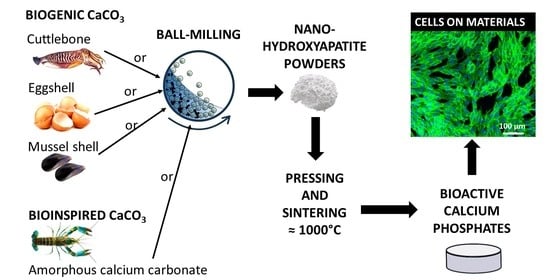
Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Materials Characterization
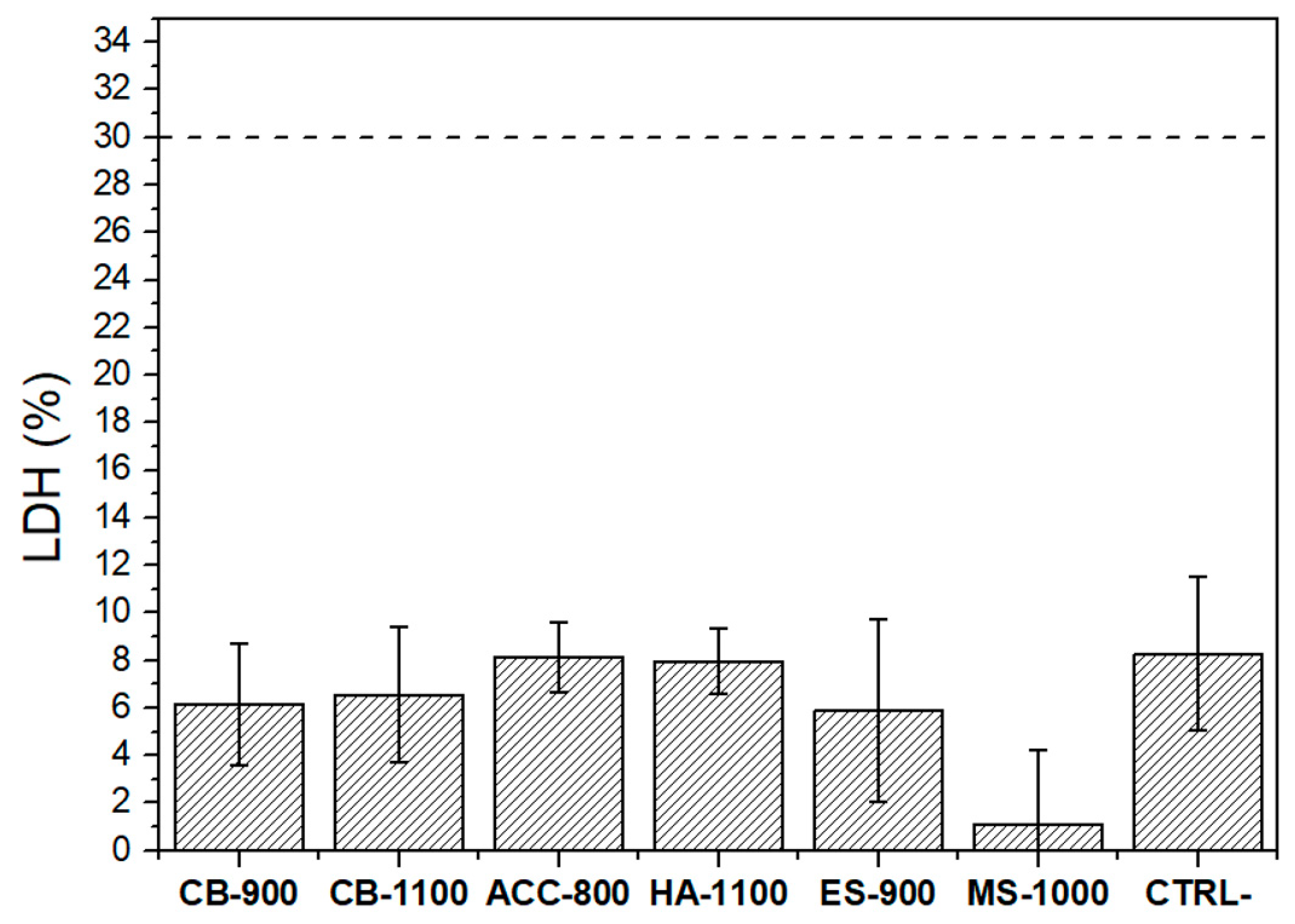
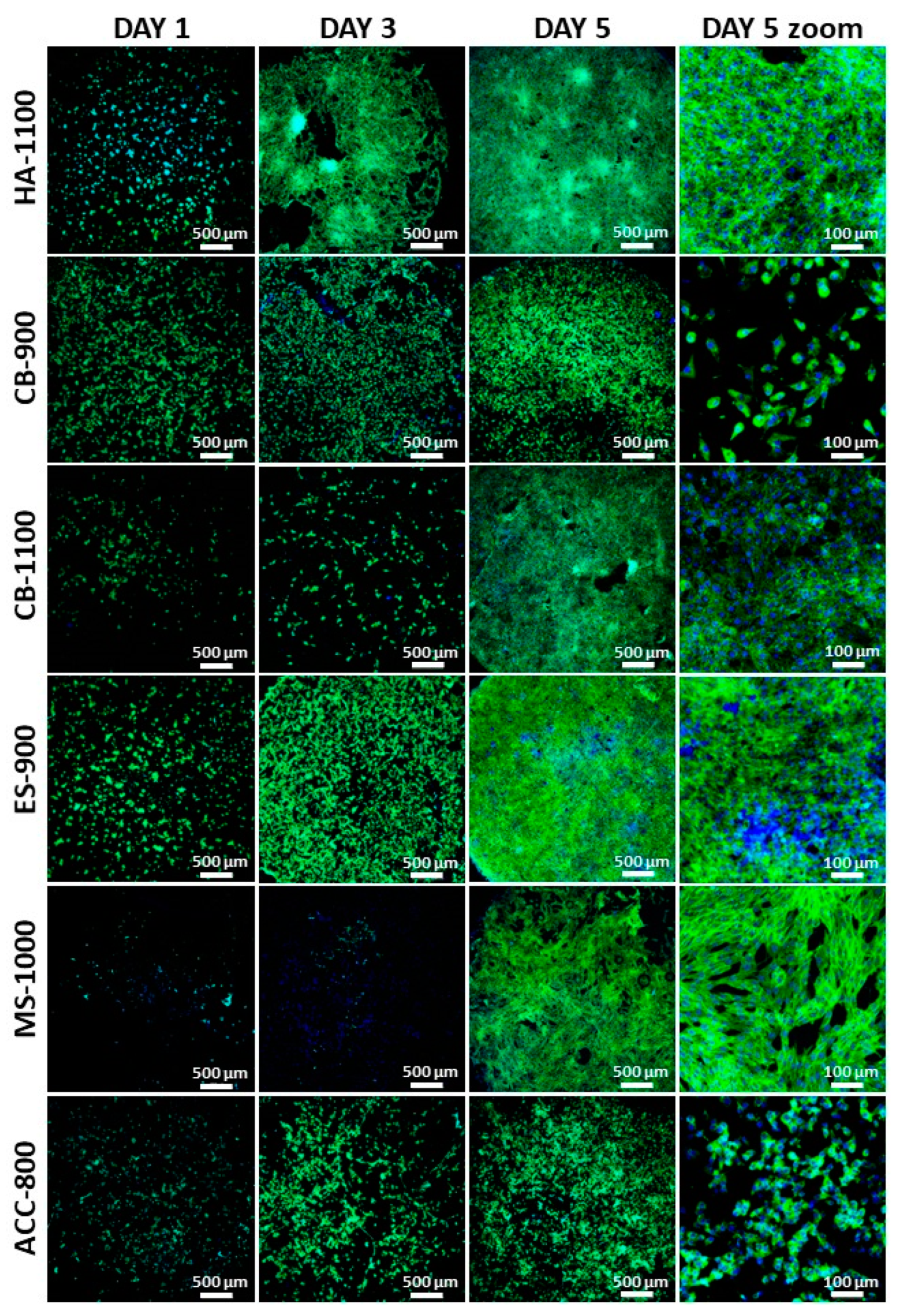
3.2. In Vitro Biological Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeGeros, R.Z.; LeGeros, J.P. Phosphate Minerals in Human Tissues. Photosphate Miner. 1984, 351–385. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Clarke, S.; Walsh, P. Marine Organisms for Bone Repair and Regeneration; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 294–318. [Google Scholar]
- Pierantozzi, D.; Scalzone, A.; Jindal, S.; Stīpniece, L.; Šalma-Ancāne, K.; Dalgarno, K.; Gentile, P.; Mancuso, E. 3D printed Sr-containing composite scaffolds: Effect of structural design and material formulation towards new strategies for bone tissue engineering. Compos. Sci. Technol. 2020, 191, 108069. [Google Scholar] [CrossRef]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Landi, E.; Uggeri, J.; Medri, V.; Guizzardi, S. Sr, Mg cosubstituted HA porous macro-granules: Potentialities as resorbable bone filler with antiosteoporotic functions. J. Biomed. Mater. Res. Part A 2013, 101, 2481–2490. [Google Scholar] [CrossRef]
- Sader, M.S.; LeGeros, R.Z.; Soares, G.A. Human osteoblasts adhesion and proliferation on magnesium-substituted tricalcium phosphate dense tablets. J. Mater. Sci. Mater. Med. 2009, 20, 521–527. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Supová, M. Isolation and preparation of nanoscale bioapatites from natural sources: A review. J. Nanosci. Nanotechnol. 2014, 14, 546–563. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.C.; Yoon, Y.S. Characteristics of calcium phosphate powders synthesized from cuttlefish bone and phosphoric acid. J. Ceram. Process. Res. 2007, 8, 427–430. [Google Scholar]
- Ivankovic, H.; Tkalčec, E.; Orlić, S.; Ferrer, G.G.; Schauperl, Z. Hydroxyapatite formation from cuttlefish bones: Kinetics. J. Mater. Sci. Mater. Electron. 2010, 21, 2711–2722. [Google Scholar] [CrossRef]
- Ivankovic, H.; Ferrer, G.G.; Tkalcec, E.; Orlic, S. Preparation of highly porous hydroxyapatite from cuttlefish bone. J. Mater. Sci. Mater. Med. 2009, 20, 1039–1046. [Google Scholar] [CrossRef]
- Tkalčec, E.; Popović, J.; Orlić, S.; Milardović, S.; Ivanković, H. Hydrothermal synthesis and thermal evolution of carbonate-fluorhydroxyapatite scaffold from cuttlefish bones. Mater. Sci. Eng. C 2014, 42, 578–586. [Google Scholar] [CrossRef]
- Reinares-Fisac, D.; Veintemillas-Verdaguer, S.; Fernández-Díaz, L. Conversion of biogenic aragonite into hydroxyapatite scaffolds in boiling solutions. CrystEngComm 2016, 19, 110–116. [Google Scholar] [CrossRef]
- Venkatesan, J.; Rekha, P.D.; Anil, S.; Bhatnagar, I.; Sudha, P.N.; Dechsakulwatana, C.; Kim, S.-K.; Shim, M.S. Hydroxyapatite from Cuttlefish Bone: Isolation, Characterizations, and Applications. Biotechnol. Bioprocess Eng. 2018, 23, 383–393. [Google Scholar] [CrossRef]
- Milovac, D.; Ferrer, G.G.; Ivankovic, M.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity. Mater. Sci. Eng. C 2014, 34, 437–445. [Google Scholar] [CrossRef]
- Rocha, J.; Lemos, A.; Agathopoulos, S.; Valério, P.; Kannan, S.; Oktar, F.; Ferreira, J.M. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef]
- Kannan, S.; Rocha, J.; Agathopoulos, S.; Ferreira, J. Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. Acta Biomater. 2007, 3, 243–249. [Google Scholar] [CrossRef]
- Rivera, E.M.; Araiza, M.; Brostow, W.; Castaño, V.M.; Díaz-Estrada, J.; Hernández, R.; Rodríguez, J. Synthesis of hydroxyapatite from eggshells. Mater. Lett. 1999, 41, 128–134. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S. Fabrication of calcium phosphate bioceramics by using eggshell and phosphoric acid. Mater. Lett. 2003, 57, 4570–4574. [Google Scholar] [CrossRef]
- Balázsi, C.; Wéber, F.; Kövér, Z.; Horváth, E.; Németh, C. Preparation of calcium–phosphate bioceramics from natural resources. J. Eur. Ceram. Soc. 2007, 27, 1601–1606. [Google Scholar] [CrossRef]
- Sanosh, K.; Chu, M.-C.; Balakrishnan, A.; Kim, T.-N.; Cho, S.-J. Utilization of biowaste eggshells to synthesize nanocrystalline hydroxyapatite powders. Mater. Lett. 2009, 63, 2100–2102. [Google Scholar] [CrossRef]
- Gergely, G.; Wéber, F.; Lukács, I.; Tóth, A.L.; Horváth, Z.E.; Mihály, J.; Balázsi, C. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Kashkarov, V.; Rumyantseva, N.; Domashevskaya, E.P.; Lenshin, A.; Agapov, B.; Domashevskaya, E. Synthesis of nanocrystalline hydroxyapatite by precipitation using hen’s eggshell. Ceram. Int. 2013, 39, 4539–4549. [Google Scholar] [CrossRef]
- Ho, W.-F.; Hsu, H.-C.; Hsu, S.-K.; Hung, C.-W.; Wu, S.-C. Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. Ceram. Int. 2013, 39, 6467–6473. [Google Scholar] [CrossRef]
- Ramesh, S.; Natasha, A.; Tan, C.; Bang, L.; Ching, C.; Chandran, H. Direct conversion of eggshell to hydroxyapatite ceramic by a sintering method. Ceram. Int. 2016, 42, 7824–7829. [Google Scholar] [CrossRef]
- Krishna, D.S.R.; Siddharthan, A.; Seshadri, S.K.; Kumar, T.S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735–1743. [Google Scholar] [CrossRef]
- Sivakumar, M.; Kumar, T.; Shantha, K.; Rao, K. Development of hydroxyapatite derived from Indian coral. Biomaterials 1996, 17, 1709–1714. [Google Scholar] [CrossRef]
- Jinawath, S.; Polchai, D.; Yoshimura, M. Low-temperature, hydrothermal transformation of aragonite to hydroxyapatite. Mater. Sci. Eng. C 2002, 22, 35–39. [Google Scholar] [CrossRef]
- Hu, J.; Russell, J.J.; Bennissan, B.; Vago, R. Production and analysis of hydroxyapatite from Australian corals via hydrothermal process. J. Mater. Sci. Lett. 2001, 20, 85–87. [Google Scholar] [CrossRef]
- Walsh, P.; Buchanan, F.; Dring, M.; Maggs, C.; Bell, S.; Walker, G. Low-pressure synthesis and characterisation of hydroxyapatite derived from mineralise red algae. Chem. Eng. J. 2008, 137, 173–179. [Google Scholar] [CrossRef]
- Lemos, A.; Rocha, J.; Quaresma, S.; Kannan, S.; Oktar, F.; Agathopoulos, S.; Ferreira, J. Hydroxyapatite nano-powders produced hydrothermally from nacreous material. J. Eur. Ceram. Soc. 2006, 26, 3639–3646. [Google Scholar] [CrossRef]
- De Paula, S.M.; Huila, M.; Araki, K.; Toma, H.E. Confocal Raman and electronic microscopy studies on the topotactic conversion of calcium carbonate from Pomacea lineate shells into hydroxyapatite bioceramic materials in phosphate media. Micron 2010, 41, 983–989. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wu, Y.-N.; Ho, W.-F. Hydroxyapatite synthesized from oyster shell powders by ball milling and heat treatment. Mater. Charact. 2011, 62, 1180–1187. [Google Scholar] [CrossRef]
- Rujitanapanich, S.; Kumpapan, P.; Wanjanoi, P. Synthesis of Hydroxyapatite from Oyster Shell via Precipitation. Energy Procedia 2014, 56, 112–117. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149–150, 607–616. [Google Scholar] [CrossRef]
- Pal, A.; Maity, S.; Chabri, S.; Bera, S.; Chowdhury, A.R.; Das, M.; Sinha, A. Mechanochemical synthesis of nanocrystalline hydroxyapatite from Mercenaria clam shells and phosphoric acid. Biomed. Phys. Eng. Express 2017, 3, 015010. [Google Scholar] [CrossRef]
- Kumar, G.S.; Girija, E.K.; Venkatesh, M.; Karunakaran, G.; Kolesnikov, E.; Kuznetsov, D. One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceram. Int. 2017, 43, 3457–3461. [Google Scholar] [CrossRef]
- Kumar, G.S.; Thamizhavel, A.; Girija, E.K. Microwave conversion of eggshells into flower-like hydroxyapatite nanostructure for biomedical applications. Mater. Lett. 2012, 76, 198–200. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Albéric, M.; Stifler, C.A.; Zou, Z.; Sun, C.-Y.; Killian, C.E.; Valencia, S.; Mawass, M.-A.; Bertinetti, L.; Gilbert, P.U.; Politi, Y. Growth and regrowth of adult sea urchin spines involve hydrated and anhydrous amorphous calcium carbonate precursors. J. Struct. Biol. X 2019, 1, 100004. [Google Scholar] [CrossRef]
- Weiss, I.M.; Tuross, N.; Addadi, L.; Weiner, S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zoo L. 2002, 293, 478–491. [Google Scholar] [CrossRef]
- Jia, S.; Guo, Y.; Zai, W.; Su, Y.; Yuan, S.; Yu, X.; Li, G. Preparation and characterization of a composite coating composed of polycaprolactone (PCL) and amorphous calcium carbonate (ACC) particles for enhancing corrosion resistance of magnesium implants. Prog. Org. Coat. 2019, 136, 105225. [Google Scholar] [CrossRef]
- Myszka, B.; Schüßler, M.; Hurle, K.; Demmert, B.; Detsch, R.; Boccaccini, A.R.; Wolf, S.E. Phase-specific bioactivity and altered Ostwald ripening pathways of calcium carbonate polymorphs in simulated body fluid. RSC Adv. 2019, 9, 18232–18244. [Google Scholar] [CrossRef]
- Cozza, N.; Monte, F.; Bonani, W.; Aswath, P.; Motta, A.; Migliaresi, C. Bioactivity and mineralization of natural hydroxyapatite from cuttlefish bone and Bioglass ® co-sintered bioceramics. J. Tissue Eng. Regen. Med. 2018, 12, e1131–e1142. [Google Scholar] [CrossRef]
- Milovac, D.; Gamboa-Martínez, T.C.; Ivankovic, M.; Ferrer, G.G.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: In vitro cell culture studies. Mater. Sci. Eng. C 2014, 42, 264–272. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of sea urchin spines to Mg-substituted tricalcium phosphate for bone implants. Acta Biomater. 2007, 3, 785–793. [Google Scholar] [CrossRef]
- Kim, B.-S.; Yang, S.-S.; Lee, J. A polycaprolactone/cuttlefish bone-derived hydroxyapatite composite porous scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 943–951. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kang, H.J.; Yang, S.-S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Dorozhkina, E.I.; Dorozhkin, S.V. Mechanism of the Solid-State Transformation of a Calcium-Deficient Hydroxyapatite (CDHA) into Biphasic Calcium Phosphate (BCP) at Elevated Temperatures. Chem. Mater. 2002, 14, 4267–4272. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Kim, H.H.; Phan, T.T.V.; Oh, J. Comparative characterization of biogenic and chemical synthesized hydroxyapatite biomaterials for potential biomedical application. Mater. Chem. Phys. 2019, 228, 344–356. [Google Scholar] [CrossRef]
- Cestari, F.; Chemello, G.; Galotta, A.; Sglavo, V.M. Low-temperature synthesis of nanometric apatite from biogenic sources. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Bortolotti, M.; Lutterotti, L.; Pepponi, G. Combining XRD and XRF analysis in one Rietveld-like fitting. Powder Diffr. 2017, 32, S225–S230. [Google Scholar] [CrossRef]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Cryst. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Zolotoyabko, E.; Caspi, E.N.; Fieramosca, J.S.; Von Dreele, R.B.; Marin, F.; Mor, G.; Addadi, L.; Weiner, S.; Politi, Y. Differences between Bond Lengths in Biogenic and Geological Calcite. Cryst. Growth Des. 2010, 10, 1207–1214. [Google Scholar] [CrossRef]
- Caspi, E.N.; Pokroy, B.; Lee, P.L.; Quintana, J.P.; Zolotoyabko, E. On the structure of aragonite. Acta Cryst. Sect. B Struct. Sci. 2005, 61, 129–132. [Google Scholar] [CrossRef]
- Ardanova, L.I.; Get’Man, E.I.; Loboda, S.N.; Prisedskii, V.V.; Tkachenko, T.V.; Marchenko, V.I.; Antonovich, V.P.; Chivireva, N.A.; Chebishev, K.A.; Lyashenko, A.S. Isomorphous Substitutions of Rare Earth Elements for Calcium in Synthetic Hydroxyapatites. Inorg. Chem. 2010, 49, 10687–10693. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Boudin, S.; Grandin, A.; Borel, M.M.; LeClaire, A.; Raveau, B. Redetermination of the β-Ca2P2O7 structure. Acta Cryst. Sect. C Cryst. Struct. Commun. 1993, 49, 2062–2064. [Google Scholar] [CrossRef]
- Fiquet, G.; Richet, P.; Montagnac, G. High-temperature thermal expansion of lime, periclase, corundum and spinel. Phys. Chem. Min. 1999, 27, 103–111. [Google Scholar] [CrossRef]
- Busing, W.R.; Levy, H.A. Neutron Diffraction Study of Calcium Hydroxide. J. Chem. Phys. 1957, 26, 563–568. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite. Nanoscale 2010, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Conforto, E.; Caillard, D.; Müller, F.A. Biomimetic apatite coatings—Carbonate substitution and preferred growth orientation. Biomol. Eng. 2007, 24, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Berndt, C.C. Thermal processing of hydroxyapatite for coating production. J. Biomed. Mater. Res. 1998, 39, 580–587. [Google Scholar] [CrossRef]
- Bigi, A.; Marchetti, F.; Ripamonti, A.; Roveri, N.; Foresti, E. Magnesium and strontium interaction with carbonate-containing hydroxyapatite in aqueous medium. J. Inorg. Biochem. 1981, 15, 317–327. [Google Scholar] [CrossRef]
- Bigi, A. Isomorphous substitutions in β-tricalcium phosphate: The different effects of zinc and strontium. J. Inorg. Biochem. 1997, 66, 259–265. [Google Scholar] [CrossRef]
- Kannan, S.; Pina, S.; Ferreira, J.M. Formation of Strontium-Stabilized?-Tricalcium Phosphate from Calcium-Deficient Apatite. J. Am. Ceram. Soc. 2006, 89, 3277–3280. [Google Scholar] [CrossRef]
- Frasnelli, M.; Sglavo, V.M. Effect of Mg2+ doping on beta–alpha phase transition in tricalcium phosphate (TCP) bioceramics. Acta Biomater. 2016, 33, 283–289. [Google Scholar] [CrossRef]
- Batra, U.; Kapoor, S.; Sharma, S. Influence of Magnesium Ion Substitution on Structural and Thermal Behavior of Nanodimensional Hydroxyapatite. J. Mater. Eng. Perform. 2013, 22, 1798–1806. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J. Mater. Sci. Mater. Electron. 1993, 4, 165–168. [Google Scholar] [CrossRef]
- Aizenberg, J.; Weiner, S.; Addadi, L. Coexistence of Amorphous and Crystalline Calcium Carbonate in Skeletal Tissues. Connect. Tissue Res. 2003, 44, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lilley, K.J.; Gbureck, U.; Wright, A.J.; Farrar, D.F.; Barralet, J.E. Cement from nanocrystalline hydroxyapatite: Effect of calcium phosphate ratio. J. Mater. Sci. Mater. Med. 2005, 16, 1185–1190. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Effect of calcium, zinc and magnesium on the attachment and spreading of osteoblast like cells onto ceramic matrices. J. Mater. Sci. Mater. Electron. 2006, 18, 699–703. [Google Scholar] [CrossRef]
- Scalera, F.; Palazzo, B.; Barca, A.; Gervaso, F. Sintering of magnesium-strontium doped hydroxyapatite nanocrystals: Towards the production of 3D biomimetic bone scaffolds. J. Biomed. Mater. Res. Part A 2019, 108, 633–644. [Google Scholar] [CrossRef]
| Raw Material | Label | Milling Time | Phosphate Provider | Drying Temperature |
|---|---|---|---|---|
| DENSITY™ | ACC | 30 min | (NH4)2HPO4 | 120 °C |
| Eggshell | ES | 4 h | H3PO4 | 150 °C |
| Mussel shell | MS | 4 h | (NH4)2HPO4 | 150 °C |
| Cuttlebone | CB | 30 min | (NH4)2HPO4 | 120 °C |
| Raw Material | Synthesized Powder | Sintered Pellet | |||
|---|---|---|---|---|---|
| Label | Phase Composition | Label | Phase Composition | Label | Phase Composition |
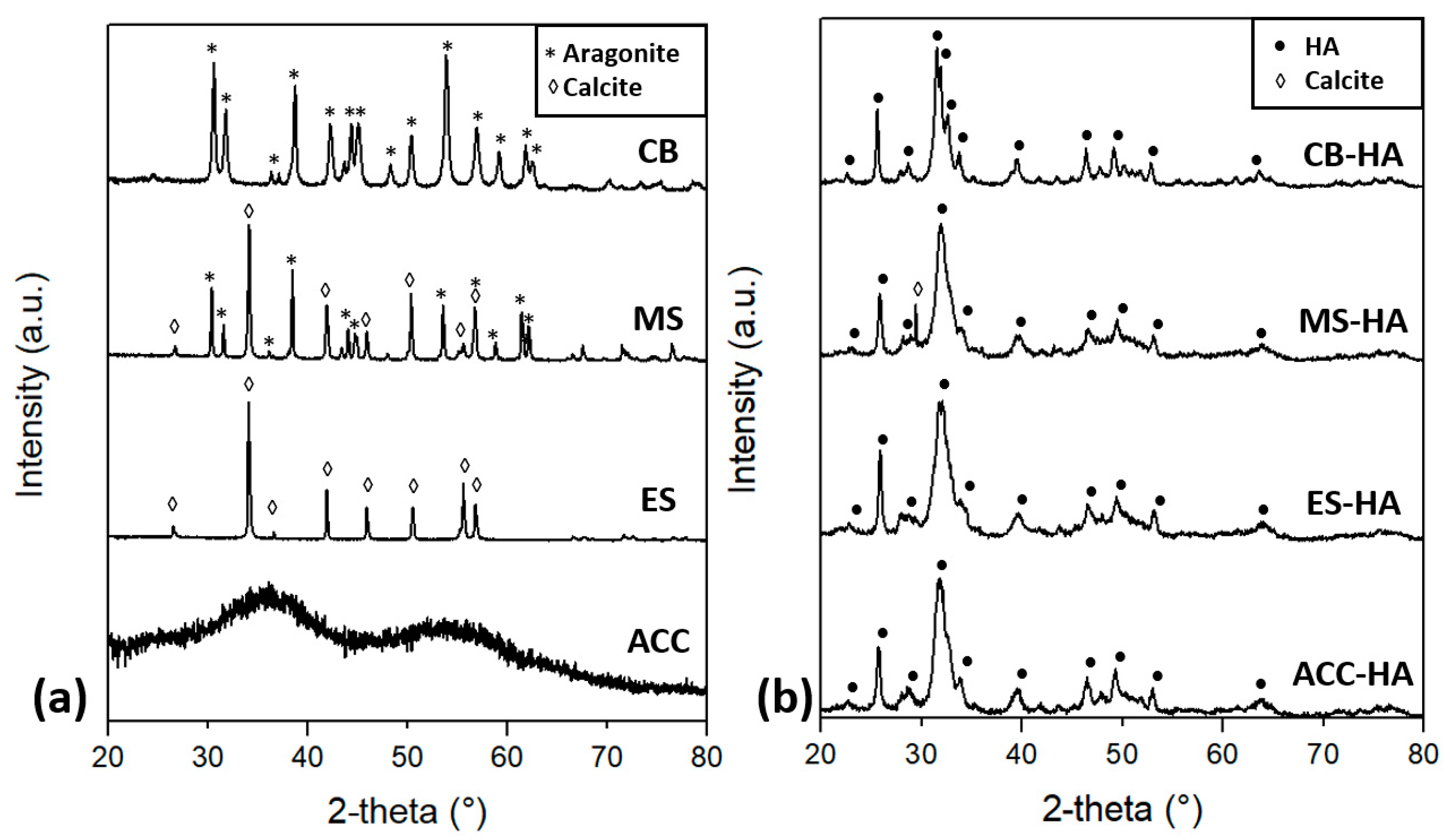
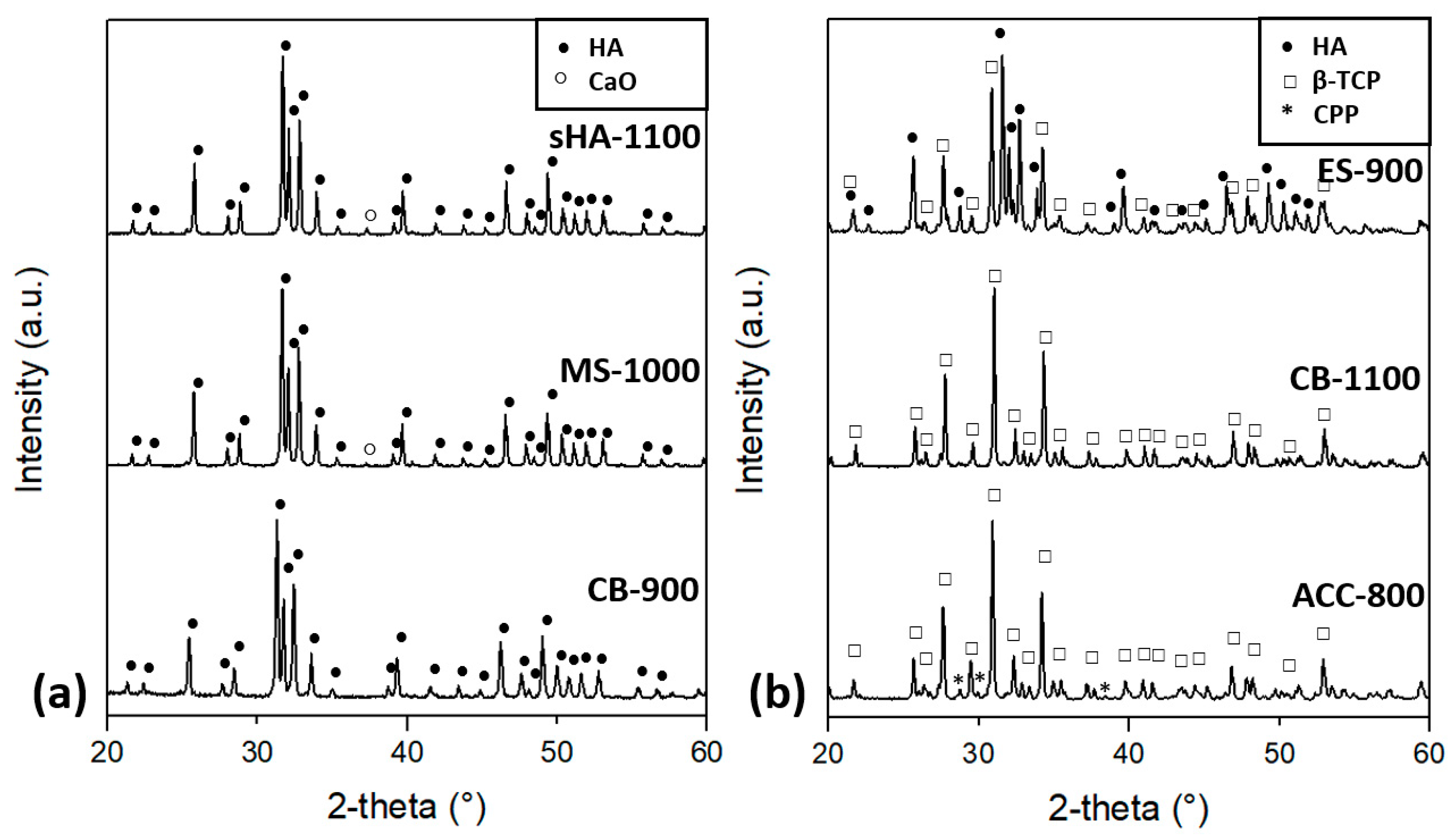
| ACC | 100% amorphous CaCO3 | ACC–HA | 100% HA | ACC-800 | ~85% β-TCP, ~15% CPP |
| ES | 100% calcite | ES–HA | 100% HA | ES-900 | ~50% HA, ~50% β-TCP |
| MS | ~70% calcite, ~30% aragonite | MS–HA | ~93% HA, ~7% calcite | MS-1000 | HA, <3% CaO |
| CB | 100% aragonite | CB–HA | 100% HA | CB-900 | 100% HA |
| CB-1100 | ~90% β-TCP, ~5% HA, ~5% CaOH | ||||
| sHA | - | - | - | sHA-1100 | HA, <3% CaO |
| P | Ca/P Molar Ratio | Na | K | Mg | Sr | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaCO3 | HA | CaCO3 | HA | CaCO3 | HA | CaCO3 | HA | CaCO3 | HA | |
| ACC | 2.0% | 1.28 ± 0.02 | 1.5% | 1.4% | <0.1% | - | - | - | <0.1% | <0.1% |
| MS | <0.2% | 1.76 ± 0.02 | 0.3% | 0.3% | <0.1% | - | 0.1% | 0.1% | 0.1% | 0.1% |
| CB | <0.2% | 1.64 ± 0.02 | 0.7% | 0.9% | 0.1% | 0.1% | <0.1% | 0.1% | 0.2% | 0.2% |
| ES | <0.2% | 1.58 ± 0.02 | 0.1% | 0.1% | 0.1% | 0.1% | 0.4% | 0.3% | <0.1% | <0.1% |
| sHA | - | 1.71 ± 0.02 | - | <0.1% | - | <0.1% | - | <0.1% | - | <0.1% |
| ACC-800 | MS-1000 | CB-900 | CB-1100 | ES-900 | sHA-1100 | |
|---|---|---|---|---|---|---|
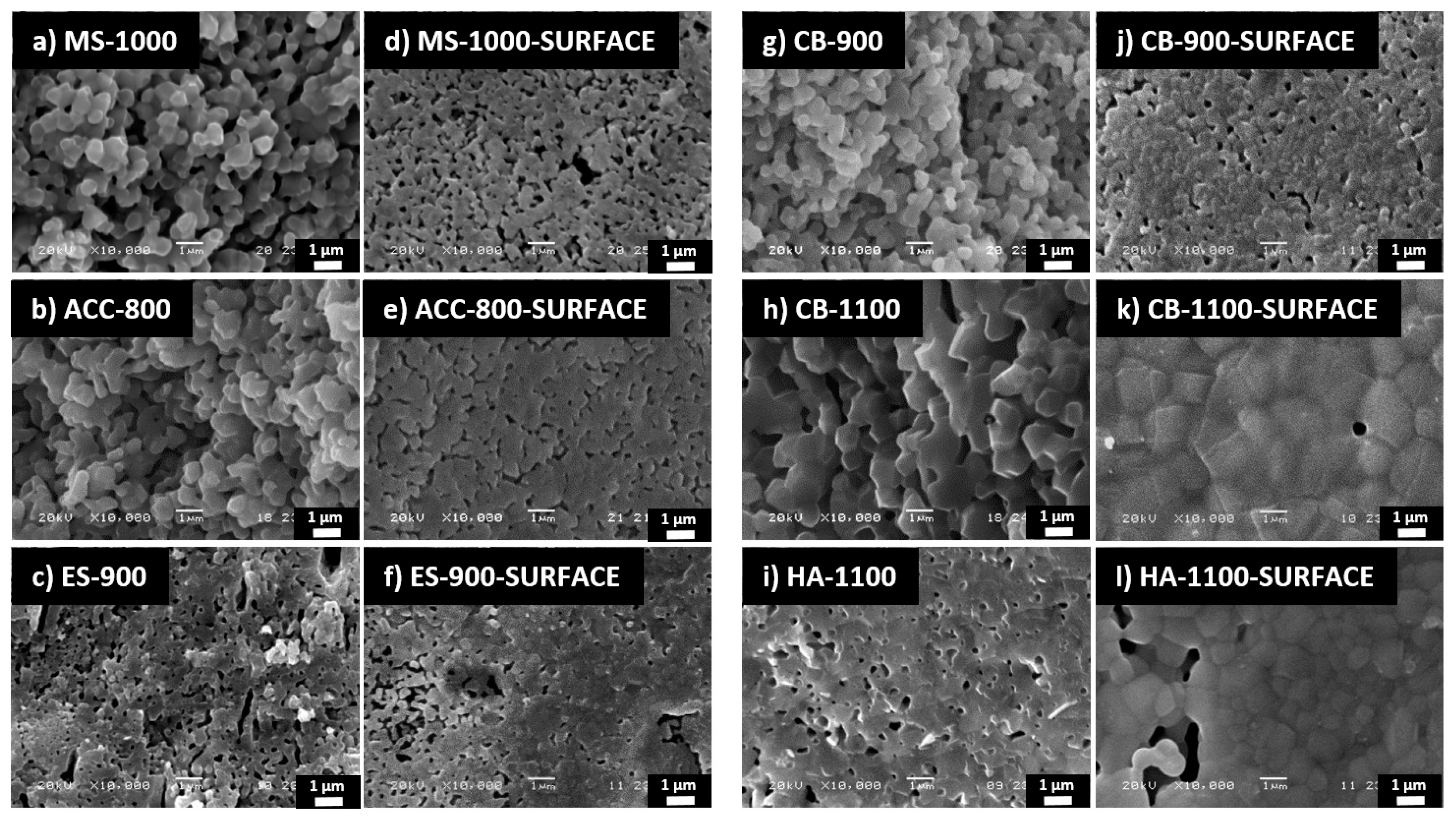
| Bulk density (g/cm3) | 2.19 ± 0.03 | 2.18 ± 0.08 | 2.33 ± 0.03 | 2.78 ± 0.03 | 2.06 ± 0.02 | 2.82 ± 0.08 |
| Relative density (%) | 71 ± 1 | 69 ± 2 | 74 ± 1 | 91 ± 1 | 66 ± 1 | 89 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; M. Sglavo, V. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials 2021, 11, 264. https://doi.org/10.3390/nano11020264
Cestari F, Agostinacchio F, Galotta A, Chemello G, Motta A, M. Sglavo V. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials. 2021; 11(2):264. https://doi.org/10.3390/nano11020264
Chicago/Turabian StyleCestari, Francesca, Francesca Agostinacchio, Anna Galotta, Giovanni Chemello, Antonella Motta, and Vincenzo M. Sglavo. 2021. "Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity" Nanomaterials 11, no. 2: 264. https://doi.org/10.3390/nano11020264
APA StyleCestari, F., Agostinacchio, F., Galotta, A., Chemello, G., Motta, A., & M. Sglavo, V. (2021). Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials, 11(2), 264. https://doi.org/10.3390/nano11020264