Targeting the “Sweet Side” of Tumor with Glycan-Binding Molecules Conjugated-Nanoparticles: Implications in Cancer Therapy and Diagnosis
Abstract
:1. Introduction
2. Targeting Nanoparticles to Cancer
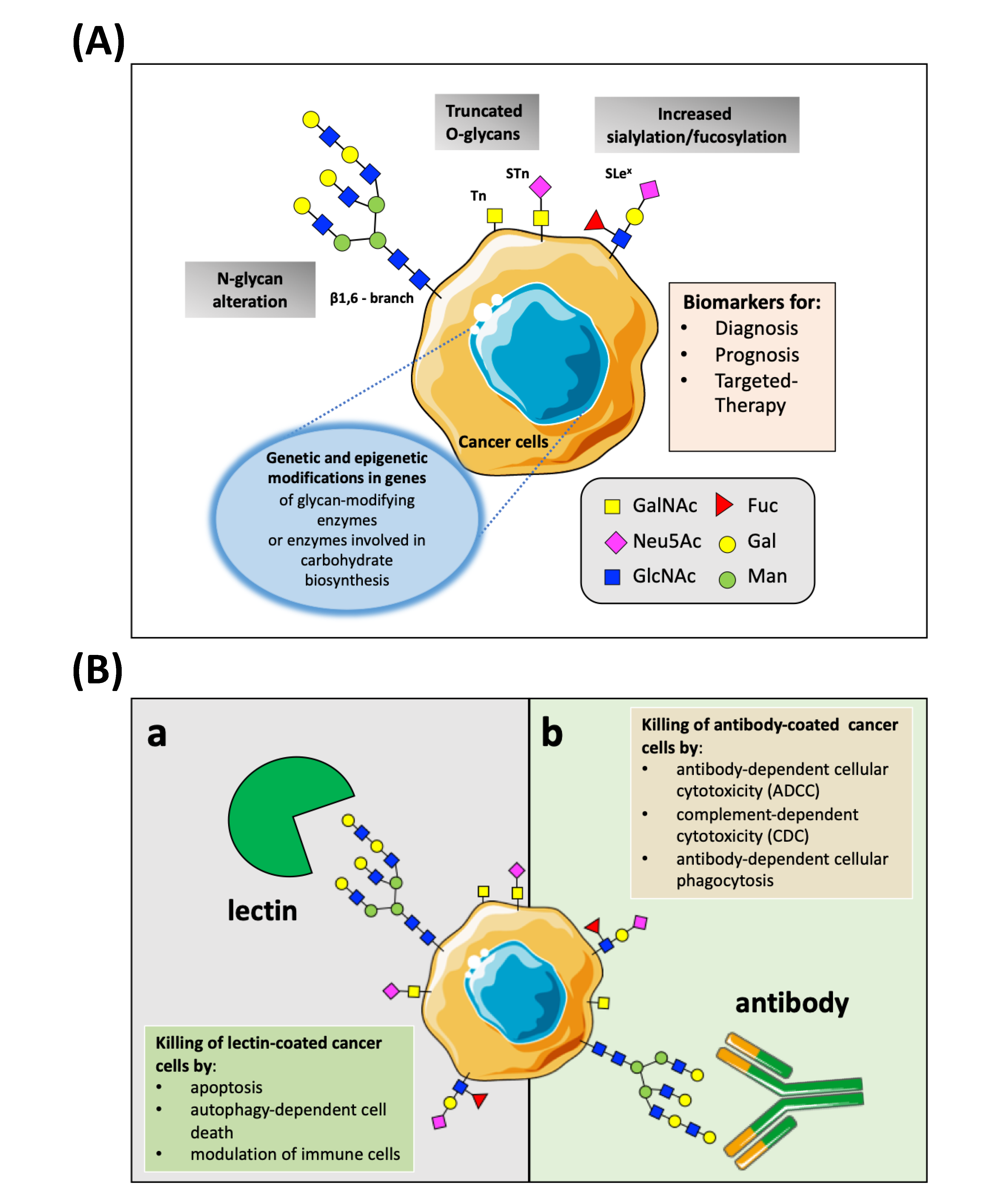
3. Aberrant Glycosylation in Cancer
4. Decorating Nanoparticles Surfaces with TACAs-Binding Molecules
4.1. NPs Conjugated with Natural Lectins: A Well-Trod Path
4.1.1. Lectins
4.1.2. Lectins-NPs
4.2. NPs Conjugated with TACAs-Binding Antibodies: An Explored Road
4.2.1. Anti-TACAs Abs
4.2.2. Anti-TACAs mAbs-NPs
4.3. Other Anti-Glycans Approaches to Target NPs towards Cancer
5. Possible Implications in Cancer Nano-Immunotherapy
6. Potentialities and Obstacles of Anti-Glycan NPs in Cancer Research
7. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Sigdel, K.; Yang, L.; Liu, Y.; Xuan, M.; Wang, X.; Gu, Z.; Wu, J.; Xie, H. Nanotechnology-Based Drug Delivery Systems for Enhanced Diagnosis and Therapy of Oral Cancer. J. Mater. Chem. B 2020, 8, 8781–8793. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Kay, B.K.; Jiang, S.; Chen, S. Nanoparticle Delivery: Targeting and Nonspecific Binding. MRS Bull. 2009, 34, 432–440. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Pearce, A.K.; O’Reilly, R.K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconj. Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug. Deliv. 2017, 2017, 9090325. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-Targeted Therapeutics in Anticancer Therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Bloise, N.; Massironi, A.; Pina, C.D.; Alongi, J.; Siciliani, S.; Manfredi, A.; Biggiogera, M.; Rossi, M.; Ferruti, P.; Ranucci, E.; et al. Extra-Small Gold Nanospheres Decorated with a Thiol Functionalized Biodegradable and Biocompatible Linear Polyamidoamine as Nanovectors of Anticancer Molecules. Front. Bioeng. Biotechnol. 2020, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.D.; Claypool, S.E.; Liu, R. The Smart Targeting of Nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clift, M.J.D.; Rothen-Rutishauser, B.; Brown, D.M.; Duffin, R.; Donaldson, K.; Proudfoot, L.; Guy, K.; Stone, V. The Impact of Different Nanoparticle Surface Chemistry and Size on Uptake and Toxicity in a Murine Macrophage Cell Line. Toxicol. Appl. Pharmacol. 2008, 232, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, A.A.; Sherwood, J.A.; Antone, A.; Coogan, K.R.; Bolding, M.S.; Deb, S.; Bao, Y.; Rao, S.S. Role of Surface Chemistry in Mediating the Uptake of Ultrasmall Iron Oxide Nanoparticles by Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 17157–17166. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Lovas, K.; Rich, M.; Yin, Q.; Lackey, K.; Bolding, M.S.; Bao, Y. Shape-Dependent Cellular Behaviors and Relaxivity of Iron Oxide-Based T1 MRI Contrast Agents. Nanoscale 2016, 8, 17506–17515. [Google Scholar] [CrossRef]
- Nag, S.; Manna, K.; Saha, K.D. Tannic Acid-Stabilized Gold Nano-Particles Are Superior to Native Tannic Acid in Inducing ROS-Dependent Mitochondrial Apoptosis in Colorectal Carcinoma Cells via the P53/AKT Axis. RSC Adv. 2019, 9, 8025–8038. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Ryu, J.H.; Park, J.; Kim, K.; Yang, J.; Lee, H. DNA/Tannic Acid Hybrid Gel Exhibiting Biodegradability, Extensibility, Tissue Adhesiveness, and Hemostatic Ability. Adv. Funct. Mater. 2015, 25, 1270–1278. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N. Single Step Natural Poly(Tannic Acid) Particle Preparation as Multitalented Biomaterial. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 824–834. [Google Scholar] [CrossRef]
- Aguilera, J.R.; Venegas, V.; Oliva, J.M.; Sayagués, M.J.; de Miguel, M.; Sánchez-Alcázar, J.A.; Arévalo-Rodríguez, M.; Zaderenko, A.P. Targeted Multifunctional Tannic Acid Nanoparticles. RSC Adv. 2016, 6, 7279–7287. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Park, J.; Lin, Q.; Weidle, J.H.; Yeo, Y. Development of Quinic Acid-Conjugated Nanoparticles as a Drug Carrier to Solid Tumors. Biomacromolecules 2013, 14, 2389–2395. [Google Scholar] [CrossRef] [Green Version]
- Varki, A. Biological Roles of Glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-M.; Ma, L.; Cao, B.; Lin, J.-Z.; Han, L.; Li, C.-Y.; Xu, R.-C.; Zhang, D.-K. Progress in Research into the Role of Abnormal Glycosylation Modification in Tumor Immunity. Immunol. Lett. 2020, 229, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Leon, F.; Rauth, S.; Batra, S.K.; Ponnusamy, M.P. A Systematic Review on the Implications of O-Linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells 2020, 9, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhu, J.; Lubman, D.M.; Gao, C. Aberrant Glycosylation and Cancer Biomarker Discovery: A Promising and Thorny Journey. Clin. Chem. Lab. Med. 2019, 57, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Song, P.; Tang, W.; Kokudo, N. Cancer-Associated Carbohydrate Antigens for Clinical Diagnostic Markers—Its Effectiveness and Limitations. Drug Discov. Ther. 2015, 9, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.F.; Campos, D.; Reis, C.A.; Gomes, C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer 2020, 6, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered Glycosylation in Cancer: A Promising Target for Biomarkers and Therapeutics. Biochim. Biophys. Acta Rev. Cancer 2020, 1875, 188464. [Google Scholar] [CrossRef]
- Padler-Karavani, V. Aiming at the Sweet Side of Cancer: Aberrant Glycosylation as Possible Target for Personalized-Medicine. Cancer Lett. 2014, 352, 102–112. [Google Scholar] [CrossRef]
- Fernandes, E.; Sores, J.; Cotton, S.; Peixoto, A.; Ferreira, D.; Freitas, R.; Reis, C.A.; Santos, L.L.; Ferreira, J.A. Esophageal, Gastric and Colorectal Cancers: Looking beyond Classical Serological Biomarkers towards Glycoproteomics-Assisted Precision Oncology. Theranostics 2020, 10, 4903–4928. [Google Scholar] [CrossRef]
- Arnaud, J.; Audfray, A.; Imberty, A. Binding Sugars: From Natural Lectins to Synthetic Receptors and Engineered Neolectins. Chem. Soc. Rev. 2013, 42, 4798–4813. [Google Scholar] [CrossRef] [PubMed]
- Tommasone, S.; Allabush, F.; Tagger, Y.K.; Norman, J.; Köpf, M.; Tucker, J.H.R.; Mendes, P.M. The Challenges of Glycan Recognition with Natural and Artificial Receptors. Chem. Soc. Rev. 2019, 48, 5488–5505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhutia, S.K.; Panda, P.K.; Sinha, N.; Praharaj, P.P.; Bhol, C.S.; Panigrahi, D.P.; Mahapatra, K.K.; Saha, S.; Patra, S.; Mishra, S.R.; et al. Plant Lectins in Cancer Therapeutics: Targeting Apoptosis and Autophagy-Dependent Cell Death. Pharmacol. Res. 2019, 144, 8–18. [Google Scholar] [CrossRef]
- Coulibaly, F.S.; Youan, B.-B.C. Current Status of Lectin-Based Cancer Diagnosis and Therapy. AIMS Mol. Sci. 2017, 4, 1–27. [Google Scholar] [CrossRef]
- Poiroux, G.; Barre, A.; Van Damme, E.J.M.; Benoist, H.; Rougé, P. Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy. Int. J. Mol. Sci. 2017, 18, 1232. [Google Scholar] [CrossRef] [Green Version]
- Bovi, M.; Cenci, L.; Perduca, M.; Capaldi, S.; Carrizo, M.E.; Civiero, L.; Chiarelli, L.R.; Galliano, M.; Monaco, H.L. BEL β-trefoil: A novel lectin with antineoplastic properties in king bolete (Boletus edulis) mushrooms. Glycobiology 2013, 23, 578–592. [Google Scholar] [CrossRef] [Green Version]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with Potential for Anti-Cancer Therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-P.; Yang, M.-C.; Liu, H.-S.; Lin, Y.-S.; Lei, H.-Y. Concanavalin A Induces Autophagy in Hepatoma Cells and Has a Therapeutic Effect in a Murine in Situ Hepatoma Model. Hepatology 2007, 45, 286–296. [Google Scholar] [CrossRef]
- Perduca, M.; Carbonare, L.D.; Bovi, M.; Innamorati, G.; Cheri, S.; Cavallini, C.; Scupoli, M.T.; Mori, A.; Valenti, M.T. Runx2 Downregulation, Migration and Proliferation Inhibition in Melanoma Cells Treated with BEL β-Trefoil. Oncol. Rep. 2017, 37, 2209–2214. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.A.; Carvalho, F.C.; Ruas, L.P.; Ricci-Azevedo, R.; Roque-Barreira, M.C. The Immunomodulatory Effect of Plant Lectins: A Review with Emphasis on ArtinM Properties. Glycoconj. J. 2013, 30, 641–657. [Google Scholar] [CrossRef] [Green Version]
- Ernst, E.; Schmidt, K.; Steuer-Vogt, M.K. Mistletoe for Cancer? A Systematic Review of Randomised Clinical Trials. Int. J. Cancer 2003, 107, 262–267. [Google Scholar] [CrossRef]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus Vulgaris Lectins: A Systematic Review of Characteristics and Health Implications. Crit. Rev. Food Sci. Nutr. 2018, 58, 70–83. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-Conjugated PH-Responsive Mesoporous Silica Nanoparticles for Targeted Bone Cancer Treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, J.; Chen, J.; Yang, M.; Wang, H.; Qiao, H.; Chen, Z.; Hu, L.; Di, L.; Li, J. Enhanced Anti-Colon Cancer Efficacy of 5-Fluorouracil by Epigallocatechin-3- Gallate Co-Loaded in Wheat Germ Agglutinin-Conjugated Nanoparticles. Nanomedicine 2019, 21, 102068. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-T.; Souris, J.S.; Cheng, S.-H.; Chu, C.-H.; Wang, Y.-C.; Konda, V.; Dougherty, U.; Bissonnette, M.; Mou, C.-Y.; Chen, C.-T.; et al. Lectin-Functionalized Mesoporous Silica Nanoparticles for Endoscopic Detection of Premalignant Colonic Lesions. Nanomedicine 2017, 13, 1941–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, A.D.; Ganganboina, A.B.; Tsai, Y.; Chiu, H.; Doong, R. Multifunctional GQDs-Concanavalin A@Fe3O4 Nanocomposites for Cancer Cells Detection and Targeted Drug Delivery. Anal. Chim. Acta 2018, 1027, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer Targeting with Biomolecules: A Comparative Study of Photodynamic Therapy Efficacy Using Antibody or Lectin Conjugated Phthalocyanine-PEG Gold Nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Liu, F.; Liu, L.; Duan, T.; Zhang, H.; Wang, Z. Lectin-Conjugated Fe2O3@Au Core@Shell Nanoparticles as Dual Mode Contrast Agents for in Vivo Detection of Tumor. Mol. Pharm. 2014, 11, 738–745. [Google Scholar] [CrossRef]
- Carvalho, M.E.T.; Oliveira, W.F.; Cunha, C.R.A.; Coelho, L.C.B.B.; Silva, M.V.; Junior, L.B.C.; Santos, B.S.; Filho, P.E.C.; Fontes, A.; Correia, M.T.S. Evaluating the Glycophenotype on Breast Cancer Tissues with Quantum Dots-Cramoll Lectin Conjugates. Int. J. Biol. Macromol. 2019, 138, 302–308. [Google Scholar] [CrossRef]
- Bhat, R.; García, I.; Aznar, E.; Arnaiz, B.; Martínez-Bisbal, M.C.; Liz-Marzán, L.M.; Penadés, S.; Martínez-Máñez, R. Lectin-Gated and Glycan Functionalized Mesoporous Silica Nanocontainers for Targeting Cancer Cells Overexpressing Lewis X Antigen. Nanoscale 2017, 10, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Giovampaola, C.D.; Capone, A.; Ermini, L.; Lupetti, P.; Vannuccini, E.; Finetti, F.; Donnini, S.; Ziche, M.; Magnani, A.; Leone, G.; et al. Formulation of Liposomes Functionalized with Lotus Lectin and Effective in Targeting Highly Proliferative Cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Soliman, C.; Yuriev, E.; Ramsland, P.A. Antibody Recognition of Aberrant Glycosylation on the Surface of Cancer Cells. Curr. Opin. Struct. Biol. 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sterner, E.; Flanagan, N.; Gildersleeve, J.C. Perspectives on Anti-Glycan Antibodies Gleaned from Development of a Community Resource Database. ACS Chem. Biol. 2016, 11, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.M.; Hill, E.M.; Al Deghaither, D.; Weiner, L.M. Mechanisms of Action of Therapeutic Antibodies for Cancer. Mol. Immunol. 2015, 67, 28–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, J.X.; Durrant, L. Monoclonal Antibodies against Tumour-Associated Carbohydrate Antigens. Carbohydrate 2017. [Google Scholar] [CrossRef] [Green Version]
- Mantuano, N.R.; Natoli, M.; Zippelius, A.; Läubli, H. Tumor-Associated Carbohydrates and Immunomodulatory Lectins as Targets for Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e001222. [Google Scholar] [CrossRef] [PubMed]
- Keyel, M.E.; Reynolds, C.P. Spotlight on Dinutuximab in the Treatment of High-Risk Neuroblastoma: Development and Place in Therapy. Biologics 2019, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Cheung, N.-K.V. Disialoganglioside GD2 as a Therapeutic Target for Human Diseases. Expert Opin. Ther. Targets 2015, 19, 349–362. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Sugarman, S.; Gelmon, K.A.; Cohen, R.; Saleh, M.; Isaacs, C.; Young, L.; Healey, D.; Onetto, N.; Slichenmyer, W. Randomized Phase II Study of BR96-Doxorubicin Conjugate in Patients with Metastatic Breast Cancer. J. Clin. Oncol. 1999, 17, 478–484. [Google Scholar] [CrossRef]
- Scott, A.M.; Geleick, D.; Rubira, M.; Clarke, K.; Nice, E.C.; Smyth, F.E.; Stockert, E.; Richards, E.C.; Carr, F.J.; Harris, W.J.; et al. Construction, Production, and Characterization of Humanized Anti-Lewis Y Monoclonal Antibody 3S193 for Targeted Immunotherapy of Solid Tumors. Cancer Res. 2000, 60, 3254–3261. [Google Scholar]
- Farrugia, W.; Scott, A.M.; Ramsland, P.A. A Possible Role for Metallic Ions in the Carbohydrate Cluster Recognition Displayed by a Lewis Y Specific Antibody. PLoS ONE 2009, 4, e7777. [Google Scholar] [CrossRef] [PubMed]
- Amon, R.; Rosenfeld, R.; Perlmutter, S.; Grant, O.C.; Yehuda, S.; Borenstein-Katz, A.; Alcalay, R.; Marshanski, T.; Yu, H.; Diskin, R.; et al. Directed Evolution of Therapeutic Antibodies Targeting Glycosylation in Cancer. Cancers 2020, 12, 2824. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, L.L.; Grim, J.C. Glycopolymer Probes of Signal Transduction. Chem. Soc. Rev. 2013, 42, 4476–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, S.; Arye, S.L.B.; Reuven, E.M.; Yu, H.; Costa, C.; Galiñanes, M.; Bottio, T.; Chen, X.; Padler-Karavani, V. Presentation Mode of Glycans Affect Recognition of Human Serum Anti-Neu5Gc IgG Antibodies. Bioconj. Chem. 2019, 30, 161–168. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Qiu, H.; Min, Y.; Rodgers, Z.; Zhang, L.; Wang, A.Z. Nanomedicine Approaches to Improve Cancer Immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1456. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Cai, X.; Huang, Q.; Xiao, J.; Cheng, Y. Targeting Nanoparticles for Diagnosis and Therapy of Bone Tumors: Opportunities and Challenges. Biomaterials 2021, 265, 120404. [Google Scholar] [CrossRef]
- Arruebo, M.; Valladares, M.; González-Fernández, Á. Antibody-Conjugated Nanoparticles for Biomedical Applications. J. Nanomater. 2009, 2009, 439389. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Nogales, C.; Noguera, R.; Couvreur, P.; Blanco-Prieto, M.J. Therapeutic Opportunities in Neuroblastoma Using Nanotechnology. J. Pharmacol. Exp. Ther. 2019, 370, 625–635. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Dolman, E.M.; Wieriks, R.; Sparidans, R.W.; Hennink, W.E.; Kok, R.J. Anti-GD2 Immunoliposomes for Targeted Delivery of the Survivin Inhibitor Sepantronium Bromide (YM155) to Neuroblastoma Tumor Cells. Pharm. Res. 2018, 35, 85. [Google Scholar] [CrossRef] [Green Version]
- Baiu, D.C.; Artz, N.S.; McElreath, M.R.; Menapace, B.D.; Hernando, D.; Reeder, S.B.; Grüttner, C.; Otto, M. High Specificity Targeting and Detection of Human Neuroblastoma Using Multifunctional Anti-GD2 Iron-Oxide Nanoparticles. Nanomedicine 2015, 10, 2973–2988. [Google Scholar] [CrossRef] [Green Version]
- Monterrubio, C.; Paco, S.; Olaciregui, N.G.; Pascual-Pasto, G.; Vila-Ubach, M.; Cuadrado-Vilanova, M.; Ferrandiz, M.M.; Castillo-Ecija, H.; Glisoni, R.; Kuplennik, N.; et al. Targeted Drug Distribution in Tumor Extracellular Fluid of GD2-Expressing Neuroblastoma Patient-Derived Xenografts Using SN-38-Loaded Nanoparticles Conjugated to the Monoclonal Antibody 3F8. J. Control. Release 2017, 255, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 Identifies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleeff, J.; Ishiwata, T.; Kumbasar, A.; Friess, H.; Büchler, M.W.; Lander, A.D.; Korc, M. The Cell-Surface Heparan Sulfate Proteoglycan Glypican-1 Regulates Growth Factor Action in Pancreatic Carcinoma Cells and Is Overexpressed in Human Pancreatic Cancer. J. Clin. Investig. 1998, 102, 1662–1673. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Fan, C.; Zhu, H.; Le, W.; Cui, S.; Chen, X.; Li, W.; Zhang, F.; Huang, Y.; Shi, D.; et al. Glypican-1-Antibody-Conjugated Gd-Au Nanoclusters for FI/MRI Dual-Modal Targeted Detection of Pancreatic Cancer. IJN 2018, 13, 2585–2599. [Google Scholar] [CrossRef] [Green Version]
- Tivnan, A.; Heilinger, T.; Ramsey, J.M.; O’Connor, G.; Pokorny, J.L.; Sarkaria, J.N.; Stringer, B.W.; Day, B.W.; Boyd, A.W.; Kim, E.L.; et al. Anti-GD2-Ch14.18/CHO Coated Nanoparticles Mediate Glioblastoma (GBM)-Specific Delivery of the Aromatase Inhibitor, Letrozole, Reducing Proliferation, Migration and Chemoresistance in Patient-Derived GBM Tumor Cells. Oncotarget 2017, 8, 16605–16620. [Google Scholar] [CrossRef]
- Jiao, P.; Otto, M.; Geng, Q.; Li, C.; Li, F.; Butch, E.R.; Snyder, S.E.; Zhou, H.; Yan, B. Enhancing Both CT Imaging and Natural Killer Cell-Mediated Cancer Cell Killing by a GD2-Targeting Nanoconstruct. J. Mater. Chem. B 2016, 4, 513–520. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, H.; Chen, X.; Song, L.; Cui, W.; Ren, S.; Wang, Y.; Guo, K.; Li, D.; Chen, R.; et al. A GPC1-Targeted and Gemcitabine-Loaded Biocompatible Nanoplatform for Pancreatic Cancer Multimodal Imaging and Therapy. Nanomedicine 2019, 14, 2339–2353. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Y.; Zhang, D.; Wu, M.; Wu, L.; Huang, A.; Yang, H.; Liu, X.; Liu, J. Glypican-3 Antibody Functionalized Prussian Blue Nanoparticles for Targeted MR Imaging and Photothermal Therapy of Hepatocellular Carcinoma. J. Mater. Chem. B 2014, 2, 3686–3696. [Google Scholar] [CrossRef]
- Tang, X.; Chen, L.; Li, A.; Cai, S.; Zhang, Y.; Liu, X.; Jiang, Z.; Liu, X.; Liang, Y.; Ma, D. Anti-GPC3 Antibody-Modified Sorafenib-Loaded Nanoparticles Significantly Inhibited HepG2 Hepatocellular Carcinoma. Drug Deliv. 2018, 25, 1484–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ma, Y.; Guo, Z.; Xing, R.; Liu, Z. Efficient Screening of Glycan-Specific Aptamers Using a Glycosylated Peptide as a Scaffold. Anal. Chem. 2020, 93, 956–963. [Google Scholar] [CrossRef]
- Nabavinia, M.S.; Gholoobi, A.; Charbgoo, F.; Nabavinia, M.; Ramezani, M.; Abnous, K. Anti-MUC1 Aptamer: A Potential Opportunity for Cancer Treatment. Med. Res. Rev. 2017, 37, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mukherjee, P. Muc1: A Multifaceted Oncoprotein with a Key Role in Cancer Progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.S.M.; Matthews, C.S.; Missailidis, S. DNA Aptamers That Bind to MUC1 Tumour Marker: Design and Characterization of MUC1-Binding Single-Stranded DNA Aptamers. Tumour. Biol. 2006, 27, 289–301. [Google Scholar] [CrossRef]
- Sayari, E.; Dinarvand, M.; Amini, M.; Azhdarzadeh, M.; Mollarazi, E.; Ghasemi, Z.; Atyabi, F. MUC1 Aptamer Conjugated to Chitosan Nanoparticles, an Efficient Targeted Carrier Designed for Anticancer SN38 Delivery. Int. J. Pharm. 2014, 473, 304–315. [Google Scholar] [CrossRef]
- Jafari, R.; Zolbanin, N.M.; Majidi, J.; Atyabi, F.; Yousefi, M.; Jadidi-Niaragh, F.; Aghebati-Maleki, L.; Shanehbandi, D.; Zangbar, M.-S.S.; Rafatpanah, H. Anti-Mucin1 Aptamer-Conjugated Chitosan Nanoparticles for Targeted Co-Delivery of Docetaxel and IGF-1R SiRNA to SKBR3 Metastatic Breast Cancer Cells. Iran. Biomed. J. 2019, 23, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Houvast, R.D.; Vankemmelbeke, M.; Durrant, L.G.; Wuhrer, M.; Baart, V.M.; Kuppen, P.J.K.; de Geus-Oei, L.-F.; Vahrmeijer, A.L.; Sier, C.F.M. Targeting Glycans and Heavily Glycosylated Proteins for Tumor Imaging. Cancers 2020, 12, 3870. [Google Scholar] [CrossRef]
- Zhao, H.; Richardson, R.; Talebloo, N.; Mukherjee, P.; Wang, P.; Moore, A. UMUC1-Targeting Magnetic Resonance Imaging of Therapeutic Response in an Orthotropic Mouse Model of Colon Cancer. Mol. Imaging Biol. 2019, 21, 852–860. [Google Scholar] [CrossRef]
- Rossez, Y.; Burtea, C.; Laurent, S.; Gosset, P.; Léonard, R.; Gonzalez, W.; Ballet, S.; Raynal, I.; Rousseaux, O.; Dugué, T.; et al. Early Detection of Colonic Dysplasia by Magnetic Resonance Molecular Imaging with a Contrast Agent Raised against the Colon Cancer Marker MUC5AC. Contrast Media Mol. Imaging 2016, 11, 211–221. [Google Scholar] [CrossRef]
- Yang, W.; Fan, H.; Gao, X.; Gao, S.; Karnati, V.V.R.; Ni, W.; Hooks, W.B.; Carson, J.; Weston, B.; Wang, B. The First Fluorescent Diboronic Acid Sensor Specific for Hepatocellular Carcinoma Cells Expressing Sialyl Lewis X. Chem. Biol. 2004, 11, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Ge, L.; Yuan, K.; Li, C.; Zhen, X.; Cai, W.; Cheng, R.; Jiang, X. Targeting and Microenvironment-Improving of Phenylboronic Acid-Decorated Soy Protein Nanoparticles with Different Sizes to Tumor. Theranostics 2019, 9, 7417–7430. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, H.; Wang, C.; Zhang, J.; Wu, W.; Jiang, X. Phenylboronic Acid-Mediated Tumor Targeting of Chitosan Nanoparticles. Theranostics 2016, 6, 1378–1392. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Li, X.; Ma, Y.; Liu, Z. Targeted Cancer Imaging and Photothermal Therapy via Monosaccharide-Imprinted Gold Nanorods. Chem. Commun. 2017, 53, 6716–6719. [Google Scholar] [CrossRef]
- Wang, S.; Yin, D.; Wang, W.; Shen, X.; Zhu, J.-J.; Chen, H.-Y.; Liu, Z. Targeting and Imaging of Cancer Cells via Monosaccharide-Imprinted Fluorescent Nanoparticles. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug Assay Using Antibody Mimics Made by Molecular Imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef]
- Fernandes, E.; Ferreira, D.; Peixoto, A.; Freitas, R.; Relvas-Santos, M.; Palmeira, C.; Martins, G.; Barros, A.; Santos, L.L.; Sarmento, B.; et al. Glycoengineered Nanoparticles Enhance the Delivery of 5-Fluoroucil and Paclitaxel to Gastric Cancer Cells of High Metastatic Potential. Int. J. Pharm. 2019, 570, 118646. [Google Scholar] [CrossRef]
- Choi, K.Y.; Yoon, H.Y.; Kim, J.-H.; Bae, S.M.; Park, R.-W.; Kang, Y.M.; Kim, I.-S.; Kwon, I.C.; Choi, K.; Jeong, S.Y.; et al. Smart Nanocarrier Based on PEGylated Hyaluronic Acid for Cancer Therapy. ACS Nano 2011, 5, 8591–8599. [Google Scholar] [CrossRef]
- Sampaolesi, S.; Nicotra, F.; Russo, L. Glycans in Nanomedicine, Impact and Perspectives. Future Med. Chem. 2018, 11, 43–60. [Google Scholar] [CrossRef]
- Turcsányi, Á.; Varga, N.; Csapó, E. Chitosan-modified hyaluronic acid-based nanosized drug carriers. Int J Biol Macromol. 2020, 1, 148:218–225. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic Acid-Based Nanomaterials for Cancer Therapy. Polymers 2018, 10, 1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, R.; Gaiteiro, C.; Peixoto, A.; Relvas-Santos, M.; Lima, L.; Santos, L.L.; Ferreira, J.A. CD44 Glycoprotein in Cancer: A Molecular Conundrum Hampering Clinical Applications. Clin. Proteom. 2018, 15, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-Type Lectin Receptors with Multivalent Carbohydrate Ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Mu, Y.; Shields, D.N.; Chauhan, S.C.; Kumar, S.; Cory, T.J.; Yallapu, M.M. Mannose-Decorated Hybrid Nanoparticles for Enhanced Macrophage Targeting. Biochem. Biophys. Rep. 2019, 17, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-Decorated Nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, Y.; Gu, X.; Li, Q. Development of a Hyaluronic Acid-Based Nanocarrier Incorporating Doxorubicin and Cisplatin as a PH-Sensitive and CD44-Targeted Anti-Breast Cancer Drug Delivery System. Front. Pharmacol. 2020, 11, 532457. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef]
- Korangath, P.; Barnett, J.D.; Sharma, A.; Henderson, E.T.; Stewart, J.; Yu, S.-H.; Kandala, S.K.; Yang, C.-T.; Caserto, J.S.; Hedayati, M.; et al. Nanoparticle Interactions with Immune Cells Dominate Tumor Retention and Induce T Cell–Mediated Tumor Suppression in Models of Breast Cancer. Sci. Adv. 2020, 6, eaay1601. [Google Scholar] [CrossRef] [Green Version]
- Baeza, A. Tumor Targeted Nanocarriers for Immunotherapy. Molecules 2020, 25, 1508. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhang, Y.S.; Hobson, D.; Hydbring, P. Nanoparticles for Immune System Targeting. Drug Discov. Today 2017, 22, 1295–1301. [Google Scholar] [CrossRef]
- Irvine, D.J.; Dane, E.L. Enhancing Cancer Immunotherapy with Nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantuano, N.R.; Oliveira-Nunes, M.C.; Alisson-Silva, F.; Dias, W.B.; Todeschini, A.R. Emerging Role of Glycosylation in the Polarization of Tumor-Associated Macrophages. Pharmacol. Res. 2019, 146, 104285. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Asahi, M. Augmented TME O-GlcNAcylation Promotes Tumor Proliferation through the Inhibition of P38 MAPK. Mol. Cancer Res. 2017, 15, 1287–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, D.C. Animal Lectins: A Historical Introduction and Overview. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 187–197. [Google Scholar] [CrossRef]
- Busold, S.; Nagy, N.A.; Tas, S.W.; van Ree, R.; de Jong, E.C.; Geijtenbeek, T.B.H. Various Tastes of Sugar: The Potential of Glycosylation in Targeting and Modulating Human Immunity via C-Type Lectin Receptors. Front. Immunol. 2020, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, L.A.M.; Van Vliet, S.J. A Bitter Sweet Symphony: Immune Responses to Altered O-Glycan Epitopes in Cancer. Biomolecules 2016, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Streng-Ouwehand, I.; Unger, W.W.J.; Van Kooyk, Y. C-Type Lectin Receptors for Tumor Eradication: Future Directions. Cancers 2011, 3, 3169–3188. [Google Scholar] [CrossRef]
- Hockl, P.F.; Wolosiuk, A.; Pérez-Sáez, J.M.; Bordoni, A.V.; Croci, D.O.; Toum-Terrones, Y.; Soler-Illia, G.J.A.A.; Rabinovich, G.A. Glyco-Nano-Oncology: Novel Therapeutic Opportunities by Combining Small and Sweet. Pharmacol. Res. 2016, 109, 45–54. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) as Major Players of the Cancer-Related Inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Bakker, T.; Harris, J.; Tsang, C.; Brown, G.D.; Wormald, M.R.; Gordon, S.; Dwek, R.A.; Rudd, P.M.; Martinez-Pomares, L. Glycosylation Influences the Lectin Activities of the Macrophage Mannose Receptor. J. Biol. Chem. 2005, 280, 32811–32820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanczak, M.A.; Siddiqui, S.S.; Trefny, M.P.; Thommen, D.S.; Boligan, K.F.; von Gunten, S.; Tzankov, A.; Tietze, L.; Lardinois, D.; Heinzelmann-Schwarz, V.; et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Investig. 2018, 128, 4912–4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galectin-3 Is a Negative Regulator of Lipopolysaccharide-Mediated Inflammation|the Journal of Immunology. Available online: https://www.jimmunol.org/content/181/4/2781 (accessed on 11 January 2021).
- Ovais, M.; Guo, M.; Chen, C. Tailoring Nanomaterials for Targeting Tumor-Associated Macrophages. Adv. Mater. 2019, 31, e1808303. [Google Scholar] [CrossRef]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel Insights into the Function of CD24: A Driving Force in Cancer. Int. J. Cancer 2021, 148, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Ayre, D.C.; Pallegar, N.K.; Fairbridge, N.A.; Canuti, M.; Lang, A.S.; Christian, S.L. Analysis of the Structure, Evolution, and Expression of CD24, an Important Regulator of Cell Fate. Gene 2016, 590, 324–337. [Google Scholar] [CrossRef]
- Polli, C.D.; Ruas, L.P.; Veronez, L.C.; Geraldino, T.H.; de Morais, F.R.; Roque-Barreira, M.C.; da-Silva, G.P. Jacalin-Activated Macrophages Exhibit an Antitumor Phenotype. Biomed. Res. Int. 2016, 2016, 2925657. [Google Scholar] [CrossRef] [Green Version]
- Bhutia, S.K.; Mallick, S.K.; Maiti, T.K. In Vitro Immunostimulatory Properties of Abrus Lectins Derived Peptides in Tumor Bearing Mice. Phytomedicine 2009, 16, 776–782. [Google Scholar] [CrossRef]
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.; Almogren, A.; Rittenhouse-Olson, K. Cancer Vaccines and Carbohydrate Epitopes. Vaccine 2011, 29, 8802–8826. [Google Scholar] [CrossRef] [Green Version]
- Polonskaya, Z.; Savage, P.B.; Finn, M.G.; Teyton, L. High-Affinity Anti-Glycan Antibodies: Challenges and Strategies. Curr. Opin. Immunol. 2019, 59, 65–71. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Villarreal, E.; Li, G.G.; Zhang, Q.; Fu, X.; Wang, H. Nanoscale Surface Curvature Effects on Ligand–Nanoparticle Interactions: A Plasmon-Enhanced Spectroscopic Study of Thiolated Ligand Adsorption, Desorption, and Exchange on Gold Nanoparticles. Nano Lett. 2017, 17, 4443–4452. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xu, C.; Yin, H.; Hill, J.; Pi, F.; Guo, P. Tuning the Size, Shape and Structure of RNA Nanoparticles for Favorable Cancer Targeting and Immunostimulation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1582. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, e1801451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, T.; Yu, W.; Ruan, S.; He, Q.; Gao, H. Ligand Size and Conformation Affect the Behavior of Nanoparticles Coated with in Vitro and in Vivo Protein Corona. ACS Appl. Mater. Interfaces 2018, 10, 9094–9103. [Google Scholar] [CrossRef] [PubMed]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef] [Green Version]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(Ethylene Glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M.; Ding, J. The Interaction between Nanoparticles-Protein Corona Complex and Cells and Its Toxic Effect on Cells. Chemosphere 2020, 245, 125624. [Google Scholar] [CrossRef]
| Lectin | Target | Cancer Type | Nanoparticles | Application | Reference |
|---|---|---|---|---|---|
| ConA | TF antigen | Bone cancer | DOX-loaded MSNs | Targeting Therapy | [43] |
| WGA | Neu5Ac GlcNac | Colon adenocarcinoma | 5-FU-EGCG -gelatin-chitosan NPs | Targeting Therapy | [44] |
| UEA I | α-L-fucose | Colorectal cancer | Fluorescent MSNs | Targeting Diagnosis | [45] |
| ConA | TF antigen | Adenocarcinoma of the cervix | DOX-loaded graphene QDs -Fe3O4 | Targeting Therapy Diagnosis | [46] |
| JCA | TF antigen | Colorectal and breast adenocarcinoma | Phthalocyanine- PEG-AuNPs | Targeting Therapy | [47] |
| ConA; RCA; WGA | TF antigen; β-D-galactose; Neu5Ac and GlcNac | Colorectal cancer | Fe2O3 -AuNPs | Targeting Diagnosis | [48] |
| Cramoll | glucose/mannose | Fibroadenoma and invasive ductal carcinoma human breast tissue | QDs | Targeting Diagnosis | [49] |
| ALL | LeX | Colon cancer | ATTO 430LS dye-loaded MSNs | Targeting Diagnosis Therapy | [50] |
| LTL | α-1,2-linked fucose | Prostate carcinoma and melanoma | DOX-loaded liposomes | Targeting Therapy | [51] |
| Antibody | Target | Cancer Type | Nanoparticles | Application | Reference |
|---|---|---|---|---|---|
| hu14.18K322A mAb | GD2 | neuroblastoma | iron-oxide NPs | Targeting Diagnosis | [71] |
| mAb 3F8 | GD2 | neuroblastoma | SN-38 loaded polymeric NPs | Targeting Therapy | [72] |
| ch14.18/CHO | GD2 | glioblastoma | PLGA nanoparticles | Targeting Therapy | [77] |
| hu14.18K322A | GD2 | neuroblastoma and melanoma cancers | AuNPs | Targeting Diagnosis Therapy | [78] |
| GPC-1 mAb | GPC-1 | pancreatic cancer | Gd-Au nanoclusters | Targeting Diagnosis | [76] |
| GPC-1 mAb | GPC-1 | pancreatic cancer | GEM-loaded multifunctional Au nanocarrier | Targeting Diagnosis Therapy | [79] |
| GPC-3 mAb | GPC-3 | hepatocellular carcinoma | PBNPs | Targeting Diagnosis Therapy | [80] |
| GPC-3 mAb | GPC-3 | hepatocellular carcinoma | SFB-loaded polymeric NPs | Targeting Therapy | [81] |
| Targeting Moiety | Target | Cancer Type | NPs | Application | Reference |
|---|---|---|---|---|---|
| Apt | MUC1 | breast cancer | chitosan NPs | Targeting Therapy | [87] |
| Heptapeptide | MUC5AC | gastric cancer | USPIOs | Targeting Diagnosis | [90] |
| Phenylboronic Acid | sialic acid | neuroblastoma | chitosan NPs | Targeting Therapy | [93] |
| Monosaccharide-imprinted polymer | sialic acid, fucose or mannose | hepatoma carcinoma and breast cancer | fluorescent silica NPs | Targeting Diagnosis | [95] |
| HA | CD44 | breast cancer | HA nanocarrier | Targeting Therapy | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, N.; Okkeh, M.; Restivo, E.; Della Pina, C.; Visai, L. Targeting the “Sweet Side” of Tumor with Glycan-Binding Molecules Conjugated-Nanoparticles: Implications in Cancer Therapy and Diagnosis. Nanomaterials 2021, 11, 289. https://doi.org/10.3390/nano11020289
Bloise N, Okkeh M, Restivo E, Della Pina C, Visai L. Targeting the “Sweet Side” of Tumor with Glycan-Binding Molecules Conjugated-Nanoparticles: Implications in Cancer Therapy and Diagnosis. Nanomaterials. 2021; 11(2):289. https://doi.org/10.3390/nano11020289
Chicago/Turabian StyleBloise, Nora, Mohammad Okkeh, Elisa Restivo, Cristina Della Pina, and Livia Visai. 2021. "Targeting the “Sweet Side” of Tumor with Glycan-Binding Molecules Conjugated-Nanoparticles: Implications in Cancer Therapy and Diagnosis" Nanomaterials 11, no. 2: 289. https://doi.org/10.3390/nano11020289
APA StyleBloise, N., Okkeh, M., Restivo, E., Della Pina, C., & Visai, L. (2021). Targeting the “Sweet Side” of Tumor with Glycan-Binding Molecules Conjugated-Nanoparticles: Implications in Cancer Therapy and Diagnosis. Nanomaterials, 11(2), 289. https://doi.org/10.3390/nano11020289







