Effects of Graphite Oxide Nanoparticle Size on the Functional Properties of Layer-by-Layer Coated Flexible Foams
Abstract
1. Introduction
2. Materials and Methods
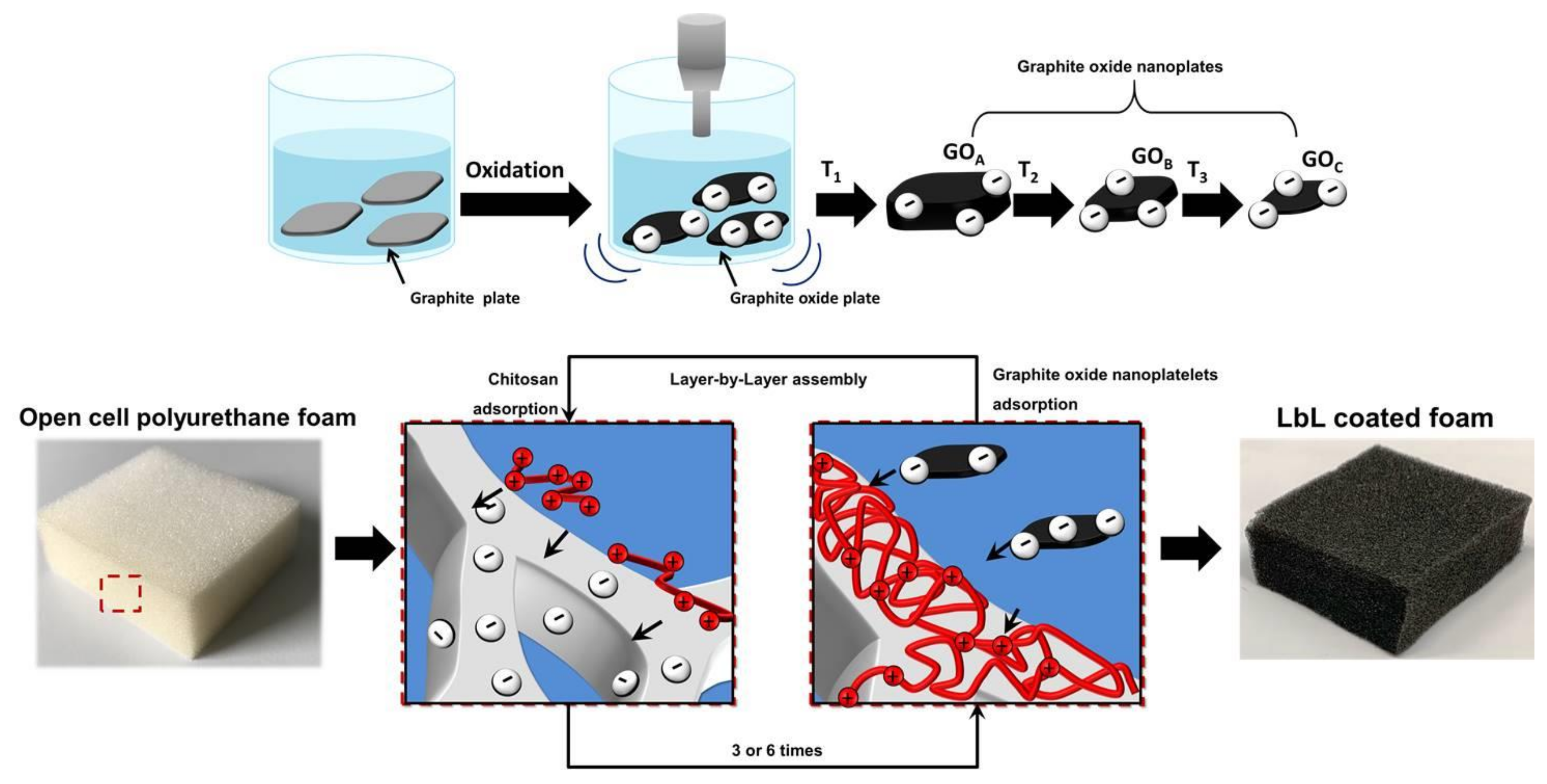
2.1. Layer-by-Layer Deposition
2.2. Characterization
3. Results
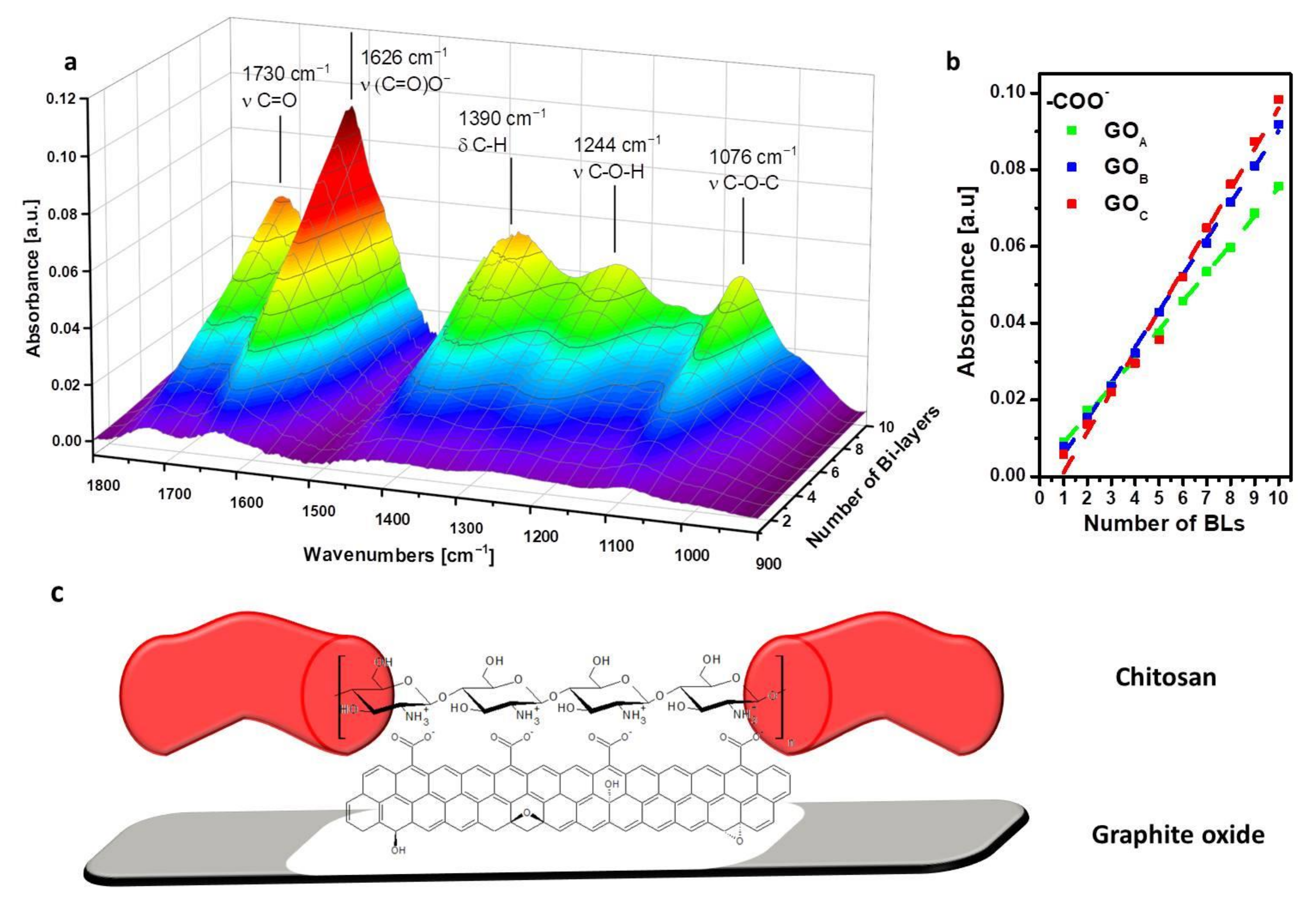
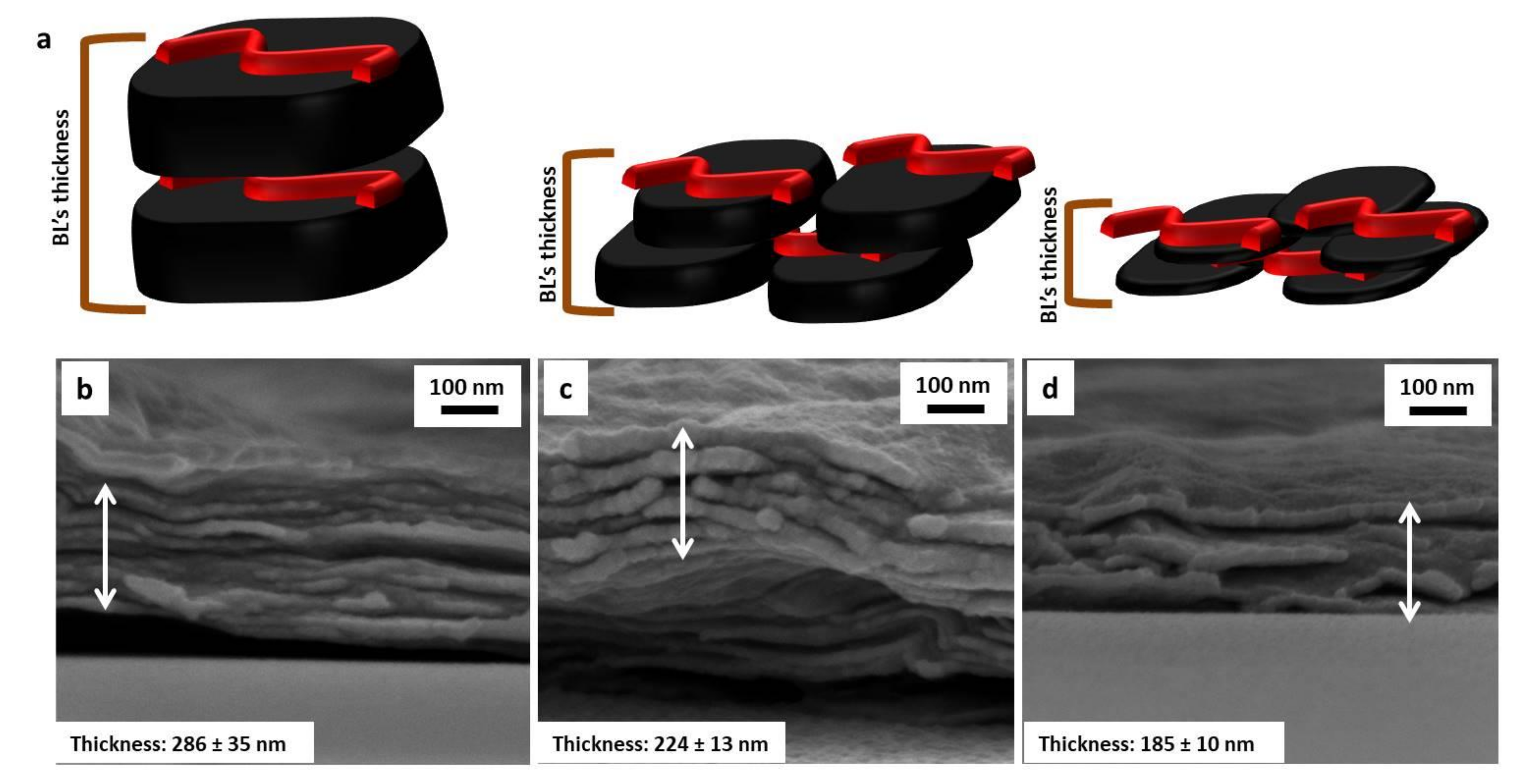
3.1. Nanoplate Dimensions Trend, Layer-by-Layer Growth and Characterization
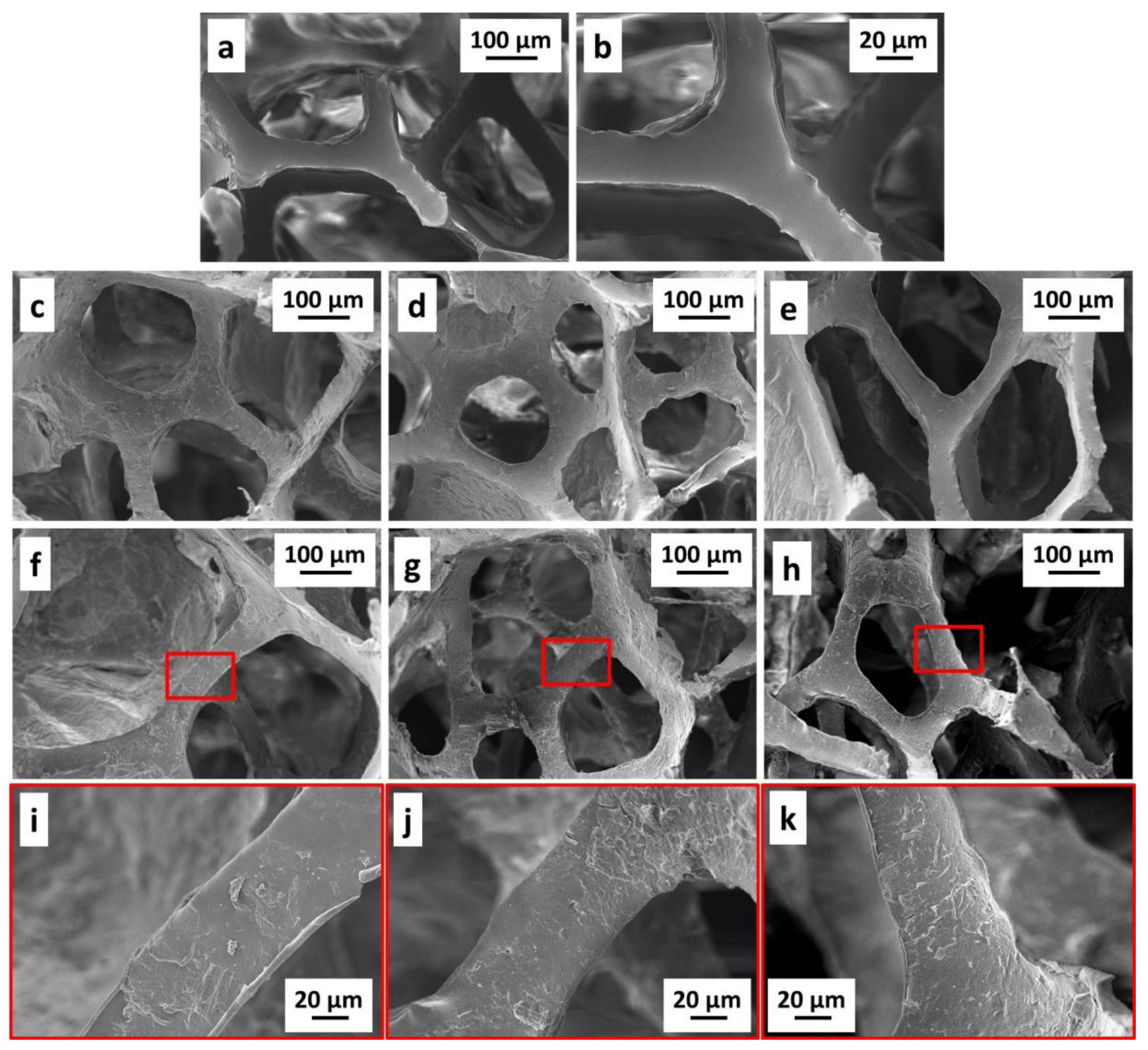
3.2. Morphology on PU Foams
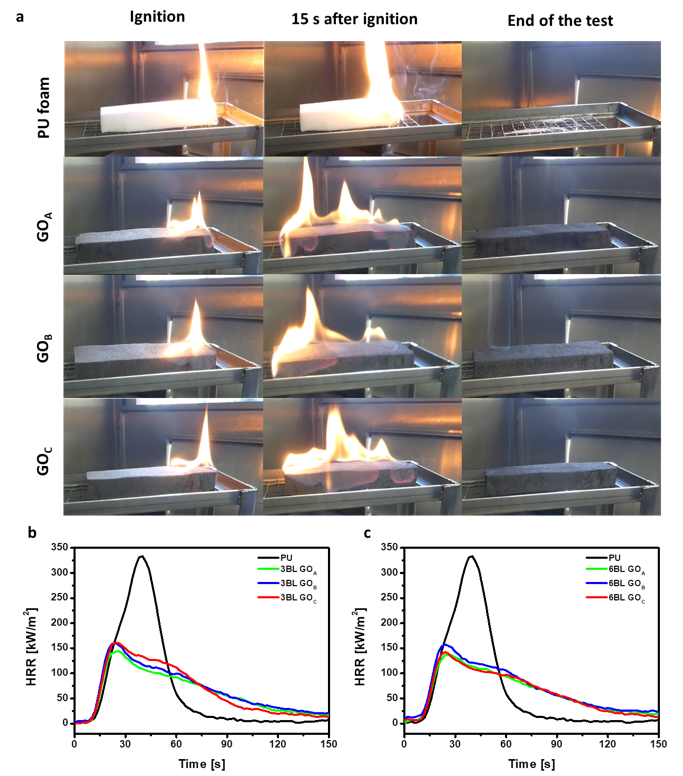
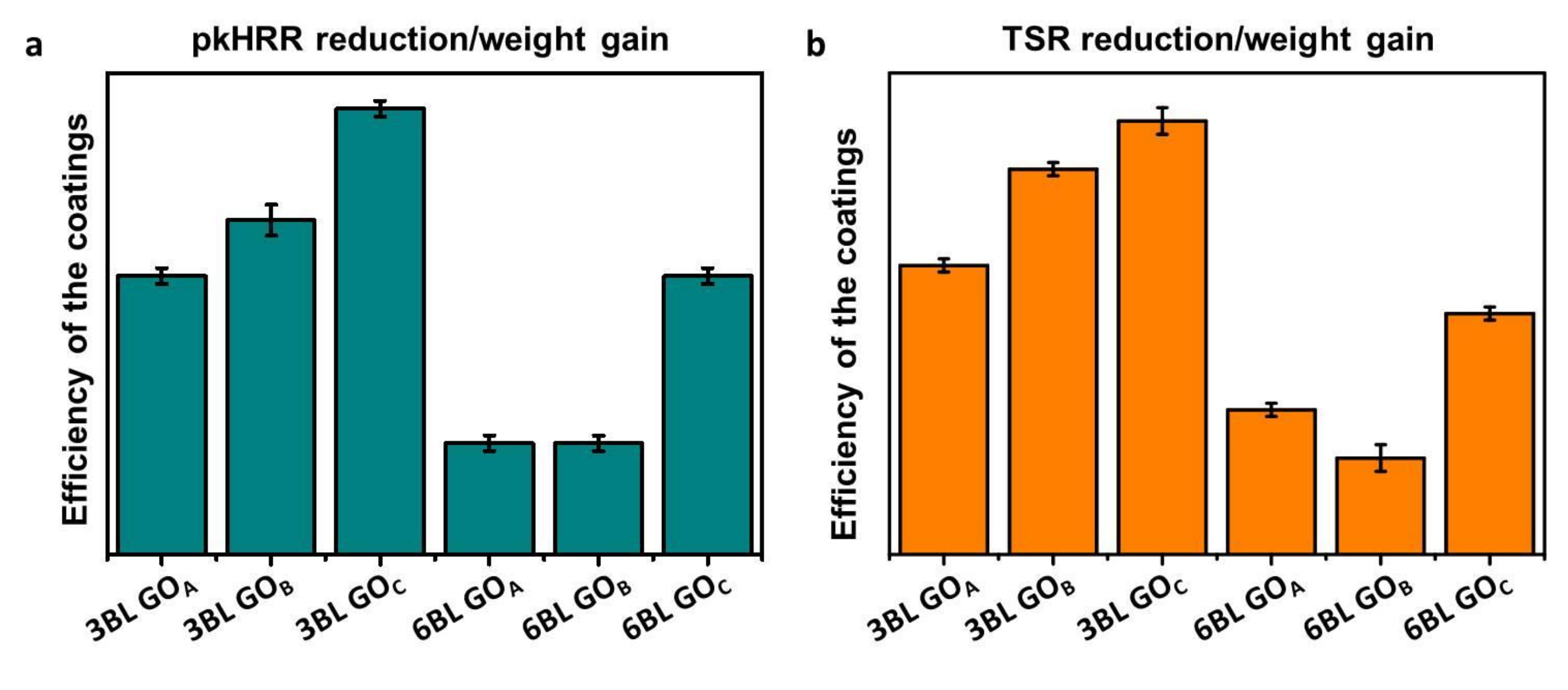
3.3. Flame-Retardant Properties
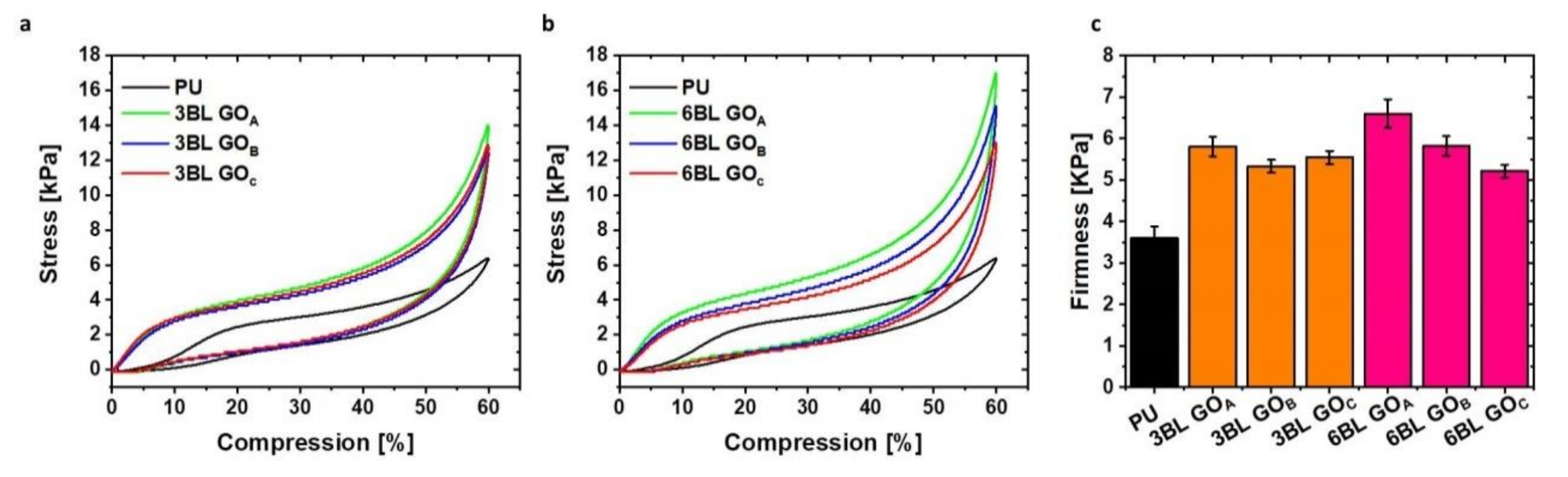
3.4. Mechanical Behavior of LbL-Treated Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randall, D.; Lee, S. The Polyurethanes Book; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Patten, W.N.; Sha, S.; Mo, C. A Vibrational Model of Open Celled Polyurethane Foam Automotive Seat Cushions. J. Sound Vib. 1998, 217, 145–161. [Google Scholar] [CrossRef]
- Kramer, R.H.; Zammarano, M.; Linteris, G.T.; Gedde, U.W.; Gilman, J.W. Heat release and structural collapse of flexible polyurethane foam. Polym. Degrad. Stab. 2010, 95, 1115–1122. [Google Scholar] [CrossRef]
- Cao, X.; James Lee, L.; Widya, T.; Macosko, C. Polyurethane/clay nanocomposites foams: Processing, structure and properties. Polymer 2005, 46, 775–783. [Google Scholar] [CrossRef]
- Harikrishnan, G.; Singh, S.N.; Kiesel, E.; Macosko, C.W. Nanodispersions of carbon nanofiber for polyurethane foaming. Polymer 2010, 51, 3349–3353. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F. 7—Flame retardancy of flexible polyurethane foams: Traditional approaches versus layer-by-layer assemblies. In Novel Fire Retardant Polymers and Composite Materials; Wang, D.-Y., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 171–200. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Malucelli, G.; Carosio, F.; Alongi, J.; Fina, A.; Frache, A.; Camino, G. Materials engineering for surface-confined flame retardancy. Mater. Sci. Eng. R Rep. 2014, 84, 1–20. [Google Scholar] [CrossRef]
- Lazar, S.; Carosio, F.; Davesne, A.-L.; Jimenez, M.; Bourbigot, S.; Grunlan, J. Extreme Heat Shielding of Clay/Chitosan Nanobrick Wall on Flexible Foam. ACS Appl. Mater. Inter 2018, 10, 31686–31696. [Google Scholar] [CrossRef] [PubMed]
- Battegazzore, D.; Frache, A.; Carosio, F. Layer-by-Layer nanostructured interphase produces mechanically strong and flame retardant bio-composites. Compos. Part B Eng. 2020, 200, 108310. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lazar, S.; Kolibaba, T.J.; Shen, R.; Quan, Y.; Wang, Q.; Chiang, H.-C.; Palen, B.; Grunlan, J.C. Environmentally Benign and Self-Extinguishing Multilayer Nanocoating for Protection of Flammable Foam. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, S.S.; Rubner, M.F. pH-Dependent Thickness Behavior of Sequentially Adsorbed Layers of Weak Polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Berndt, P.; Kurihara, K.; Kunitake, T. Adsorption of poly(styrenesulfonate) onto an ammonium monolayer on mica: A surface forces study. Langmuir 1992, 8, 2486–2490. [Google Scholar] [CrossRef]
- Carosio, F.; Negrell-Guirao, C.; Di Blasio, A.; Alongi, J.; David, G.; Camino, G. Tunable thermal and flame response of phosphonated oligoallylamines layer by layer assemblies on cotton. Carbohydr. Polym. 2015, 115, 752–759. [Google Scholar] [CrossRef]
- Tan, H.L.; McMurdo, M.J.; Pan, G.; Van Patten, P.G. Temperature Dependence of Polyelectrolyte Multilayer Assembly. Langmuir 2003, 19, 9311–9314. [Google Scholar] [CrossRef]
- McAloney, R.A.; Sinyor, M.; Dudnik, V.; Goh, M.C. Atomic Force Microscopy Studies of Salt Effects on Polyelectrolyte Multilayer Film Morphology. Langmuir 2001, 17, 6655–6663. [Google Scholar] [CrossRef]
- Carosio, F.; Di Pierro, A.; Alongi, J.; Fina, A.; Saracco, G. Controlling the melt dripping of polyester fabrics by tuning the ionic strength of polyhedral oligomeric silsesquioxane and sodium montmorillonite coatings assembled through Layer by Layer. J. Colloid Interface Sci. 2018, 510, 142–151. [Google Scholar] [CrossRef]
- Carosio, F.; Maddalena, L.; Gomez, J.; Saracco, G.; Fina, A. Graphene Oxide Exoskeleton to Produce Self-Extinguishing, Nonignitable, and Flame Resistant Flexible Foams: A Mechanically Tough Alternative to Inorganic Aerogels. Adv. Mater. Interfaces 2018, 5, 1801288. [Google Scholar] [CrossRef]
- Maddalena, L.; Carosio, F.; Gomez, J.; Saracco, G.; Fina, A. Layer-by-layer assembly of efficient flame retardant coatings based on high aspect ratio graphene oxide and chitosan capable of preventing ignition of PU foam. Polym. Degrad. Stab. 2018, 152, 1–9. [Google Scholar] [CrossRef]
- Cain, A.A.; Nolen, C.R.; Li, Y.-C.; Davis, R.; Grunlan, J.C. Phosphorous-filled nanobrick wall multilayer thin film eliminates polyurethane melt dripping and reduces heat release associated with fire. Polym. Degrad. Stab. 2013, 98, 2645–2652. [Google Scholar] [CrossRef]
- Holder, K.M.; Huff, M.E.; Cosio, M.N.; Grunlan, J.C. Intumescing multilayer thin film deposited on clay-based nanobrick wall to produce self-extinguishing flame retardant polyurethane. J. Mater. Sci. 2015, 50, 2451–2458. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay–Chitosan Nanobrick Walls: Completely Renewable Gas Barrier and Flame-Retardant Nanocoatings. ACS Appl. Mater. Interfaces 2012, 4, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Neisius, N.M.; Gaan, S. Recent developments in flame retardant polymeric coatings. Prog. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Cain, A.A.; Plummer, M.G.B.; Murray, S.E.; Bolling, L.; Regev, O.; Grunlan, J.C. Iron-containing, high aspect ratio clay as nanoarmor that imparts substantial thermal/flame protection to polyurethane with a single electrostatically-deposited bilayer. J. Mater. Chem. A 2014, 2, 17609–17617. [Google Scholar] [CrossRef]
- Liu, X.; Qin, S.; Li, H.; Sun, J.; Gu, X.; Zhang, S.; Grunlan, J.C. Combination Intumescent and Kaolin-Filled Multilayer Nanocoatings That Reduce Polyurethane Flammability. Macromol. Mater. Eng. 2019, 304, 1800531. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R.; et al. Production and processing of graphene and related materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Dideikin, A.T.; Kirilenko, D.A.; Baidakova, M.V.; Shnitov, V.V.; Roth, F.; Konyakhin, S.V.; Besedina, N.A.; Pavlov, S.I.; Kuricyn, R.A.; et al. Facile reduction of graphene oxide suspensions and films using glass wafers. Sci. Rep. 2018, 8, 14154. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Villaro, E.; Navas, A.; Recio, I. Testing the influence of the temperature, RH and filler type and content on the universal power law for new reduced graphene oxide TPU composites. Mater. Res. Express 2017, 4, 105020. [Google Scholar] [CrossRef]
- Gonçalves, G.; Vila, M.; Bdikin, I.; de Andrés, A.; Emami, N.; Ferreira, R.A.S.; Carlos, L.D.; Grácio, J.; Marques, P.A.A.P. Breakdown into nanoscale of graphene oxide: Confined hot spot atomic reduction and fragmentation. Sci. Rep. 2014, 4, 6735. [Google Scholar] [CrossRef] [PubMed]
- Rudyak, V.Y.; Krasnolutskii, S.L. Dependence of the viscosity of nanofluids on nanoparticle size and material. Phys. Lett. A 2014, 378, 1845–1849. [Google Scholar] [CrossRef]
- Tesfai, W.; Singh, P.; Shatilla, Y.; Iqbal, M.Z.; Abdala, A.A. Rheology and microstructure of dilute graphene oxide suspension. J. Nanopart. Res. 2013, 15, 1989. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Silva, S.M.; Braga, C.R.; Fook, M.V.; Raposo, C.M.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies—Table and Charts; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hu, M.; Mi, B. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Li, Y.-C.; Kim, Y.S.; Shields, J.; Davis, R. Controlling polyurethane foam flammability and mechanical behaviour by tailoring the composition of clay-based multilayer nanocoatings. J. Mater. Chem. A 2013, 1, 12987–12997. [Google Scholar] [CrossRef]
- Carosio, F.; Ghanadpour, M.; Alongi, J.; Wågberg, L. Layer-by-layer-assembled chitosan/phosphorylated cellulose nanofibrils as a bio-based and flame protecting nano-exoskeleton on PU foams. Carbohydr. Polym. 2018, 202, 479–487. [Google Scholar] [CrossRef]
| Sample | pH | Viscosity (mPa∙s) | D50 * (µm) |
|---|---|---|---|
| GOA | 1.58 ± 0.08 | 10.8 ± 0.4 | 61 ± 2 |
| GOB | 1.51 ± 0.03 | 30.5 ± 0.7 | 39 ± 2 |
| GOC | 1.39 ± 0.06 | 77.5 ± 3.5 | 34 ± 3 |
| Sample | Flammability | Cone Calorimetry | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight Gain (%) | Melt Dripping | Flame Spread Rate (mm/s) | Residue (%) | TTI ± σ (s) | pkHRR (kW/m2) | THR (MJ/m2) | TSR (m2/m2) | Residue (%) | |
| PU | Yes | 4.9 ± 0.4 | - | 3 ± 1 | 308 ± 25 | 10 ± 1 | 170 ± 11 | 7 ± 1 | |
| 3BL CHIT/GOA | 11 ± 2 | No | 7.3 ± 0.3 | 67 ± 1 | 2 ± 1 | 149 ± 3 | 9 ± 0.1 | 54 ± 1 | 11 ± 1 |
| 3BL CHIT/GOB | 8 ± 1 | No | 6.5 ± 0.4 | 67 ± 1 | 2 ± 1 | 163 ± 19 | 10 ± 2 | 56 ± 12 | 10 ± 1 |
| 3BL CHIT/GOC | 6 ± 1 | No | 5.9 ± 0.7 | 63 ± 1 | 2 ± 1 | 163 ± 11 | 9 ± 0.3 | 81 ± 10 | 8 ± 1 |
| 6BL CHIT/GOA | 27 ± 2 | No | 4.2 ± 0.1 | 80 ± 1 | 2 ± 1 | 143 ± 4 | 9 ± 0.6 | 40 ± 3 | 12 ± 1 |
| 6BL CHIT/GOB | 24 ± 2 | No | 4.3 ± 0.1 | 80 ± 1 | 2 ± 1 | 154 ± 9 | 11 ± 1.5 | 74 ± 27 | 12 ± 1 |
| 6BL CHIT/GOC | 12 ± 1 | No | 5.5 ± 0.6 | 70 ± 1 | 2 ± 1 | 143 ± 7 | 10 ± 1 | 64 ± 8 | 11 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddalena, L.; Gomez, J.; Fina, A.; Carosio, F. Effects of Graphite Oxide Nanoparticle Size on the Functional Properties of Layer-by-Layer Coated Flexible Foams. Nanomaterials 2021, 11, 266. https://doi.org/10.3390/nano11020266
Maddalena L, Gomez J, Fina A, Carosio F. Effects of Graphite Oxide Nanoparticle Size on the Functional Properties of Layer-by-Layer Coated Flexible Foams. Nanomaterials. 2021; 11(2):266. https://doi.org/10.3390/nano11020266
Chicago/Turabian StyleMaddalena, Lorenza, Julio Gomez, Alberto Fina, and Federico Carosio. 2021. "Effects of Graphite Oxide Nanoparticle Size on the Functional Properties of Layer-by-Layer Coated Flexible Foams" Nanomaterials 11, no. 2: 266. https://doi.org/10.3390/nano11020266
APA StyleMaddalena, L., Gomez, J., Fina, A., & Carosio, F. (2021). Effects of Graphite Oxide Nanoparticle Size on the Functional Properties of Layer-by-Layer Coated Flexible Foams. Nanomaterials, 11(2), 266. https://doi.org/10.3390/nano11020266







