Co-Zn-MOFs Derived N-Doped Carbon Nanotubes with Crystalline Co Nanoparticles Embedded as Effective Oxygen Electrocatalysts
Abstract
1. Introduction
2. Materials and Methods
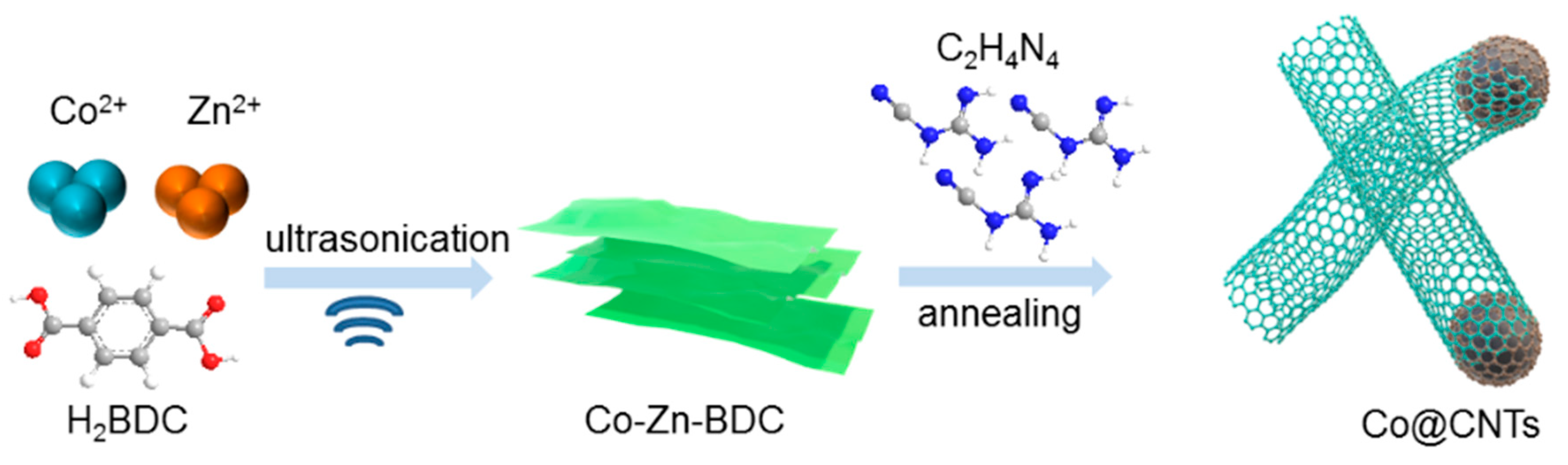
2.1. Preparation of Co-Zn-BDC
2.2. Preparation of Co@CNTs-T and Control Samples
2.3. Sample Characterization Methods
2.4. Electrochemical Measurement
3. Results
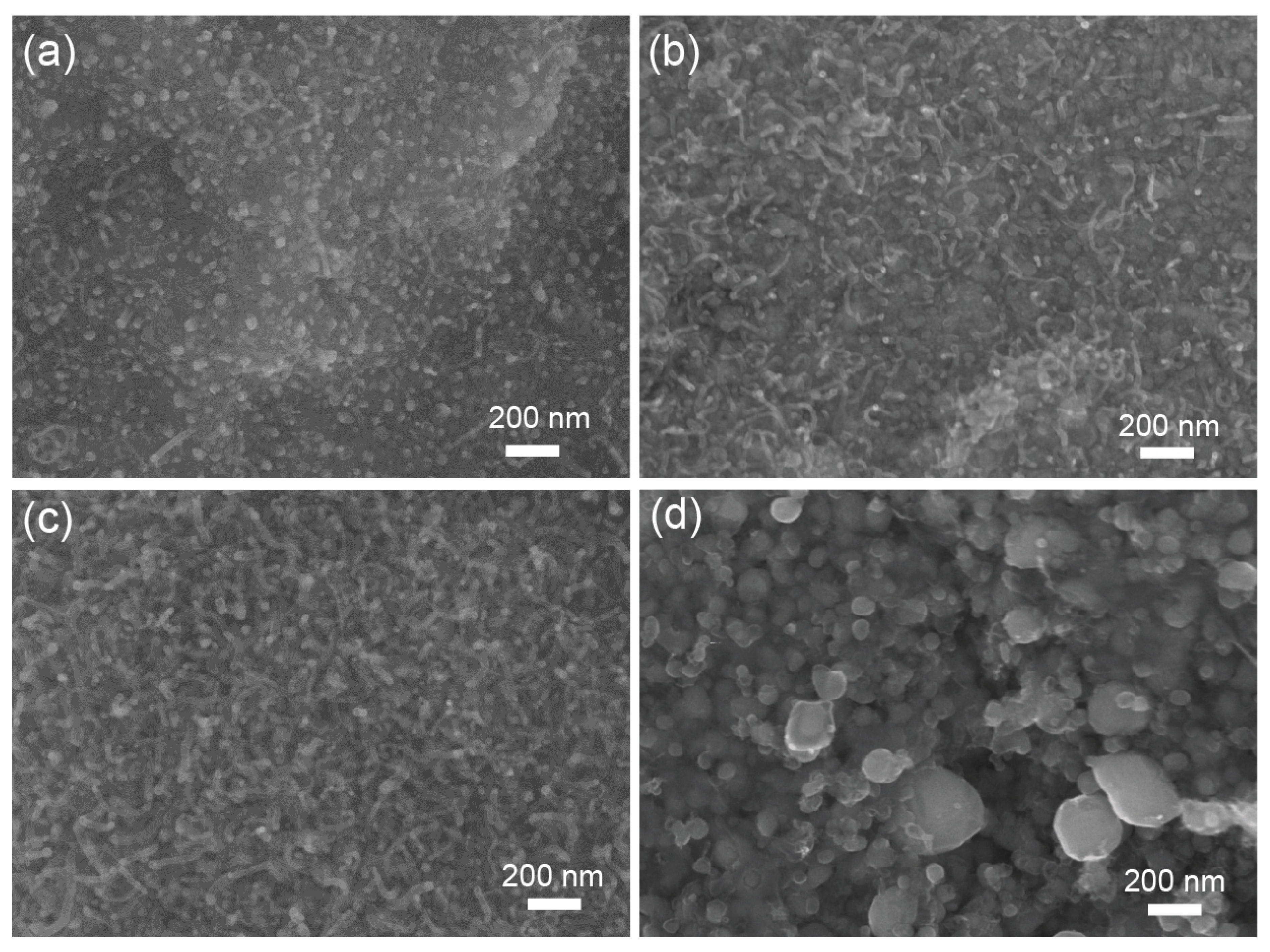
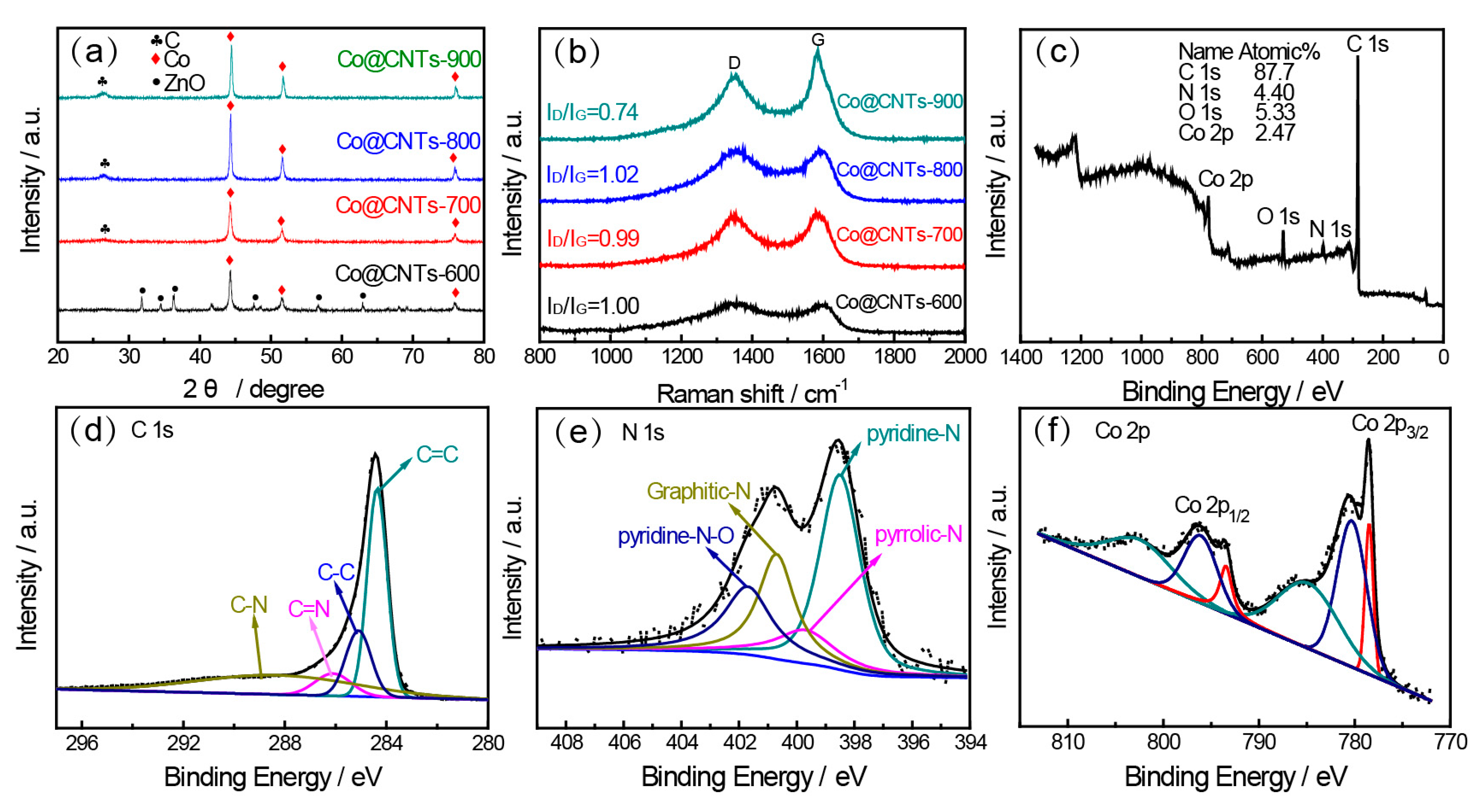
3.1. Structural Properties of the Co@CNTs-T
3.2. Electrocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, J.; Ye, D.; Zhu, X.; Liao, Q.; Zheng, J. Optimization of inner diameter of tubular bamboo charcoal anode for a microbial fuel cell. Int. J. Hydrog. Energ. 2014, 39, 19242–19248. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Li, M.; Zhang, Q.; Wang, Z.; Dai, Y.; Zhang, X.; Liu, Y.; Wang, M.H.; Huang, B. Adsorption of gaseous ethylene via induced polarization on plasmonic photocatalyst Ag/AgCl/TiO2 and subsequent photodegradation. Appl. Catal. B-Environ. 2018, 220, 356–361. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Self-supported earth-abundant nanoarrays as efficient and robust electrocatalysts for energy-related reactions. ACS Catal. 2018, 8, 6707–6732. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent progress in oxygen electrocatalysts for zinc-air batteries. Small. 2017, 1, 1700209–1700225. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.N.; Cui, H.; Zhou, Z. Bifunctional electrocatalysts for rechargeable Zn-air batteries. Chin. J. Catal. 2019, 40, 1298–1310. [Google Scholar] [CrossRef]
- LV, H.; Mu, S. Nano-ceramic support materials for low temperature fuel cell catalysts. Nanoscale 2014, 6, 5063–5074. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Wang, Y.; Jiang, L.; Wu, Z.; Liu, D.J.; Zhuang, L.; et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Xu, M.J.; Liu, J.; Ge, J.J.; Liu, C.P.; Xing, W. Research Progress of Metal-Nitrogen-Carbon Catalysts toward Oxygen Reduction Reaction in Changchun Institute of Applied Chemistry. J. Electrochem. 2020, 26, 464–473. [Google Scholar]
- Zhang, B.; Zhang, J.; Tan, X.; Tan, D.; Shi, J.; Zhang, F.; Liu, L.; Su, Z.; Han, B.; Zheng, L.; et al. One-step synthesis of ultrathin α-Co (OH)2 nanomeshes and their high electrocatalytic activity toward the oxygen evolution reaction. Chem. Commun. 2018, 54, 4045–4048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qiao, J.; Yang, L.; Zhang, J. A review of graphene-based nanostructural materials for both catalyst supports and metal-free catalysts in PEM fuel cell oxygen reduction reactions. Adv. Energy Mater. 2014, 4, 1301523. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov., J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Cai, Q.; Vasileff, A.; Li, L.; Han, Y.; Chen, Y.; Qiao, S. Molecule-level g-C3N4 coordinated transition metals as a new class of electrocatalysts for oxygen electrode reactions. J. Am. Chem. Soc. 2017, 139, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.Q.; Yang, L.J.; Zou, L.L.; Zou, Z.Q.; Chen, C.; Hu, Z.; Yang, H. Single Cobalt Atom and N Codoped Carbon Nanofibers as Highly Durable Electrocatalyst for Oxygen Reduction Reaction. ACS Catal. 2017, 7, 6864–6871. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.M.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2019, 12, 250–260. [Google Scholar] [CrossRef]
- Gao, K.; Wang, B.; Tao, L.; Cunning, B.V.; Zhang, Z.; Wang, S.; Ruoff, R.; Qu, L. Efficient Metal-Free Electrocatalysts from N-Doped Carbon Nanomaterials: Mono-Doping and Co-Doping. Adv. Mater. 2019, 31, 1805121. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Dai, S.; Qiao, S. Graphene oxide-polydopamine derived N, S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.C.; Pan, L.; Shen, G.-Q.; Mahmood, N.; Ma, Y.-H.; Shi, Y.; Jia, W.; Wang, L.; Zhang, X.; et al. Engineering cobalt defects in cobalt oxide for highly efficient electrocatalytic oxygen evolution. ACS Catal. 2018, 8, 3803–3811. [Google Scholar] [CrossRef]
- Hu, C.; Yi, Z.; She, W.; Wang, J.; Xiao, J.; Wang, S. Urchin-like non-precious-metal bifunctional oxygen electrocatalysts: Boosting the catalytic activity via the In-situ growth of heteroatom (N, S)-doped carbon nanotube on mesoporous cobalt sulfide/carbon spheres. J. Colloid Interf. Sci. 2018, 524, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Feldblyum, J.I.; Wong-Foy, A.G.; Matzger, A.J. Non-interpenetrated IRMOF-8: Synthesis, activation, and gas sorption. Chem. Commun. 2012, 79, 9828–9830. [Google Scholar] [CrossRef] [PubMed]
- Dau, P.V.; Tanabea, K.K.; Cohen, S.M. Functional group effects on metal–organic framework topology. Chem. Commun. 2012, 48, 9370–9372. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, S.B.; Wiktor, C.; Ramakrishnan, A.; Weßing, J.; Schneemann, R.; Tendeloob, G.V.; Fischer, R.A. Lewis base mediated efficient synthesis and solvation-like host-guest chemistry of covalent organic framework-1. Chem. Commun. 2013, 49, 463–465. [Google Scholar] [CrossRef]
- Li, Y.; Jia, B.; Fan, Y.; Zhu, K.; Li, G.; Su, C.-Y. Bimetallic zeolitic imidazolite framework derived carbon nanotubes embedded with Co nanoparticles for efficient bifunctional oxygen electrocatalyst. Adv. Energy Mater. 2018, 8, 1702048. [Google Scholar] [CrossRef]
- Peera, S.G.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Sustainable Synthesis of Co@NC Core Shell Nanostructures from Metal Organic Frameworks via Mechanochemical Coordination Self-Assembly: An Efficient Electrocatalyst for Oxygen Reduction Reaction. Small 2018, 14, 1800441. [Google Scholar] [CrossRef]
- Wang, H.F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Han, B.; Ou, X.; Deng, Z.; Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.-J.; et al. Ni Metal-Organic Frameworks Monolayers for Photoreduction of Diluted CO2: Metal Nodes-Dependent Activity and Selectivity. Angew. Chem. In. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 6149. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Min, S.; Xue, Y.; Tian, L.; Lei, Y.; Wang, F. In situ growth and activation of an amorphous MoS x catalyst on Co-containing metal-organic framework nanosheets for highly efficient dye-sensitized H2 evolution. New J. Chem. 2019, 43, 4152–4159. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Feng, T.; Qin, H.; Zhang, M. Co@C Nanoparticle Embedded Hierarchically Porous N-Doped Hollow Carbon for Efficient Oxygen Reduction. Chem. Eur. J. 2018, 24, 10178–10185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, F.; Zhang, J.; Chen, Z.; Xu, Q.; Guo, S. Cobalt-Carbon Core-Shell Nanoparticles Aligned on Wrinkle of N-Doped Carbon Nanosheets with Pt-Like Activity for Oxygen Reduction. Small 2016, 12, 2839–2845. [Google Scholar] [CrossRef]
- Meng, Z.; Cai, S.; Wang, R.; Tang, H.; Song, S.; Tsiakarascde, P. Bimetallic−Organic Framework-derived Hierarchically Porous Co-Zn-N-C as Efficient Catalyst for Acidic Oxygen Reduction Reaction. Appl. Catal. B-Environ. 2019, 244, 120–127. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Kashyap, V.; Bhange, S.N.; Baek, J.B.; Kurungot, S. Nanoporous graphene enriched with Fe/Co-N active sites as a promising oxygen reduction electrocatalyst for anion exchange membrane fuel cells. Adv. Funct. Mater. 2016, 26, 2150–2162. [Google Scholar] [CrossRef]
- Yu, H.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.I.N.; Zhao, Y.; Zhou, C.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Nitrogen-Doped Porous Carbon Nanosheets Templated from g-C3N4 as Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Z.; Jiang, F.; Yang, L.; Qian, J.; Zhou, Y.; Li, W.; Hong, M. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions. Nanoscale 2014, 6, 6590–6602. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, X.; Cao, Y.; Chen, H.-S.; Yang, P. Ni/Ni3C core–shell nanoparticles encapsulated in N-doped bamboo-like carbon nanotubes towards efficient overall water splitting. Inorg. Chem. Front. 2019, 6, 1073–1080. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Parwaiz, S.; Lecoeuvre, F.; Schmidt, J.; Pradhan, D.; Van Der Voort, P. Illustrating the Role of Quaternary-N of BINOL Covalent Triazine-Based Frameworks in Oxygen Reduction and Hydrogen Evolution Reactions. ACS Appl. Mater Inter. 2020, 12, 44689–44699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Qin, D.; Xue, Z.; Lu, X. Fabrication of iron-doped cobalt oxide nanocomposite films by electrodeposition and application as electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2014, 320, 73–82. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano-Jiménez, M.J.; Ye, G.; Kim, N.; Samuel, E.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, J.; Xu, Y.; Wang, X.; Ni, J.; Li, Y.; Niemantsverdriet, H. Cobalt and cobalt carbide on alumina/NiAl (110) as a catalyst model. Catal. Sci Technol. 2017, 7, 5893–5899. [Google Scholar] [CrossRef]
- Han, H.; Bai, Z.; Wang, X.; Chao, S.; Liu, J.; Kong, Q.; Yang, X.; Yang, L. Highly Dispersed Co Nanoparticles Inlayed in S, N-doped Hierarchical Carbon Nanoprisms Derived from Co-MOFs as Efficient Electrocatalysts for Oxygen Reduction Reaction. Catal. Today 2018, 318, 126–131. [Google Scholar] [CrossRef]
- Lu, H.S.; Zhang, H.; Liu, R.; Zhang, X.; Zhao, H.; Wang, G. Macroscale cobalt-MOFs derived metallic Co nanoparticles embedded in N-doped porous carbon layers as efficient oxygen electrocatalysts. Appl. Surf. Sci. 2017, 392, 402–409. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Chen, L.; Liu, Z.; Long, L.; Wang, S.; Liu, C.; Dong, S.; Jia, J. Graphitic Carbon Nitride (g-C3N4)-Derived Bamboo-Like Carbon Nanotubes/Co Nanoparticles Hybrids for Highly Efficient Electrocatalytic Oxygen Reduction. ACS Appl. Mater. Inter. 2020, 12, 4463–4472. [Google Scholar] [CrossRef]
- Liu, X.; Zhuo, M.; Zhang, W.; Gao, M.; Liu, X.-H.; Sun, B.; Wu, J. One-step ultrasonic synthesis of Co/Ni-catecholates for improved performance in oxygen reduction reaction. Ultrason. Sonochem. 2020, 67, 105179. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liu, X.; Gao, M.; Shang, H.; Liu, X. Co-Zn-MOFs Derived N-Doped Carbon Nanotubes with Crystalline Co Nanoparticles Embedded as Effective Oxygen Electrocatalysts. Nanomaterials 2021, 11, 261. https://doi.org/10.3390/nano11020261
Zhang W, Liu X, Gao M, Shang H, Liu X. Co-Zn-MOFs Derived N-Doped Carbon Nanotubes with Crystalline Co Nanoparticles Embedded as Effective Oxygen Electrocatalysts. Nanomaterials. 2021; 11(2):261. https://doi.org/10.3390/nano11020261
Chicago/Turabian StyleZhang, Wendi, Xiaoming Liu, Man Gao, Hong Shang, and Xuanhe Liu. 2021. "Co-Zn-MOFs Derived N-Doped Carbon Nanotubes with Crystalline Co Nanoparticles Embedded as Effective Oxygen Electrocatalysts" Nanomaterials 11, no. 2: 261. https://doi.org/10.3390/nano11020261
APA StyleZhang, W., Liu, X., Gao, M., Shang, H., & Liu, X. (2021). Co-Zn-MOFs Derived N-Doped Carbon Nanotubes with Crystalline Co Nanoparticles Embedded as Effective Oxygen Electrocatalysts. Nanomaterials, 11(2), 261. https://doi.org/10.3390/nano11020261




