Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry
Abstract
1. Introduction
2. Antimicrobial Properties
2.1. Gaseous Ozone, Ozonated Water and Ozonated Oil
2.2. Titanium Dioxide Nanoparticles
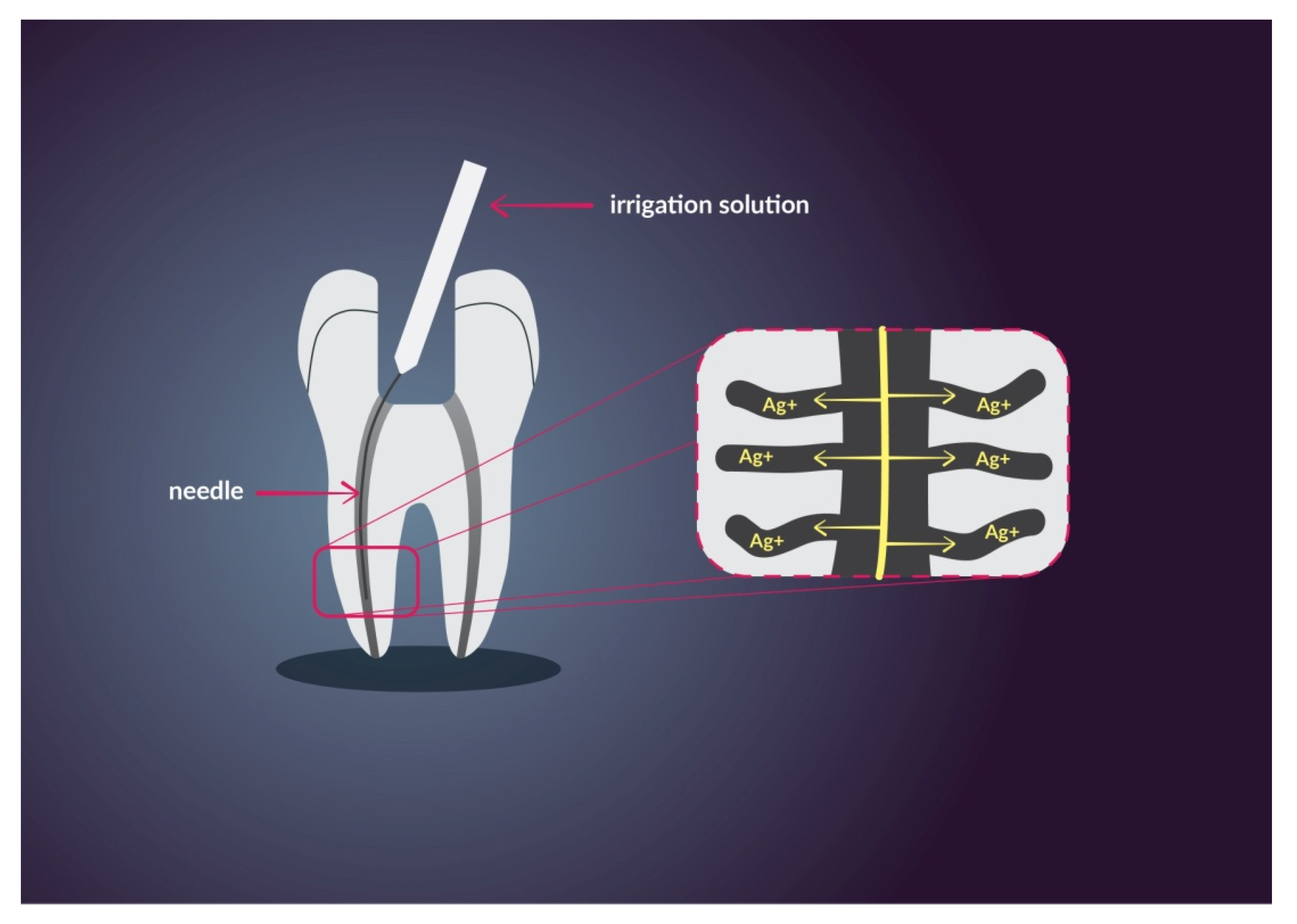
2.3. Silver Nanoparticles and Ions
2.4. Platinum Nanoparticles
2.5. Copper Oxide
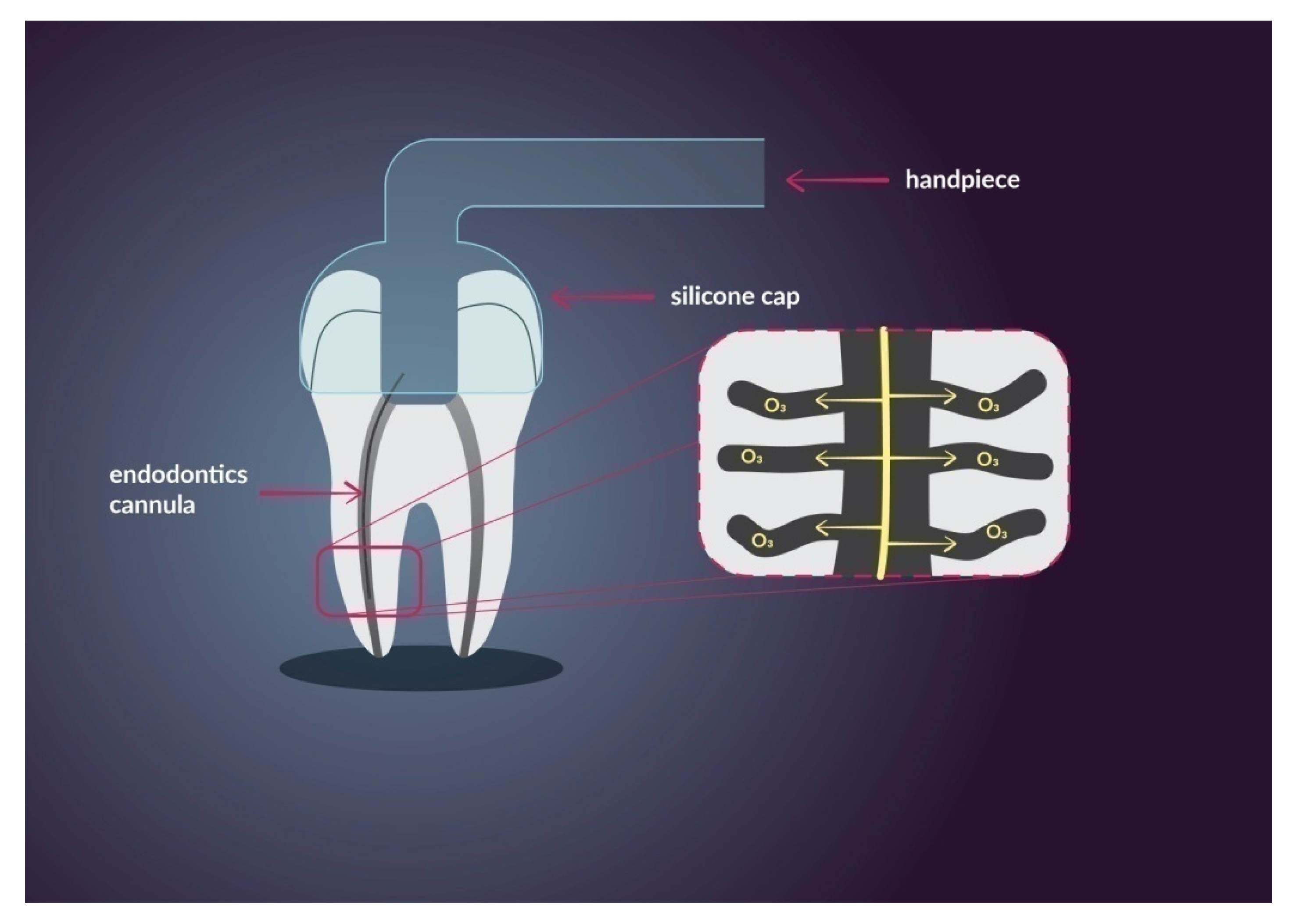
3. The Use of Ozone and Nanoparticles in Dentistry
3.1. Restorative Dentistry
3.2. Endodontics
3.3. Dental Surgery
3.4. Prosthetic
4. Cytotoxicity
4.1. Ozone
4.2. Titanium Dioxide
4.3. Silver
4.4. Platinum
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naik, S.V.; Rajeshwari, K.; Kohli, S.; Zohabhasan, S.; Bhatia, S. Ozone—A biological therapy in dentistry—Reality or Myth????? Open Dent. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bhateja, S. The miraculous healing therapy—Ozone therapy in dentistry. J. Dent. 2012, 3, 150–155. [Google Scholar] [CrossRef]
- Elvis, A.; Ekta, J. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhagawati, S.; Tyagi, P.; Kumar, P. Current interpretations and scientific rationale of the ozone usage in dentistry: A systematic review of literature. Eur. J. Gen. Dent. 2014, 3. [Google Scholar] [CrossRef]
- Nagarakanti, S.; Athuluru, D. Ozone: A new revolution in dentistry. WebmedCentral Dent. 2011. [Google Scholar] [CrossRef]
- Garg, R. Ozone: A new face of dentistry. Internet J. Dent. Sci. 2012, 7. [Google Scholar] [CrossRef]
- Sun, Y. Controlled synthesis of colloidal silver nanoparticles in organic solutions: Empirical rules for nucleation engineering. Chem. Soc. Rev. 2013, 42, 2497–2511. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Duran, G.; Galembeck, A.; Cogo-Mueller, K.; Franz-Montan, M. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, S.J.; Kook, J.K.; Lim, B.S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 2009, 25, 206–213. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Khanna, S.N. A systematic framework and nanoperiodic concept for unifying nanoscience: Hard/soft nanoelements, superatoms, meta-Atoms, new emerging properties, periodic property patterns, and predictive mendeleev-like nanoperiodic tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dai, T.; Xuan, Y.; Tegos, G.P.; Hamblin, M.R. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: Efficacy against bacterial burn infections. Antimicrob. Agents Chemother. 2011, 55, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Antony, J.J.; Sivalingam, P.; Chen, B. Toxicological effects of silver nanoparticles. Environ. Toxicol. Pharmacol. 2015, 40, 729–732. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Giorgio Zambonin, P.; Traversa, E. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Cava, R.J. Structural chemistry and the local charge picture of copper oxide superconductors. Science 1990, 247, 656–662. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Amiri, M.; Etemadifar, Z.; Daneshkazemi, A.; Nateghi, M. Antimicrobial effect of copper oxide nanoparticles on some oral bacteria and candida species. J. Dent. Biomater. 2017, 4, 347–352. [Google Scholar] [PubMed]
- Battez, A.H.; Viesca, J.L.; González, R.; Blanco, D.; Asedegbega, E.; Osorio, A. Friction reduction properties of a CuO nanolubricant used as lubricant for a NiCrBSi coating. Wear 2010, 268, 325–328. [Google Scholar] [CrossRef]
- Pan, X.; Redding, J.E.; Wiley, P.A.; Wen, L.; McConnell, J.S.; Zhang, B. Mutagenicity evaluation of metal oxide nanoparticles by the bacterial reverse mutation assay. Chemosphere 2010, 79, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Eshed, M.; Lellouche, J.; Matalon, S.; Gedanken, A.; Banin, E. Sonochemical coatings of ZnO and CuO nanoparticles inhibit streptococcus mutans biofilm formation on teeth model. Langmuir 2012, 28, 12288–12295. [Google Scholar] [CrossRef]
- Antibacterial Effect of TiO2 Nanoparticles on Pathogenic Strain of E. coli. Available online: https://www.researchgate.net/publication/230683452_Antibacterial_effect_of_TiO2_nanoparticles_on_pathogenic_strain_of_E_coli (accessed on 11 September 2020).
- Sun, H.; Bai, Y.; Cheng, Y.; Jin, W.; Xu, N. Preparation and characterization of visible-light-driven carbon—Sulfur-codoped TiO2 photocatalysts. Ind. Eng. Chem. Res. 2006, 45, 4971–4976. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef]
- Rosenberg, B.; van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Chwalibog, A.; Sawosz, E.; Hotowy, A.; Szeliga, J.; Sokolowska, A. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomed. 2010, 5, 1085–1094. [Google Scholar] [CrossRef]
- Watanabe, A.; Kajita, M.; Kim, J.; Kanayama, A.S.; Miyamoto, Y. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Shuhei, H.; Futami, N.; Toru, T.; Takatsumi, I.; Takahiro, W.; Kiyotaka, A. Effect of application time of colloidal platinum nanoparticles on the microtensile bond strength to dentin. Dent. Mater. J. 2010, 29, 682–689. [Google Scholar] [CrossRef]
- Itohiya, H.; Matsushima, Y.; Shirakawa, S.; Kajiyama, S.; Yashima, A.; Nagano, T.; Gomi, K. Organic resolution function and effects of platinum nanoparticles on bacteria and organic matter. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L. Pharmaceutical intermediate-modified gold nanoparticles: Against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Ceci, M.; Preda, F.; Poggio, C.; Colombo, M. Evaluation of the antibacterial activity of a new ozonized olive oil against oral and periodontal pathogens. J. Clin. Exp. Dent. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tuncay, O.; Dinçer, A.N.; Kuştarci, A.; Er, O.; Dinç, G.; Demirbuga, S. Effects of ozone and photo-activated disinfection against Enterococcus faecalis biofilms in vitro. Niger. J. Clin. Pract. 2015, 18, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Alonso, F.; Salmerón-Lozano, P.; Martínez-Beneyto, Y. Effects of photodynamic therapy, 2% chlorhexidine, triantibiotic mixture, propolis and ozone on root canals experimentally infected with Enterococcus faecalis: An in vitro study. Odontology 2017, 105, 338–346. [Google Scholar] [CrossRef]
- Sancakli, G.Y.; Siso, S.H.; Yildiz, S.O. Antibacterial effect of surface pretreatment techniques against streptococcus mutans. Niger J. Clin. Pr. 2018, 21, 170–175. [Google Scholar]
- Krunić, J.; Nikola, S.; Dukic, L.; Jelena, R.; Branka, P.; Ivana, S. Clinical antibacterial effectiveness and biocompatibility of gaseous ozone after incomplete caries removal. Clin. Oral Investig. 2019, 23, 785–792. [Google Scholar] [CrossRef]
- Ajeti, N.; Pustina-Krasniqi, T.; Apostolska, S.; Xhajanka, E. The effect of gaseous ozone in infected root canal. Open Access Maced. J. Med. Sci. 2018, 6, 389–396. [Google Scholar] [CrossRef]
- Anumula, L.; Kumar, K.V.S.; Krishna, C.H.N.V.M.; Lakshmi, K.S. Antibacterial activity of freshly prepared ozonated water and chlorhexidine on Mutans Streptococcus when used as an oral rinse—A randomised clinical study. J. Clin. Diagnostic Res. 2017, 11, ZC05–ZC08. [Google Scholar] [CrossRef]
- Sodagar, A.; Sadegh, M.S.A.; Bahador, A.; Jalali, Y.F.; Behzadi, Z.; Elhaminejad, F. Mirhashemi, A.H. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in orthodontics. Dent. Press J. Orthod. 2017, 22, 67–74. [Google Scholar] [CrossRef]
- Chen, R.; Han, A.; Huang, Z.; Karki, J.; Wang, C. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Györgyey, Á.; Janovák, L.; Ádám, A.; Kopniczky, J.; Tóth, K.; Deák, Á.; Panayotov, I.; Cuisinier, F.; Dékány, I.; Turzó, K. Investigation of the in vitro photocatalytic antibacterial activity of nanocrystalline TiO2 and coupled TiO2/Ag containing copolymer on the surface of medical grade titanium. J. Biomater. Appl. 2016, 31, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Lavaee, F.; Faez, K.; Faez, K.; Hadi, N.; Modaresi, F. Antimicrobial and antibiofilm activity of silver, titanium dioxide and iron nano particles. Am. J. Dent. 2016, 29, 315–320. [Google Scholar] [PubMed]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted hydroxyapatites with antibacterial properties. Biomed. Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J.Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Liu, X.; Gan, K.; Liu, H.; Song, X.; Chen, T.; Liu, C. Antibacterial properties of nano-silver coated PEEK prepared through magnetron sputtering. Dent. Mater. 2017, 33, e348–e360. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.W.; Lim, H.P.; Park, C.; Yun, K.D. Biocompatibility evaluation of feldspathic porcelain with nano-sized silver ion particles. J. Nanosci. Nanotechnol. 2018, 18, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Pourhashemi, S.J.; Sadegh, M.; Salehi, Y.; Fard, M.J.K. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Lo, E.C.M.; Tang, J.; Li, Q. Synthesis and characterization of fluoridated silver nanoparticles and their potential as a non-staining anti-caries agent. Int. J. Nanomed. 2020, 15, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Badea, F. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef]
- Lee, J.H.; El-Fiqi, A.; Mandakhbayar, N.; Lee, H.H.; Kim, H.W. Drug/ion co-delivery multi-functional nanocarrier to regenerate infected tissue defect. Biomaterials 2017, 142, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed Alkheraif, A.A.; Haleemg, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Li, Q.-L.; Tang, J.; Chu, C.-H. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch. Oral Biol. 2019, 102, 106–112. [Google Scholar] [CrossRef]
- Nam, K.Y. Characterization and bacterial anti-adherent effect on modified PMMA denture acrylic resin containing platinum nanoparticles. J. Adv. Prosthodont. 2014, 6, 207–214. [Google Scholar] [CrossRef]
- Grigore, M.; Biscu, E.; Holban, A.; Gestal, M.; Grumezescu, A. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Yang, L.; Long, J.; Zheng, Y.; Liu, J.; Xiao, S.; Jia, J. Activation of Erk and p53 regulates copper oxide nanoparticle-induced cytotoxicity in keratinocytes and fibroblasts. Int. J. Nanomed. 2014, 9, 4763. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Zhao, J.; White, J.C.; Qu, P.; Xing, B. CuO nanoparticle interaction with human epithelial cells: Cellular uptake, location, export, and genotoxicity. Chem. Res. Toxicol. 2012, 25, 1512–1521. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Kumar, S.; Alhadlaq, H.A.; Alrokayan, S.A.; Abu-Salah, K.M.; Ahamed, M. Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol. Ind. Health 2016, 32, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Assadian, E.; Zarei, M.H.; Gilani, A.G.; Farshin, M.; Degampanah, H.; Pourahmad, J. Toxicity of copper oxide (CuO) nanoparticles on human blood lymphocytes. Biol. Trace Elem. Res. 2018, 184, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yan, Y.; Zhao, Y.; Guo, F.; Jiang, C. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS ONE 2012, 7, e43442. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Partida, E.; Valdez-Salas, B.; Valdez-Salas, E.; Pérez-Cortéz, G.; Nedev, N. Synthesis, characterization, and in situ antifungal and cytotoxicity evaluation of ascorbic acid-capped copper nanoparticles. J. Nanomater. 2019. [Google Scholar] [CrossRef]
- Ramazanzadeh, B.; Jahanbin, A.; Yaghoubi, M.; Shahtahmassbi, N.; Shafaee, H. Comparison of antibacterial effects of ZnO and CuO nanoparticles coated brackets against streptococcus mutans. J. Dent. 2015, 16, 200–205. [Google Scholar]
- Toodehzaeim, M.H.; Zandi, H.; Meshkani, H.; Firouzabadi, A.H. The effect of CuO nanoparticles on antimicrobial effects and shear bond strength of orthodontic adhesives. J. Dent. 2018, 19, 1–5. [Google Scholar]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Blánaid, D.; Renato, V.; Mathur, M.R.; Stefan, L.; Roger Keller, C.; Guarnizo-Herreo, C.C.; Cristin, K. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Ximenes, M.; Cardoso, M.; Astorga, F.; Arnold, R.; Pimenta, L.A.; Viera, R.D. Antimicrobial activity of ozone and NaF-chlorhexidine on early childhood caries. Braz. Oral Res. 2017, 31. [Google Scholar] [CrossRef][Green Version]
- Hauser-Gerspach, I.; Pfäffli-Savtchenko, V.; Dähnhardt, J.E.; Meyer, J.; Lussi, A. Comparison of the immediate effects of gaseous ozone and chlorhexidine gel on bacteria in cavitated carious lesions in children in vivo. Clin. Oral Investig. 2009, 13, 287–291. [Google Scholar] [CrossRef]
- Duangthip, D.; Jiang, M.; Chu, C.H.; Lo, E.C.M. Non-surgical treatment of dentin caries in preschool children—Systematic review. BMC Oral Health 2015, 15. [Google Scholar] [CrossRef]
- Kalniņa, J. Ozone Therapy in Prevention and Treatment of in Caries in Permanent Teeth. 2017. Available online: www.rsu.lv (accessed on 2 January 2020).
- Cehreli, S.B.; Yalcinkaya, Z.; Guven-Polat, G.; Çehreli, Z.C. Effect of ozone pretreatment on the microleakage of pit and fissure sealants. J. Clin. Pediatr. Dent. 2010, 35, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Dalkilic, E.E.; Arisu, H.D.; Kivanc, B.H.; Uctasli, M.B.; Omurlu, H. Effect of different disinfectant methods on the initial microtensile bond strength of a self-etch adhesive to dentin. Lasers Med. Sci. 2012, 27, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Reddy, N.; Dinapadu, S.; Reddy, M.; Pasari, S. Role of ozone therapy in minimal intervention dentistry and endodontics—A review. J. Int. Oral Heal. 2013, 5, 102–108. [Google Scholar]
- Almaz, M.E.; Sönmez, I.Ş. Ozone therapy in the management and prevention of caries. J. Formos. Med. Assoc. 2015, 114, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, V.A.; Tabari, K.; Sheikh-Al-Eslamian, S.M.; Abrandabadi, A.N. Physiochemical properties of experimental nano-hybrid MTA. J. Med. Life 2018, 11, 51–56. [Google Scholar]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Kanda, Y.; Nakajima, H.; Sakagami, H. Effects of TiO2 nano glass ionomer cements against normal and cancer oral cells. In Vivo 2014, 28, 895–908. [Google Scholar]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M.; Montiel-Company, J.M. Antibacterial properties of nanoparticles in dental restorative materials. A systematic review and meta-analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Marangoni, V.S.; Bernardi, A.C.d.; Rastelli, A.N.d.; Hernandes, A.C. Synthesis, characterization and application of Ag doped ZnO nanoparticles in a composite resin. Mater. Sci. Eng. C 2019, 96, 391–401. [Google Scholar] [CrossRef]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109. [Google Scholar] [CrossRef]
- Koohpeima, F.; Mokhtari, M.J.; Khalafi, S. The effect of silver nanoparticles on composite shear bond strength to dentin with different adhesion protocols. J. Appl. Oral Sci. 2017, 25, 367–373. [Google Scholar] [CrossRef]
- Vazquez-Garcia, F.; Tanomaru-Filho, M.; Chávez-Andrade, G.M.; Bosso-Martelo, R.; Basso-Bernardi, M.I.; Guerreiro-Tanomaru, J.M. Effect of silver nanoparticles on physicochemical and antibacterial properties of calcium silicate cements. Braz. Dent. J. 2016, 27, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.M.; El-Sayed, H.S.; Abdelnabi, A. The antibacterial effect of two cavity disinfectants against one of cariogenic pathogen: An in vitro comparative study. Contemp. Clin. Dent. 2018, 9, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, B.B. In vitro evaluation of the remineralizing potential and antimicrobial activity of a cariostatic agent with silver nanoparticles. Braz. Dent. J. 2017, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, K.S.; Egusa, H.; Ngo, H.C.; Panduwawala, C.; Pesee, S.; Samaranayake, L.P. Clinical efficacy and the antimicrobial potential of silver formulations in arresting dental caries: A systematic review. BMC Oral Health 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Tuncay, Ö.; Er, Ö.; Demirbuga, S.; Zorba, Y.O.; Topçuoǧlu, H.S. Effect of gaseous ozone and light-activated disinfection on the surface hardness of resin-based root canal sealers. Scanning 2016, 38, 141–147. [Google Scholar] [CrossRef]
- César Sumita, T.C.; Junqueira, J.C.; Jorge, A.O.C.; do Rego, M.A. Antimicrobial effects of ozonated water on the sanitization of dental instruments contaminated with E. coli, S. aureus, C. albicans, or the spores of B. Atrophaeus. J. Infect. Public Health 2012, 5, 269–274. [Google Scholar] [CrossRef]
- Tandan, M.; Gupta, S.; Tandan, P. Ozone in conservative dentistry & endodontics: A. Review. Int. J. Clin. Prev. Dent. 2012, 8, 29–35. [Google Scholar]
- Thote, A.; Ikhar, A.; Chandak, M.; Nikhade, P. Ozone an endodontic bliss: A. review. Int. J. Adv. Res. 2018, 6, 951–956. [Google Scholar] [CrossRef]
- Cardoso, M.G.; de Oliveira, L.D.; Koga-Ito, C.Y.; Jorge, A.O.C. Effectiveness of ozonated water on Candida albicans, Enterococcus faecalis, and endotoxins in root canals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105. [Google Scholar] [CrossRef]
- Noguchi, F.; Kitamura, C.; Nagayoshi, M.; Chen, K.K.; Terashita, M.; Nishihara, T. Ozonated water improves lipopolysaccharide-induced responses of an odontoblast-like cell line. J. Endod. 2009, 35, 668–672. [Google Scholar] [CrossRef]
- Hubbezoglu, I.; Zan, R.; Tunc, T.; Sumer, Z. Antibacterial efficacy of aqueous ozone in root canals infected by Enterococcus faecalis. Jundishapur J. Microbiol. 2014, 7, 11411. [Google Scholar] [CrossRef] [PubMed]
- Hems, R.S.; Gulabivala, K.; Ng, Y.L.; Ready, D.; Spratt, D.A. An in vitro evaluation of the ability of ozone to kill a strain of Enterococcus faecalis. Int. Endod. J. 2005, 38, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, M.; Kitamura, C.; Fukuizumi, T.; Nishihara, T.; Terashita, M. Antimicrobial effect of ozonated water on bacteria invading dentinal tubules. J. Endod. 2004, 30, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Kist, S.; Kollmuss, M.; Jung, J.; Schubert, S.; Hickel, R.; Huth, K.C. Comparison of ozone gas and sodium hypochlorite/chlorhexidine two-visit disinfection protocols in treating apical periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2017, 21, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Estrela, C.R.A.; Decurcio, D.A.; Hollanda, A.C.B.; Silva, J.A. Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hypochlorite and chlorhexidine in infected human root canals. Int. Endod. J. 2007, 40, 85–93. [Google Scholar] [CrossRef]
- Zan, R.; Hubbezoglu, I.; Sümer, Z.; Tunç, T.; Tanalp, J. Antibacterial effects of two different types of laser and aqueous ozone against enterococcus faecalis in root canals. Photomed. Laser Surg. 2013, 31, 150–154. [Google Scholar] [CrossRef]
- Noites, R.; Pina-Vaz, C.; Rocha, R.; Carvalho, M.F.; Gonçalves, A.; Pina-Vaz, I. Synergistic antimicrobial action of chlorhexidine and ozone in endodontic treatment. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Prado, M.C.; Soares, D.N.; Hecksher, F.; Martins, J.N.R.; Fidalgo, T.K.S. The effect of ozone therapy in root canal disinfection: A systematic review. Int. Endod. J. 2019. [Google Scholar] [CrossRef]
- Antonijevic, D.; Milovanovic, P.; Brajkovic, D.; Ilic, D.; Hahn, M.; Amling, M. Microstructure and wettability of root canal dentine and root canal filling materials after different chemical irrigation. Appl. Surf. Sci. 2015, 355, 369–378. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Yaripour, S.; Shalavi, S.; Palazzi, F.; Asgary, S. Root canal irrigants and dentin bonding: An update. Iran. Endod. J. 2017, 12, 131. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of antimicrobial nanoparticles in dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.N.; Jo, U.B.; Ryu, H.Y.; Kim, Y.S.; Song, K.S.; Yu, I.J. Histochemical study of intestinal mucins after administration of silver nanoparticles in Sprague-Dawley rats. Arch. Toxicol. 2010, 84, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shantiaee, Y.; Dianat, O.; Mohammadkhani, H.; Baghban, A.A. Cytotoxicity comparison of nanosilver coated gutta-percha with guttaflow and normal Gutta-Percha On L929 fibroblast with MTT assay. J. Dent. Sch. Shahid Beheshti Univ. Med. Sci. 2011, 29, 63–69. [Google Scholar]
- Lotfi, M.; Vosoughhosseini, S.; Ranjkesh, B.; Khani, S. Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis. Afr. J. Biotechnol. 2011, 10, 6799–6803. [Google Scholar]
- González-Luna, P.I.; Martínez-Castañon, G.-A.; Zavala-Alonso, A.; Patiño-Marin, N.; Nino, N.; Moran, J.; Ramírez-González, J.-H. Bactericide effect of silver nanoparticles as a final irrigation agent in endodontics on enterococcus faecalis: An ex vivo study. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Nabavizadeh, M.; Gholami, A.; Aleyasin, Z.S.; Dorostkar, S.; Saliminasab, M.; Ghasemi, Y.; Hemmateenejad, B.; Sharghi, H. Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: A promising disinfectant in root canal treatment. Int. Endod. J. 2015, 48, 790–800. [Google Scholar] [CrossRef]
- Samiei, M.; Aghazadeh, M.; Lotfi, M.; Shakoei, S.; Aghazadeh, Z.; Pakdel, S.M.V. Antimicrobial efficacy of mineral trioxide aggregate with and without silver nanoparticles. Iran. Endod. J. 2013, 8, 166–170. [Google Scholar] [CrossRef]
- Seung, J.; Weir, M.D.; Melo, M.A.S.; Romberg, E.; Nosrat, A.; Xu, H.H.K.; Tordik, P.A. A modified resin sealer: Physical and antibacterial properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef]
- Monzavi, A.; Eshraghi, S.; Hashemian, R.; Momen-Heravi, F. In vitro and ex vivo antimicrobial efficacy of nano-MgO in the elimination of endodontic pathogens. Clin. Oral Investig. 2015, 19, 349–356. [Google Scholar] [CrossRef]
- Grumezescu, A.M. Nanobiomaterials in dentistry: Applications of nanobiomaterials. Nanobiomater. Dent. Appl. Nanobiomater. 2016, 11, 1–467. [Google Scholar]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Aberasturi, D.J.D.; Larramendi, I.R.D.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Argueta-Figueroa, L.; Morales-Luckie, R.A.; Scougall-Vilchis, R.J.; Olea-Mejía, O.F. Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu-Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 2014, 24, 321–328. [Google Scholar] [CrossRef]
- Aun, D.P.; Peixoto, I.F.D.C.; Houmard, M.; Buono, V.T.L. Enhancement of NiTi superelastic endodontic instruments by TiO2 coating. Mater. Sci. Eng. C 2016, 68, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Łazarz-Półkoszek, M.J.; Loster, J.E.; Wiśniewska, G. Application of ozone to various fields of dentistry—Review of literature. Protet. Stomatol. 2020, 70, 90–106. [Google Scholar] [CrossRef]
- Madi, M.; Htet, M.; Zakaria, O.; Alagl, A.; Kasugai, S. Re-osseointegration of dental implants after periimplantitis treatments: A systematic review. Implant. Dent. 2018, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Isler, S.C.; Soysal, F.; Akca, G.; Bakirarar, B.; Ozcan, G.; Unsal, B. The effects of decontamination methods of dental implant surface on cytokine expression analysis in the reconstructive surgical treatment of peri-implantitis. Odontology 2020. [Google Scholar] [CrossRef]
- Kazancioglu, H.O.; Kurklu, E.; Ezirganli, S. Effects of ozone therapy on pain, swelling, and trismus following third molar surgery. Int. J. Oral Maxillofac. Surg. 2014, 43, 644–648. [Google Scholar] [CrossRef]
- Kazancioglu, H.O.; Ezirganli, S.; Demirtas, N. Comparison of the influence of ozone and laser therapies on pain, swelling, and trismus following impacted third-molar surgery. Lasers Med. Sci. 2014, 29, 1313–1319. [Google Scholar] [CrossRef]
- Shi, X.; Xu, L.; Le, T.B.; Zhou, G.; Zheng, C.; Tsure, K.; Ishikawa, K. Partial oxidation of TiN coating by hydrothermal treatment and ozone treatment to improve its osteoconductivity. Mater. Sci. Eng. C 2016, 59, 542–548. [Google Scholar] [CrossRef]
- Hauser-Gerspach, I.; Vadaszan, J.; Deronjic, I.; Gass, C.; Meyer, J.; Dard, M.; Waltimo, T.; Stübinger, S.; Mauth, C. Influence of gaseous ozone in peri-implantitis: Bactericidal efficacy and cellular response. An in vitro study using titanium and zirconia. Clin. Oral Investig. 2012, 16, 1049–1059. [Google Scholar] [CrossRef]
- Isler, S.C.; Unsal, B.; Soysal, F.; Ozcan, G.; Peker, E.; Karaca, I.R. The effects of ozone therapy as an adjunct to the surgical treatment of peri-implantitis. J. Periodontal Implant. Sci. 2018, 48, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Gunputh, U.F.; Le, H.; Handy, R.D.; Tredwin, C. Anodised TiO2 nanotubes as a scaffold for antibacterial silver nanoparticles on titanium implants. Mater. Sci. Eng. C 2018, 91, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-light active titanium dioxide nanomaterials with bactericidal properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discovery Today 2019, 24, 85–98. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Leal, B.F.; Hubler, R.; de Oliveira, S.D.; Teixeira, E.R. Antibacterial potential associated with drug-delivery built TiO2 nanotubes in biomedical implants. AMB Express 2019, 9, 51. [Google Scholar] [CrossRef]
- Azzawi, Z.G.M.; Hamad, T.I.; Kadhim, S.A.; Naji, G.A.H. Osseointegration evaluation of laser-deposited titanium dioxide nanoparticles on commercially pure titanium dental implants. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef]
- Gunputh, U.F.; Le, H.; Lawton, K.; Besinis, A.; Tredwin, C.; Handy, R.D. Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology 2020, 14, 97–110. [Google Scholar] [CrossRef]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is metal particle release associated with peri-implant bone destruction? An emerging concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Shen, X.; Al-Baadani, M.A.; He, H.; Cai, L.; Liu, J. Antibacterial and osteogenesis performances of LL37-loaded titania nanopores in vitro and in vivo. Int. J. Nanomedicine 2019, 14, 3043–3054. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Arita, M. Microbicidal efficacy of ozonated water om candida albicans adhered to avrylic plates. Oral Microbiol. Immunol. 2005, 6, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Mizuguchi, M.; Hattori, M.; Ito, Y.; Kawai, T.; Hasegawa, J. Effect of denture cleaner using ozone against methicillin-resistant Staphylococcus aureus and E. coli T1 phage. Dent. Mater. J. 2002, 21, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Oizumi, M.; Furuya, J.; Okamoto, Y.; Rosenstiel, S.F. Influence of ozone on oxidation of dental alloys. Int. J. Prosthodont. 1999, 12, 179–183. [Google Scholar] [PubMed]
- AlZarea, B.K. Management of denture-related traumatic ulcers using ozone. J. Prosthet. Dent. 2019, 121, 76–82. [Google Scholar] [CrossRef]
- Suganya, S.; Ahila, S.C.; Kumar, M.B.; Kumar, V.M. Evaluation and comparison of anti-Candida effect of heat cure polymethylmethacrylate resin enforced with silver nanoparticles and conventional heat cure resins: An in vitro study. J. Dent. Res. 2014, 25, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Lan, J.; Qi, Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef]
- Ginjupalli, K.; Alla, R.K.; Tellapragada, C.; Gupta, L.; Perampalli, N.U. Antimicrobial activity and properties of irreversible hydrocolloid impression materials incorporated with silver nanoparticles. J. Prosthet. Dent. 2016, 115, 722–728. [Google Scholar] [CrossRef]
- Şuhani, M.F.; Băciutcedil, G.; Băciutcedil, M.; Şuhani, R.; Bran, S. Current perspectives regarding the application and incorporation of silver nanoparticles into dental biomaterials. Clujul Med. 2018, 91, 274–279. [Google Scholar] [CrossRef]
- Köroğlu, A.; Şahın, O.; Kürkçüoğlu, I.; Dede, D.Ö.; Özdemır, T.; Hazer, B. Silver nanoparticle incorporation effect on mechanical and thermal properties of denture base acrylic resins. J. Appl. Oral Sci. 2016, 24, 590–596. [Google Scholar] [CrossRef]
- Matsuura, T.; Abe, Y.; Sato, Y.; Okamoto, K.; Ueshige, M.; Akagawa, Y. Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J. Dent. 1997, 25, 373–377. [Google Scholar] [CrossRef]
- Fujieda, T.; Uno, M.; Ishigami, H.; Kurachi, M.; Wakamatsu, N.; Doi, Y. Effects of dental porcelain containing silver nanoparticles on static fatigue. Dent. Mater. J. 2013, 32, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, T.; Uno, M.; Ishigami, H.; Kurachi, M.; Wakamatsu, N.; Doi, Y. Addition of platinum and silver nanoparticles to toughen dental porcelain. Dent. Mater. J. 2012, 31, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Nonogaki, R.; Fujieda, T.; Ishigami, H.; Kurachi, M.; Kamemizu, H.; Wakamatsu, N.; Doi, Y. Toughening of CAD/CAM all-ceramic crowns by staining slurry. Dent. Mater. J. 2012, 31, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Fouad, H.; Hashem, M.; Niazy, A.A.; AlBadah, A. Titanium Oxide (TiO2)/polymethylmethacrylate (PMMA) denture base nanocomposites: Mechanical, viscoelastic and antibacterial behavior. Materials 2018, 11, 1096. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The fututre in dental care for elderly edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef]
- Tsuji, H.; Sugahara, H.; Gotoh, Y.; Ishikawa, J. Metal negative-ion implantation into rutile TiO2 and enhancement of photocatalytic property under irradiation of fluorescent light. In Proceedings of the 14th International Conference on Ion Implantation Technology, Taos, NM, USA, 22–27 September 2002; Volume 2002, pp. 705–708. [Google Scholar] [CrossRef]
- Borges, G.Á.; Elias, S.T.; da Silva, S.M.M.; Magalhães, P.O.; Macedo, S.B.; Ribeiro, A.P.D.; Guerra, E.N.S. In vitro evaluation of wound healing and antimicrobial potential of ozone therapy. J. Cranio Maxillofac. Surg. 2017, 45, 364–370. [Google Scholar] [CrossRef]
- Huth, K.C.; Jakob, F.M.; Saugel, B.; Cappello, C.; Brand, K. Effect of ozone on oral cells compared with established antimicrobials. Eur. J. Oral Sci. 2006, 114, 435–440. [Google Scholar] [CrossRef]
- Colombo, M.; Ceci, M.; Felisa, E.; Poggio, C.; Pietrocola, G. Cytotoxicity evaluation of a new ozonized olive oil. Eur. J. Dent. 2018, 12, 585–589. [Google Scholar] [CrossRef]
- Kashiwazaki, J.; Nakamura, K.; Hara, Y.; Harada, R.; Wada, I.; Kanemitsu, K. Evaluation of the cytotoxicity of various hand disinfectants and ozonated water to human keratinocytes in a cultured epidermal model. Adv. Skin Wound Care 2020, 33, 313–318. [Google Scholar] [CrossRef]
- Kuroda, K.; Azuma, K.; Mori, T.; Kawamoto, K.; Murahata, Y.; Tsuka, T.; Osaki, T.; Ito, N.; Imagawa, T.; Itoh, F.; et al. The safety and anti-tumor effects of ozonated water in vivo. Int. J. Mol. Sci. 2015, 16, 25108–25120. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, R.; Kan, F.; Jiang, F. Synthesis and characterization of TiO2 Nanoparticles. Asian J. Chem. 2014, 26, 655–659. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Kharat, A.N.; Fatholahi, L.; Beiranvand, Z.M. A review on synthesis of nano-TiO2 via different methods. J. Nanostructures 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Buraso, W.; Lachom, V.; Siriya, P.; Laokul, P. Synthesis of TiO2 nanoparticles via a simple precipitation method and photocatalytic performance. Mater. Res. Express 2018, 5, 115003. [Google Scholar] [CrossRef]
- Jeantelot, G.; Samy, O.C.; Julien, S.K.; Edy, A.H.; Anjum, D.H.; Sergei, L.; Moussab, H.; Luigi, C.; Jean-Marie, B. Morphology control of anatase TiO2 for well-defined surface chemistry. Phys. Chem. Chem. Phys. 2018, 20, 14362–14373. [Google Scholar] [CrossRef] [PubMed]
- Heringa, M.B.; Geraets, L.; van Eijkeren, J.C.H.; Vandebriel, R.J.; de Jong, W.H.; Oomen, A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology 2016, 10, 1515–1525. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Formation of crystalline nanosized titania in reverse micelles at room temperature. J. Mater. Chem. 2002, 12, 3677–3680. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatology Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef]
- Kurzmann, C.; Verheyen, J.; Coto, M.; Kumar, R.V.; Divitini, G.; Shokoohi-Tabrizi, H.A.; Verheyen, P.; de Moor, R.J.G.; Moritz, A.; Agis, H. In vitro evaluation of experimental light activated gels for tooth bleaching. Photochem. Photobiol. Sci. 2019, 18, 1009–1019. [Google Scholar] [CrossRef]
- Shirkavad, S.; Moslehifard, E. Effect of TiO2 nanoparticles on tensile strength of dental acrylic resins. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 197–203. [Google Scholar] [CrossRef]
- Meena, R.; Rani, M.; Pal, R.; Rajamani, P. Nano-TiO2-induced apoptosis by oxidative stress-mediated DNA damage and activation of p53 in human embryonic kidney cells. Appl. Biochem. Biotechnol. 2012, 167, 791–808. [Google Scholar] [CrossRef]
- Shukla, R.K.; Kumar, A.; Gurbani, D.; Pandey, A.K.; Singh, S.; Dhawan, A. TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology 2013, 7, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Rahman, Q.; Kashyap, M.; Singh, A.; Jain, G.; Jahan, S.; Lohani, M.; Lantow, M. A Pant. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum. Exp. Toxicol. 2013, 32, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand-Hammarström, B.; Akfur, C.M.; Andersson, P.O.; Lejon, C.; Österlund, L.; Bucht, A. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology 2012, 6, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Sharma, V.; Pandey, A.K.; Singh, S.; Sultana, S.; Dhawan, A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. Vitr. 2011, 25, 231–241. [Google Scholar] [CrossRef]
- Xue, C.; Wu, J.; Lan, F.; Liu, W.; Yang, X.; Zeng, F.; Xu, H. Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J. Nanosci. Nanotechnol. 2010, 10, 8500–8507. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, X.; Wang, Y.; Peng, S.; Yue, B.; Fan, C.; Chen, W.; Li, X. Comparative toxicities of bismuth oxybromide and titanium dioxide exposure on human skin keratinocyte cells. Chemosphere 2015, 135, 83–93. [Google Scholar] [CrossRef]
- Wright, C.; Iyer, A.K.V.; Wang, L.; Wu, N.; Yakisich, J.S.; Rojanasakul, Y.; Azad, N. Effects of titanium dioxide nanoparticles on human keratinocytes. Drug Chem. Toxicol. 2017, 40, 90–100. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.; Paulraj, R. Role of macrophage (M1 and M2) in titanium-dioxide nanoparticle-induced oxidative stress and inflammatory response in rat. Appl. Biochem. Biotechnol. 2016, 180, 1257–1275. [Google Scholar] [CrossRef]
- El-Bassyouni, G.T.; Eshak, M.G.; Barakat, I.A.H.; Khalil, W.K.B. Immunotoxicity evaluation of novel bioactive composites in male mice as promising orthopaedic implants. Cent. Eur. J. Immunol. 2017, 42, 54–67. [Google Scholar] [CrossRef]
- Jawaad, R.S.; Sultan, K.F.; Al-Hamadani, A.H. Synthesis of silver nanoparticles. J. Eng. Appl. Sci. 2014, 9, 586–592. [Google Scholar] [CrossRef]
- Habouti, S.; Solterbeck, C.H.; Es-Souni, M. Synthesis of silver nano-fir-twigs and application to single molecules detection. J. Mater. Chem. 2010, 20, 5215–5219. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; MlOzNiak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced electrical conductivity of silver nanoparticles for high frequency electronic applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater.Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.G.; Gonçalves, P.J.R.d.; Ottoni, C.A.; Ruiz, R.d.; Morgano, M.A.; de Araújo, W.L.; de Melo, I.S.; de Souza, A.O. Functional textiles impregnated with biogenic silver nanoparticles from Bionectria ochroleuca and its antimicrobial activity. Biomed. Microdevices 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, Y.; Cui, J.; Gan, L. The antibacterial properties and safety of a nanoparticle-coated parquet floor. Coatings 2019, 9, 403. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Hoet, P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Boegli, L.; James, G.; Velasquillo, C.; Sanchez-Sanchez, R.; Martinez-Martinez, R.E.; Martínez-Castañón, G.A.; Martinez-Gutierrez, F. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C 2015, 55, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Salaie, R.N.; Besinis, A.; Le, H.; Tredwin, C.; Handy, R.D. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Mater. Sci. Eng. C 2020, 107, 110210. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.; Woodward, B. A healthy future: Platinum in medical applications platinum group metals enhance the quality of life of the global population. Platin. Metals Rev. 2011, 55, 98–107. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Meyer-Lueckel, H. Systematic review on noninvasive treatment of root caries lesions. J. Dent. Res. 2015, 94, 261–271. [Google Scholar] [CrossRef]
- Ramirez, E.; Eradès, L.; Philippot, K.; Lecante, P.; Chaudret, B. Shape control of platinum nanoparticles. Adv. Funct. Mater. 2007, 17, 2219–2228. [Google Scholar] [CrossRef]
- Bigall, N.C.; Härtling, T.; Klose, M.; Simon, P.; Eng, L.M.; Eychmüller, A. Monodisperse platinum nanospheres with adjustable diameters from 10 to 100 nm: Synthesis and distinct optical properties. Nano Lett. 2008, 8, 4588–4592. [Google Scholar] [CrossRef]
- Islam, M.A.; Bhuiya, M.A.K.; Islam, M.S. A review on chemical synthesis process of platinum nanoparticles. Asia Pacific J. Energy Environ. 2014, 1, 103–116. [Google Scholar] [CrossRef]
- Loan, T.T.; Do, L.T.; Yoo, H. Platinum nanoparticles induce apoptosis on raw 264.7 macrophage cells. J. Nanosci. Nanotechnol. 2018, 18, 861–864. [Google Scholar] [CrossRef]
- Konieczny, P.; Goralczyk, A.G.; Szmyd, R.; Skalniak, L.; Koziel, J.; Filon, F.L.; Crosera, M.; Cierniak, A.; Zuba-Surma, E.K.; Borowczyk, J.; et al. Effects triggered by platinum nanoparticles on primary keratinocytes. Int. J. Nanomed. 2013, 8, 3963. [Google Scholar] [CrossRef]
- Labrador-Rached, C.J.; Browning, R.T.; Braydich-Stolle, L.K.; Comfort, K.K. Toxicological implications of platinum nanoparticle exposure: Stimulation of intracellular stress, inflammatory response, and akt signaling in vitro. J. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-X.; Gu, J.-L.; Cao, J.-M. The acute toxic effects of platinum nanoparticles on ion channels, transmembrane potentials of cardiomyocytes in vitro and heart rhythm in vivo in mice. Int. J. Nanomed. 2019, 14, 5595–5609. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A. Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Ali, D.; Alarifi, S.; Alkahtani, S.; Almansour, M. Green platinum nanoparticles interaction with HEK293 cells: Cellular toxicity, apoptosis, and genetic damage. Dose Response 2018, 16. [Google Scholar] [CrossRef]
- Şahin, B.; Aygün, A.; Gündüz, H.; Şahin, K.; Demir, E.; Akocak, S.; Şen, F. Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids Surf. B Biointerfaces 2018, 163, 119–124. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Oladipo, A.O.; Msagati, T.A.M.; Lebelo, S.L.; Meddows-Taylor, S.; More, G.K. Novel silver-platinum bimetallic nanoalloy synthesized from Vernonia mespilifolia extract: Antioxidant, antimicrobial, and cytotoxic activities. Arab. J. Chem. 2020, 13, 6639–6648. [Google Scholar] [CrossRef]
- Suh, Y.; Patel, S.; Kaitlyn, R.; Gandhi, J.; Joshi, G.; Smith, N.L.; Khan, S.A. Clinical utility of ozone therapy in dental and oral medicine. Med. Gas. Res. 2019, 9, 163–167. [Google Scholar] [CrossRef]
- Hashimoto, M.; Sasaki, J.I.; Yamaguchi, S.; Kawai, K.; Kawakami, H.; Iwasaki, Y.; Imazato, S. Gold nanoparticles inhibit matrix metalloproteases without cytotoxicity. J. Dent. Res. 2015, 94, 1085–1091. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubojanski, A.; Dobrzynski, M.; Nowak, N.; Rewak-Soroczynska, J.; Sztyler, K.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Wiglusz, K.; et al. Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry. Nanomaterials 2021, 11, 259. https://doi.org/10.3390/nano11020259
Lubojanski A, Dobrzynski M, Nowak N, Rewak-Soroczynska J, Sztyler K, Zakrzewski W, Dobrzynski W, Szymonowicz M, Rybak Z, Wiglusz K, et al. Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry. Nanomaterials. 2021; 11(2):259. https://doi.org/10.3390/nano11020259
Chicago/Turabian StyleLubojanski, Adam, Maciej Dobrzynski, Nicole Nowak, Justyna Rewak-Soroczynska, Klaudia Sztyler, Wojciech Zakrzewski, Wojciech Dobrzynski, Maria Szymonowicz, Zbigniew Rybak, Katarzyna Wiglusz, and et al. 2021. "Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry" Nanomaterials 11, no. 2: 259. https://doi.org/10.3390/nano11020259
APA StyleLubojanski, A., Dobrzynski, M., Nowak, N., Rewak-Soroczynska, J., Sztyler, K., Zakrzewski, W., Dobrzynski, W., Szymonowicz, M., Rybak, Z., Wiglusz, K., & Wiglusz, R. J. (2021). Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry. Nanomaterials, 11(2), 259. https://doi.org/10.3390/nano11020259









