---|---|---|---|
| of Beryllium Atoms | of Gold Atoms | |||||
| In Silicon | In Quartz | In Beryllium | In Silicon | In Quartz | In Gold | |
| 5 | 0.3 | 0.2 | 0.2 | 0.6 | 0.6 | 0.2 |
| 10 | 0.4 | 0.3 | 0.3 | 0.8 | 0.7 | 0.2 |
| 40 | 0.8 | 0.7 | 0.6 | 1.3 | 1.2 | 0.3 |
| 100 | 1.4 | 1.2 | 1.0 | 1.8 | 1.7 | 0.4 |
| 300 | 2.8 | 2.0 | 2.3 | 2.7 | 2.6 | 0.6 |
| 320 | 2.9 | 2.6 | 2.5 | 2.8 | 2.7 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharko, S.A.; Serokurova, A.I.; Novitskii, N.N.; Ketsko, V.A.; Smirnova, M.N.; Almuqrin, A.H.; Sayyed, M.I.; Trukhanov, S.V.; Trukhanov, A.V. A New Approach to the Formation of Nanosized Gold and Beryllium Films by Ion-Beam Sputtering Deposition. Nanomaterials 2022, 12, 470. https://doi.org/10.3390/nano12030470
Sharko SA, Serokurova AI, Novitskii NN, Ketsko VA, Smirnova MN, Almuqrin AH, Sayyed MI, Trukhanov SV, Trukhanov AV. A New Approach to the Formation of Nanosized Gold and Beryllium Films by Ion-Beam Sputtering Deposition. Nanomaterials. 2022; 12(3):470. https://doi.org/10.3390/nano12030470
Chicago/Turabian StyleSharko, Sergei A., Aleksandra I. Serokurova, Nikolai N. Novitskii, Valerii A. Ketsko, Maria N. Smirnova, Aljawhara H. Almuqrin, M. I. Sayyed, Sergei V. Trukhanov, and Alex V. Trukhanov. 2022. "A New Approach to the Formation of Nanosized Gold and Beryllium Films by Ion-Beam Sputtering Deposition" Nanomaterials 12, no. 3: 470. https://doi.org/10.3390/nano12030470
APA StyleSharko, S. A., Serokurova, A. I., Novitskii, N. N., Ketsko, V. A., Smirnova, M. N., Almuqrin, A. H., Sayyed, M. I., Trukhanov, S. V., & Trukhanov, A. V. (2022). A New Approach to the Formation of Nanosized Gold and Beryllium Films by Ion-Beam Sputtering Deposition. Nanomaterials, 12(3), 470. https://doi.org/10.3390/nano12030470








