Facile Synthesis of Cobalt Ferrite (CoFe2O4) Nanoparticles in the Presence of Sodium Bis (2-ethyl-hexyl) Sulfosuccinate and Their Application in Dyes Removal from Single and Binary Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods and Equipment
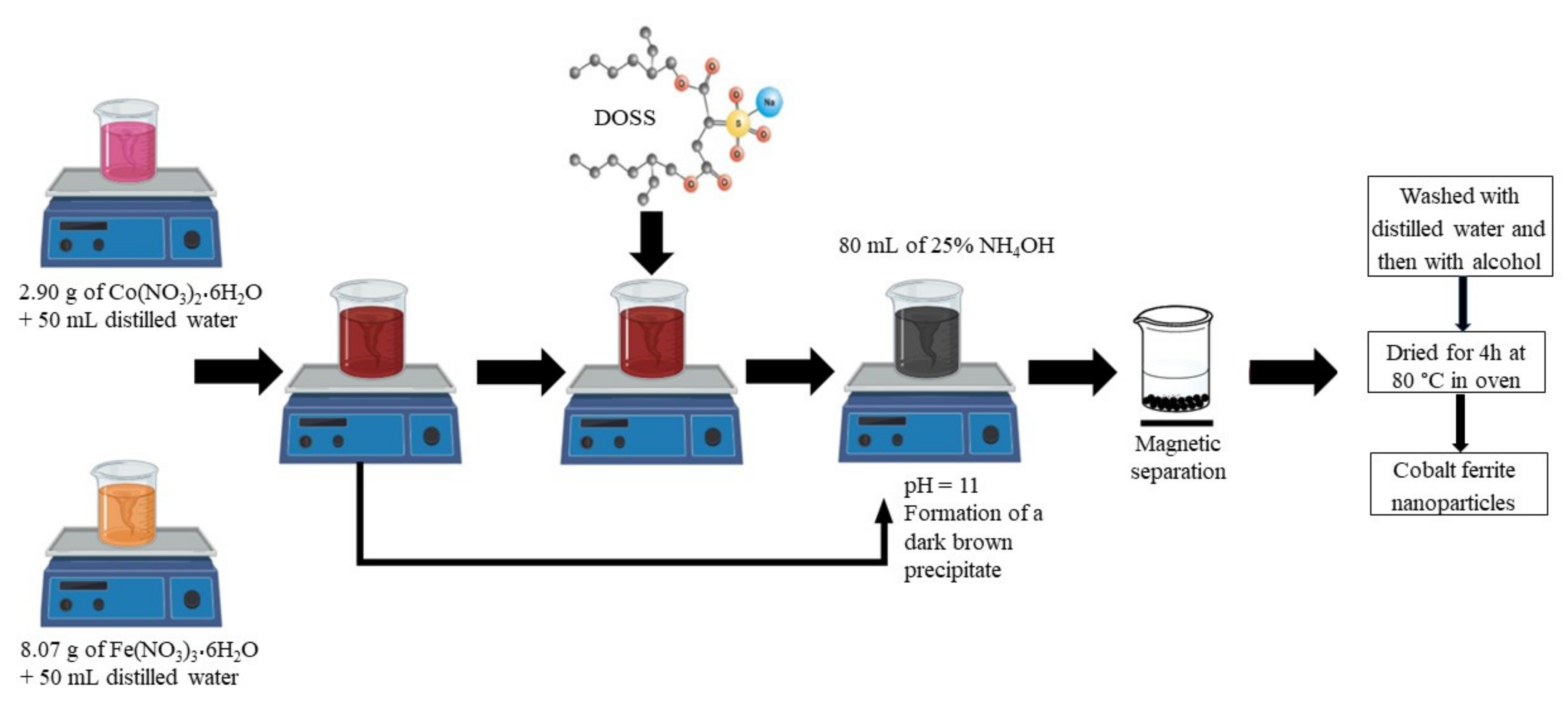
2.3. Synthesis Protocol
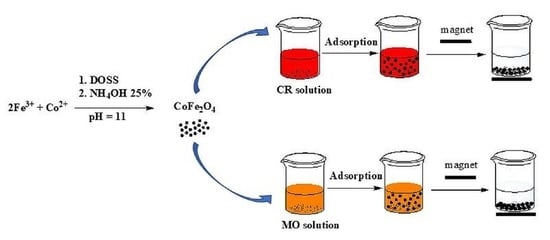
2.4. The Adsorption and Desorption Test
3. Results and Discussion
3.1. Spectroscopic Characterization of CoFe2O4 Samples (FTIR Spectroscopy)
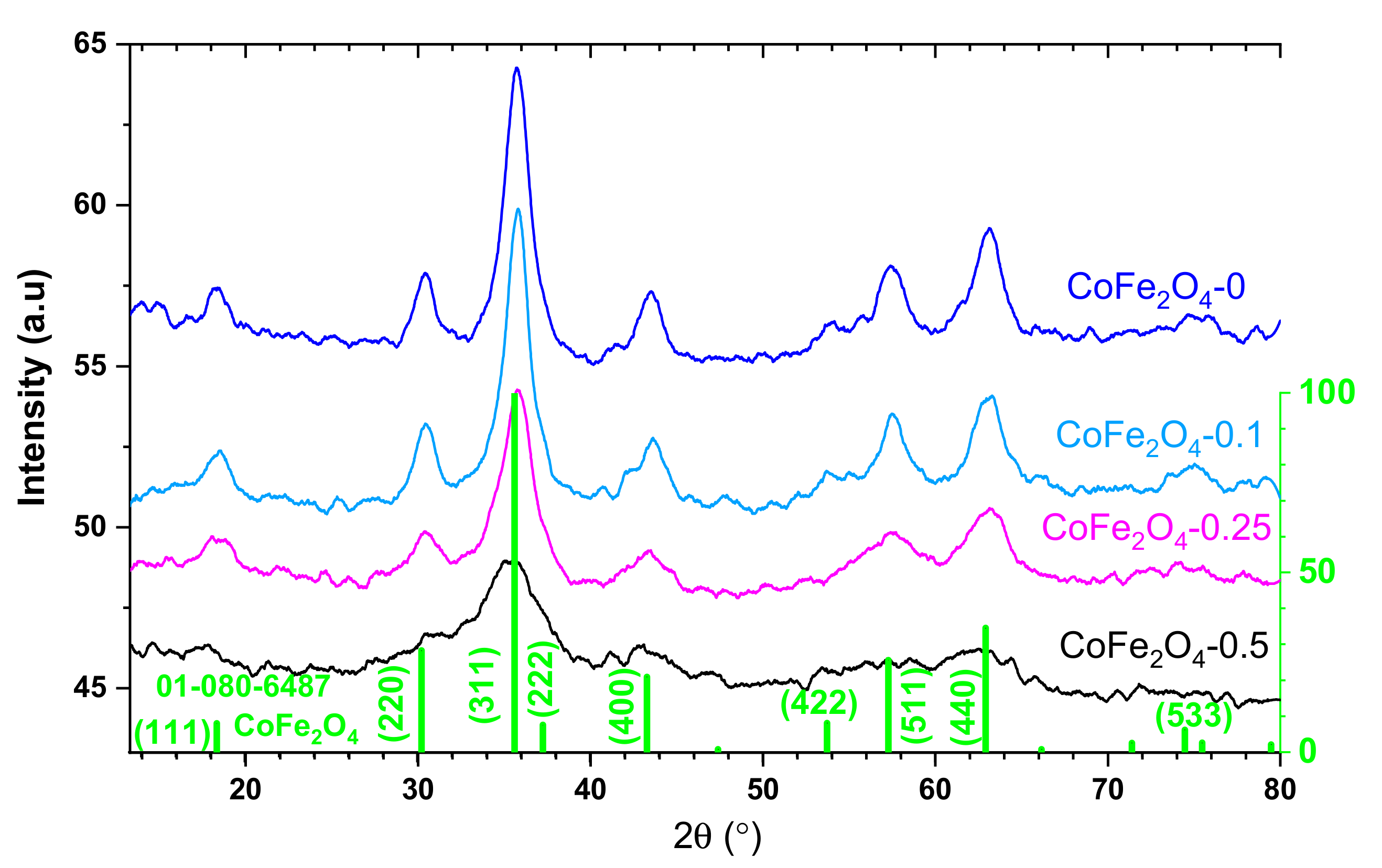
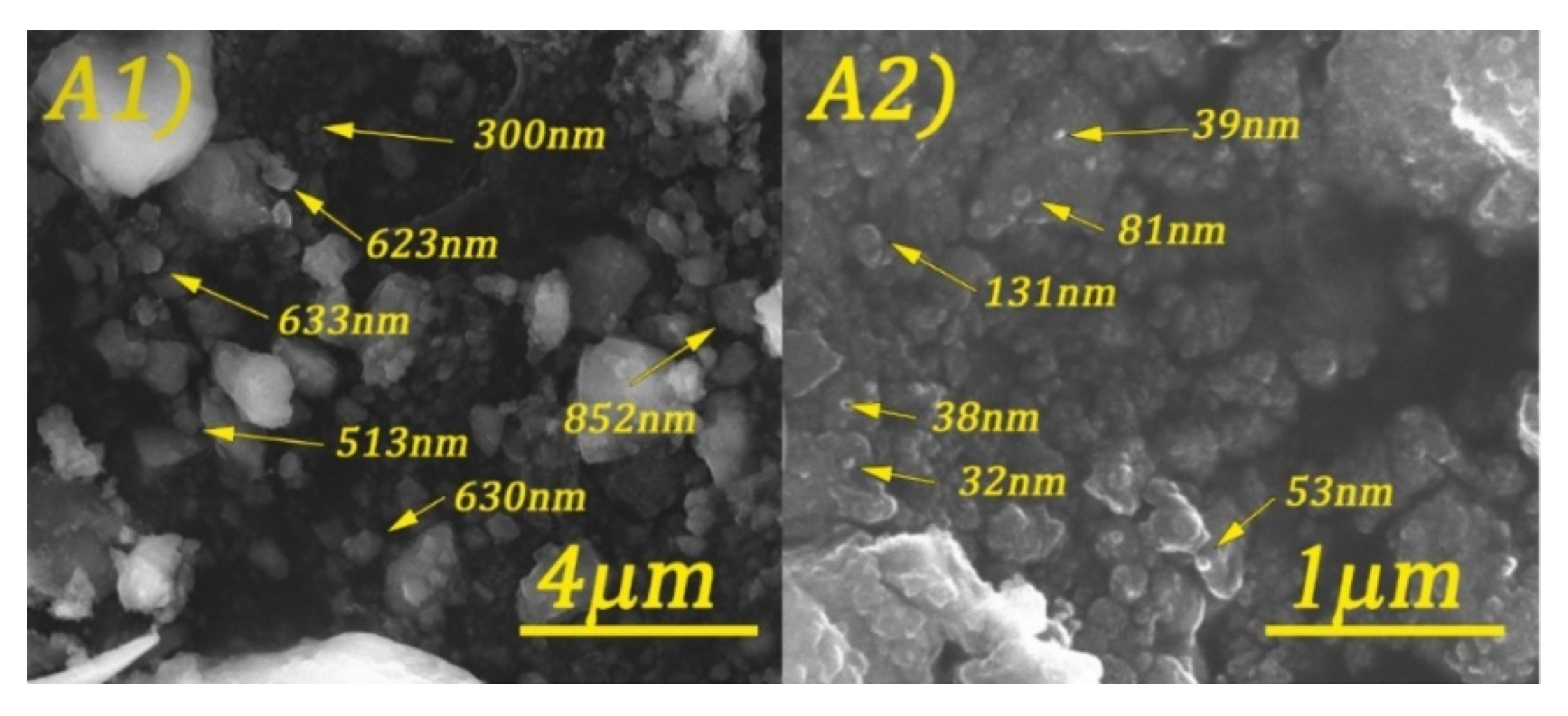
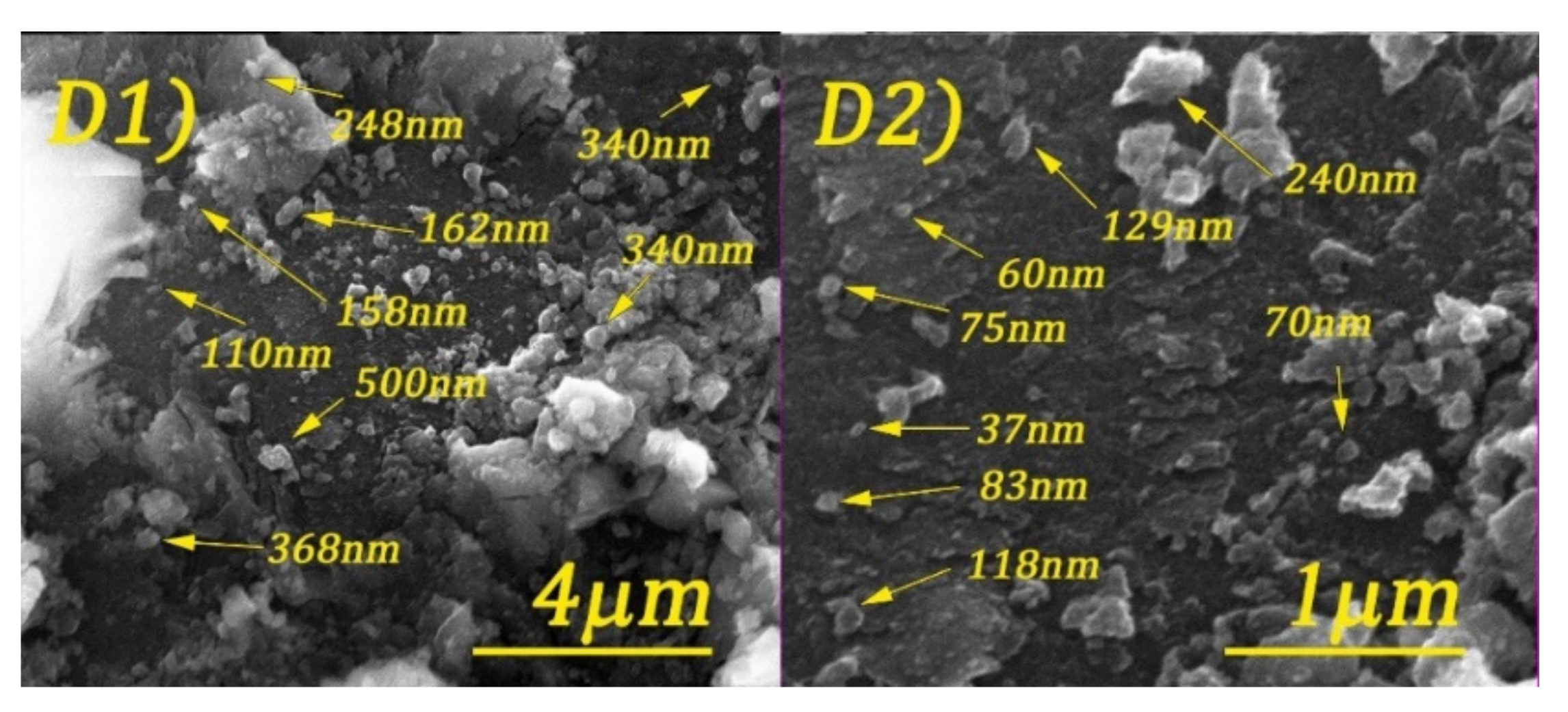
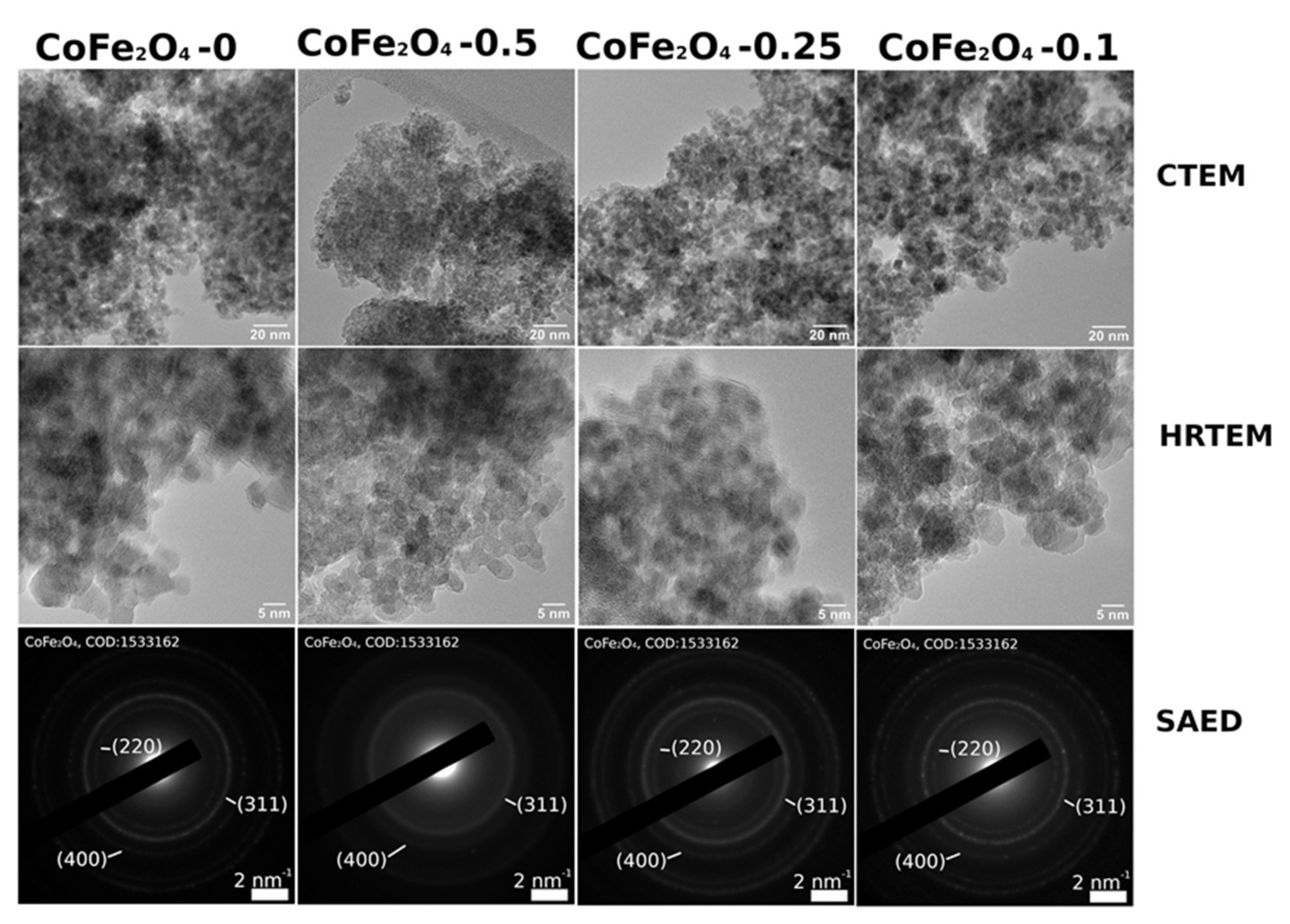
3.2. Structural and Morphological Characterization of CoFe2O4 Samples
3.3. Textural Properties of CoFe2O4 Samples
3.4. Magnetic Characterization of CoFe2O4 Samples
3.5. Removal of Anionic Dyes from Single and Binary Solutions by Adsorption on CoFe2O4 Samples
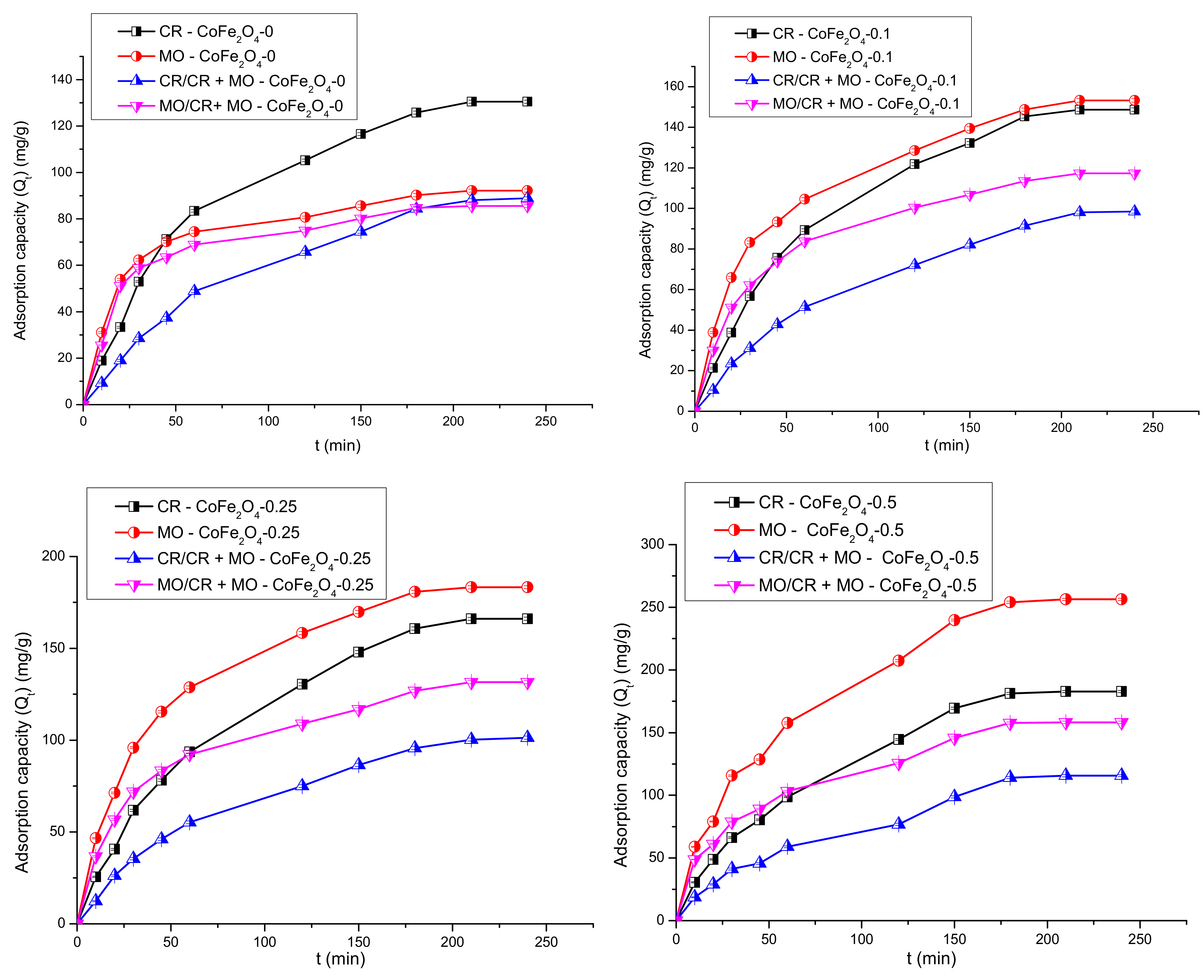
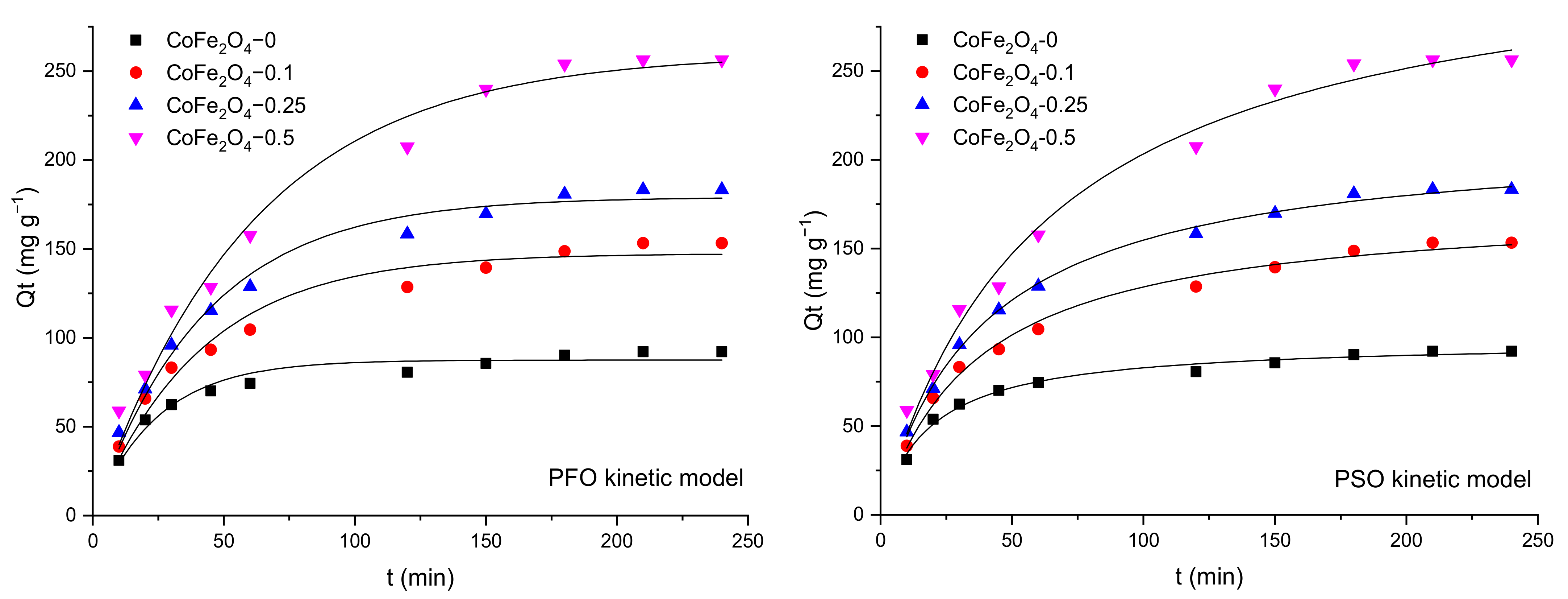
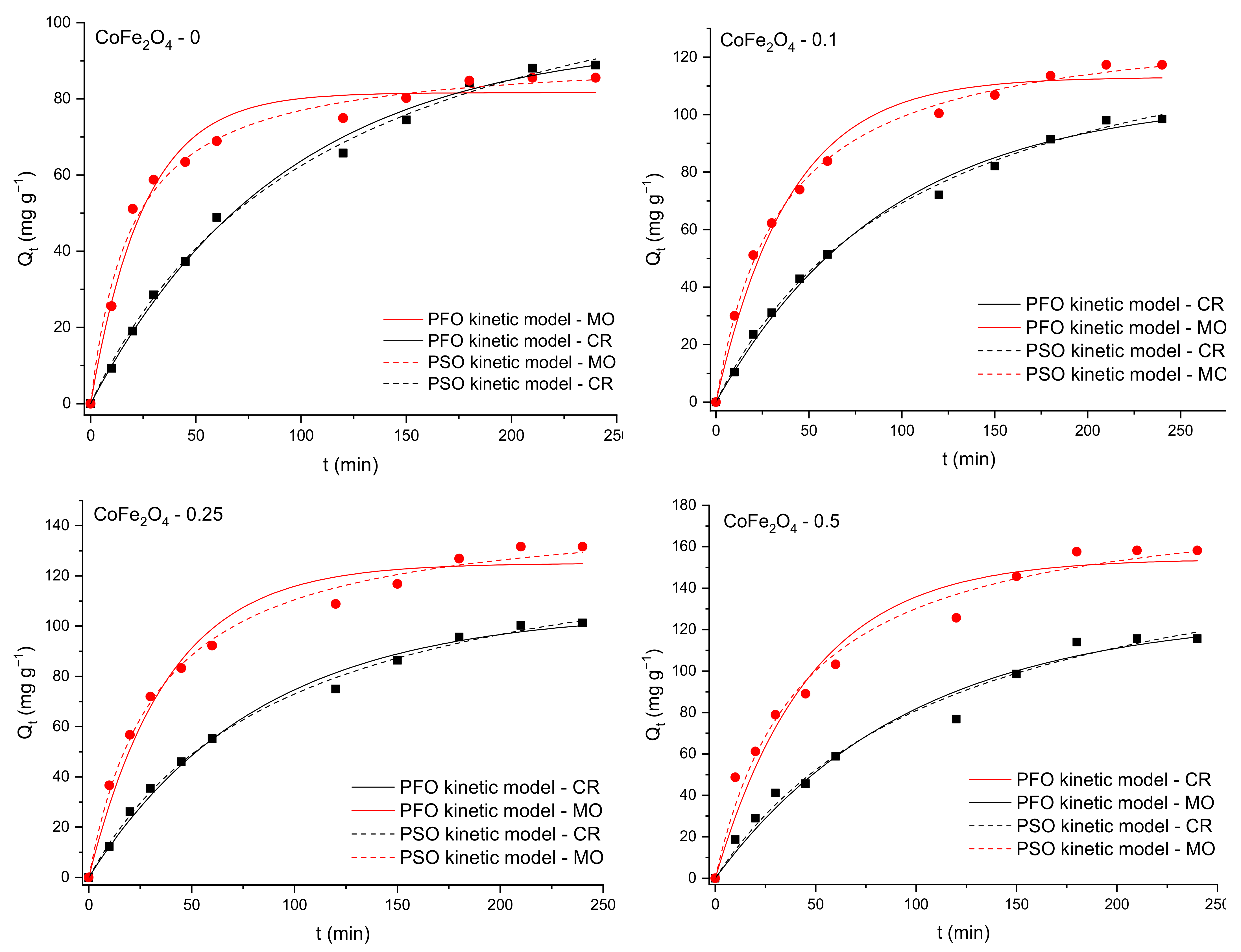
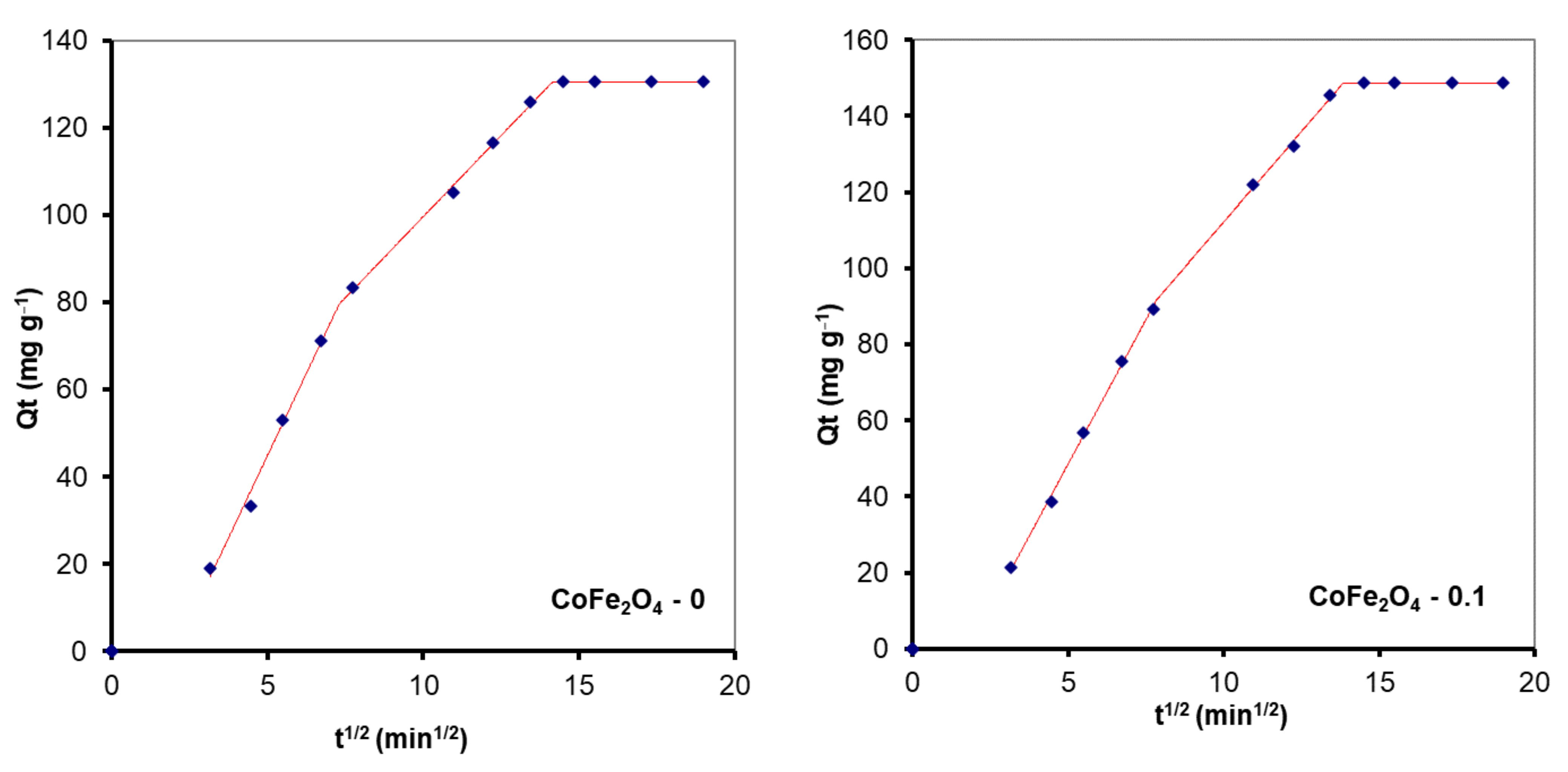
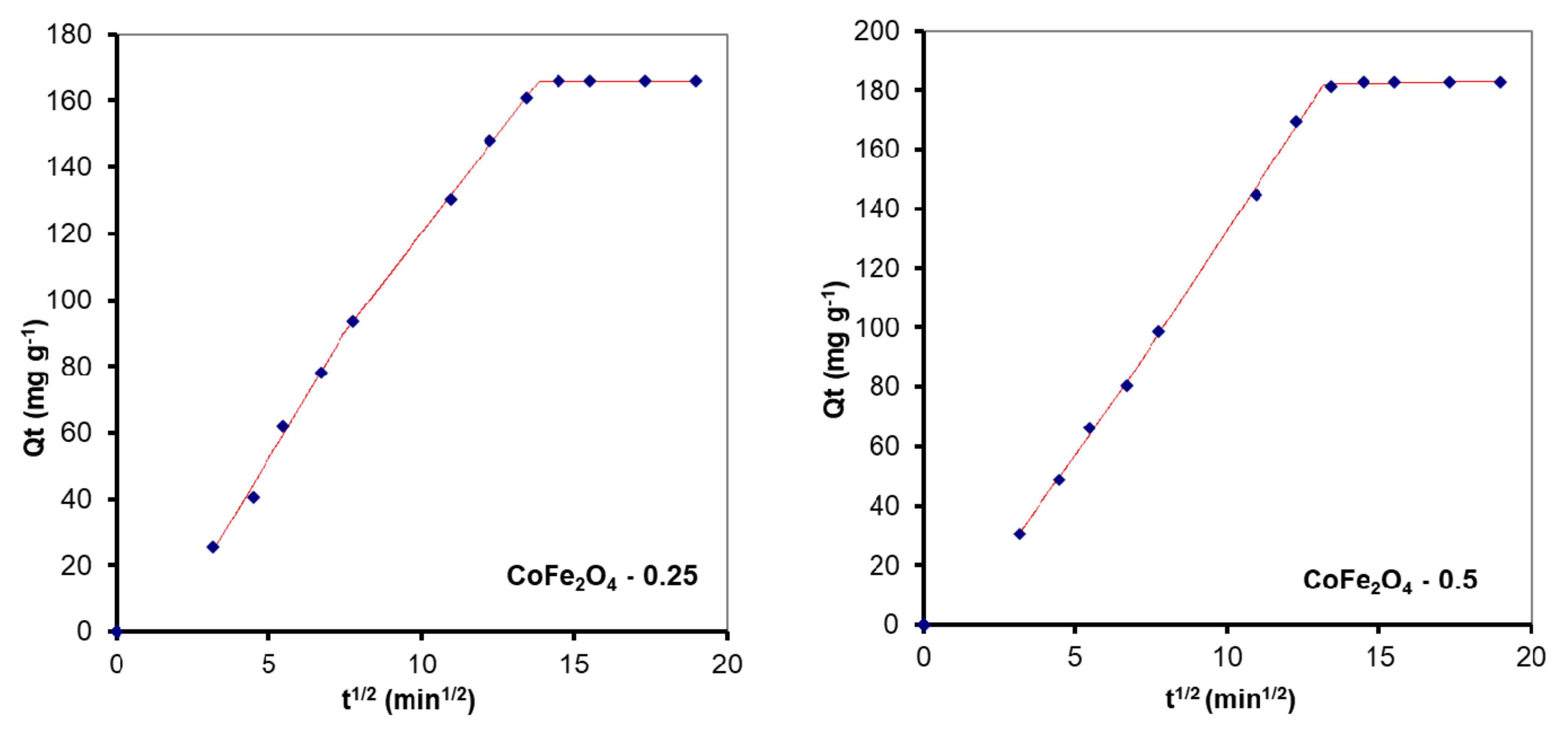
3.5.1. The Effect of the Contact Time and Fitting of Kinetic Models to the Adsorption Data
3.5.2. The Effect of pH on the Adsorption Performance
3.5.3. The Adsorption Isotherms
3.5.4. Desorption Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive amputation of hazardous azo dye Congo red from wastewater: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 14810–14853. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Alam, M.S.; Khanom, R.; Rahman, M.A. Removal of Congo Red Dye from Industrial Wastewater by Untreated Sawdust. Am. J. Environ. Prot. 2015, 4, 207–213. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Garvasis, J.; Prasad, A.R.; Shamsheera, K.O.; Jaseela, P.K.; Joseph, A. Efficient removal of Congo red from aqueous solutions using phytogenic aluminum sulfate nano coagulant. Mater. Chem. Phys. 2020, 251, 123040. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Joo, T.C.; Salleh, A.; Ang, B.C.; Afifi, A.M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr. Polym. 2017, 157, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, A.; Benhiti, R.; Ichou, A.A.; Carja, G.; Soudani, A.; Zerbet, M.; Sinan, F.; Chiban, M. Characterization and application of MgAl layered double hydroxide for methyl orange removal from aqueous solution. Mater. Today Proc. 2021, 37, 3793–3797. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Y.; Wang, J.; Zhang, Y.; Gao, Z.; Yang, Y.; Yang, K. Ultrasound-assisted adsorption of Congo red from aqueous solution using Mg-Al-CO3 layered double hydroxide. Appl. Clay Sci. 2019, 174, 100–109. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Adlii, A.; Bakry, B.M. Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/CH@Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr(VI) from water. Int. J. Biol. Macromol. 2019, 126, 402–413. [Google Scholar] [CrossRef]
- Sathishkumara, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B Biol. 2019, 200, 111655. [Google Scholar] [CrossRef]
- Masalvad, S.K.S.; Sakare, P.K. Application of photo Fenton process for treatment of textile Congo-red dye solution. Mater. Today Proc. 2021, 46, 5291–5297. [Google Scholar] [CrossRef]
- Yao, K.; Liu, Y.; Yang, H.; Yuan, J.; Shan, S. Polyaniline-modified 3D-spongy SnS composites for the enhanced visible light photocatalytic degradation of methyl orange. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125240. [Google Scholar] [CrossRef]
- Zwane, S.; Masheane, M.L.; Kuvarega, A.T.; Vilakati, G.D.; Mamba, B.B.; Nyoni, H.; Mhlanga, S.D.; Dlamini, D.S. Polyethersulfone/Chromolaena odorata (PES/CO) adsorptive membranes for removal of Congo red from water. J. Water Process Eng. 2019, 30, 100498. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Liu, L.; Gao, M.; Han, Z. A stable metal-organic framework nanofibrous membrane as photocatalyst for simultaneous removal of methyl orange and formaldehyde from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126359. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Makete, E. Removal of Congo red dye from aqueous media using Litchi seeds powder: Equilibrium, kinetics and thermodynamics. Phys. Chem. Earth 2021, 123, 103007. [Google Scholar] [CrossRef]
- Khaniabadi, Y.O.; Heydari, R.; Nourmoradi, H.; Basiri, H.; Basiri, H. Low-cost sorbent for the removal of aniline and methyl orange from liquid-phase: Aloe Vera leaves wastes. J. Taiwan Inst. Chem. Eng. 2016, 68, 90–98. [Google Scholar] [CrossRef]
- Goswami, M.; Chaturvedi, P.; Sonwani, R.K.; Gupta, A.D.; Singhania, R.R.; Giri, B.S.; Rai, B.N.; Singh, H.; Yadav, S.; Singh, R.S. Application of Arjuna (Terminalia arjuna) seed biochar in hybrid treatment system for the bioremediation of Congo red dye. Bioresour. Technol. 2020, 307, 123203. [Google Scholar] [CrossRef]
- Sonwani, R.K.; Swain, G.; Giri, B.S.; Singh, R.S.; Rai, B.N. Biodegradation of Congo red dye in a moving bed biofilm reactor: Performance evaluation and kinetic modeling. Bioresour. Technol. 2020, 302, 122811. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Abdou, A.E.H.; Shehata, A.K.; Header, H.M.A.; Hamed, E.A. Sustainable super fast adsorptive removal of Congo red dye from water by a novel technique based on microwave-enforced sorption process. J. Ind. Eng. Chem. 2018, 57, 28–36. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Pang, Y.L.; Lim, S.; Chong, C.W. Sonocatalytic degradation of Congo red by using green synthesized silver doped zinc oxide nanoparticles. Mater. Today Proc. 2021, 46, 1948–1953. [Google Scholar] [CrossRef]
- Ma, M.; Ying, H.; Cao, F.; Wang, Q.; Ai, N. Adsorption of congo red on mesoporous activated carbon prepared by CO2 physical activation. Chin. J. Chem. Eng. 2020, 28, 1069–1076. [Google Scholar] [CrossRef]
- Yönten, V.; Sanyürek, N.K.; Kivanç, M.R. A thermodynamic and kinetic approach to adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Vitis vinifera L. Surf. Interfaces 2020, 20, 100529. [Google Scholar] [CrossRef]
- Yu, K.L.; Lee, X.J.; Ong, H.C.; Chen, W.-H.; Chang, J.-S.; Lin, C.-S.; Show, P.L.; Ling, T.C. Adsorptive removal of cationic methylene blue and anionic Congo red dyes using wet-torrefied microalgal biochar: Equilibrium, kinetic and mechanism modeling. Environ. Pollut. 2021, 272, 115986. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/chitosan modified biochar composite for enhanced methyl orange removal based on adsorption and photo-Fenton process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126104. [Google Scholar] [CrossRef]
- Arab, C.; El Kurdi, R.; Patra, D. Efficient removal of Congo red using curcumin conjugated zinc oxide nanoparticles as new adsorbent complex. Chemosphere 2021, 276, 130158. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ur Rehman, H.; Ali, Z.; Qadeer, A.; Haseeb, A.; Ajmal, Z. Solvent assisted synthesis of hierarchical magnesium oxide flowers for adsorption of phosphate and methyl orange: Kinetic, isotherm, thermodynamic and removal mechanism. Surf. Interfaces 2021, 23, 100953. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Qian, G.; Wu, D.; Frost, R.L. Removal of methyl orange from aqueous solutions through adsorption by calcium aluminate hydrates. J. Colloid Interface Sci. 2014, 426, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, M.A.; Adebomi, J.I.; Abe, T.O.; Areo, F.I. Removal of aqueous Congo red and malachite green using ackee apple seed–bentonite composite. Colloids Interface Sci. Commun. 2020, 38, 100311. [Google Scholar] [CrossRef]
- Zayed, A.M.; Abdel Waheda, M.S.M.; Mohamed, E.A.; Sillanpää, M. Insights on the role of organic matters of some Egyptian clays in methyl orange adsorption: Isotherm and kinetic studies. Appl. Clay Sci. 2018, 166, 49–60. [Google Scholar] [CrossRef]
- Borth, K.W.; Galdino, C.W.; de Carvalho Teixeira, V.; Anaissi, F.J. Iron oxide nanoparticles obtained from steel waste recycling as a green alternative for Congo red dye fast adsorption. Appl. Surf. Sci. 2021, 546, 149126. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Saraf, R. Ag-doped hydroxyapatite as efficient adsorbent for removal of Congo red dye from aqueous solution: Synthesis, kinetic and equilibrium adsorption isotherm analysis. Micropor. Mesopor. Mater. 2016, 219, 134–144. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.S.D.; Banu, H.A.T.; Farzana, M.H.; Park, C.M. Hydrothermal synthesis of hydroxyapatite-reduced graphene oxide (1D-2D) hybrids with enhanced selective adsorption properties for methyl orange and hexavalent chromium from aqueous solutions. Chemosphere 2021, 276, 130200. [Google Scholar] [CrossRef]
- Raza, S.; Wen, H.; Peng, Y.; Zhang, J.; Li, X.; Liu, C. Fabrication of SiO2 modified biobased hydrolyzed hollow polymer particles and their applications as a removal of methyl orange dye and bisphenol-A. Eur. Polym. J. 2021, 144, 110199. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Zaini, M.A.A.; Arsad, A. Evaluation of dyes removal by beta-cyclodextrin adsorbent. Mater. Today Proc. 2021, 39, 907–910. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Liu, S.; Su, G.; Liu, X.; Tang, A.; Xue, J.; Zeng, M. Synthesis of dithiocarbamate-modified crosslinked poly(β−cyclodextrin-co-triethylenetetramine) micro-spheres for simultaneous and highly efficient removal of Cu2+ and methyl orange/thymol blue from wastewater. React. Funct. Polym. 2021, 159, 104809. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, A.; Chatterjee, S.; Nayek, H.P. Elucidation of selective adsorption study of Congo red using new Cadmium(II) metal-organic frameworks: Adsorption kinetics, isotherm and thermodynamics. J. Solid State Chem. 2021, 296, 121929. [Google Scholar] [CrossRef]
- Tor, A.; Cengeloglu, Y. Removal of congo red from aqueous solution by adsorption onto acid activated red mud. J. Hazard. Mater. 2006, B138, 409–415. [Google Scholar] [CrossRef]
- Kumari, D.; Mazumder, P.; Kumar, M.; Deka, J.P.; Shim, J. Simultaneous removal of Cong red and Cr(VI) in aqueous solution by using Mn powder extracted from battery waste solution. Groundw. Sustain. Dev. 2018, 7, 459–464. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.S.; Debnath, A.K.; Muthe, K.P.; Das, N.; Parhi, P. Cobalt ferrite nanoparticles prepared by microwave hydrothermal synthesis and adsorption efficiency for organic dyes: Isotherms, thermodynamics and kinetic studies. Adv. Powder Technol. 2020, 31, 4552–4562. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, Y.; Zhao, L.; Jiang, O. Adsorption capability for Congo red on nanocrystalline MFe2O4 (M = Mn, Fe Co, Ni) spinel ferrites. Chem. Eng. J. 2012, 181, 72–79. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Mohamed, A.A. Effective removal of indigo carmine dye from wastewaters by adsorption onto mesoporous magnesium ferrite nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100550. [Google Scholar] [CrossRef]
- An, S.; Liu, X.; Yang, L.; Zhang, L. Enhancement removal of crystal violet dye using magnetic calcium ferrite nanoparticle: Study insingle- and binary-solute systems. Chem. Eng. Res. Des. 2015, 94, 726–735. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Bastani, D. Direct dyes removal using modified magnetic ferrite nanoparticle. J. Environ. Health Sci. Eng. 2014, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Humelnicu, A.-C.; Cojocaru, C.; Pascariu Dorneanu, P.; Samoila, P.; Harabagiu, V. Novel chitosan-functionalized samarium-doped cobalt ferrite for adsorptive removal of anionic dye from aqueous solutions. Compets Rendus Chim. 2017, 20, 1026–1036. [Google Scholar] [CrossRef]
- Moghaddam, A.Z.; Ghiamati, E.; Pourashuri, A.; Allahresani, A. Modified nickel ferrite nanocomposite/functionalized chitosan as a novel adsorbent for the removal of acidic dyes. Int. J. Biol. Macromol. 2018, 120, 1714–1725. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Jeyanthi, J. Dynamic modelling of Alginate—Cobalt ferrite nanocomposite for removal of binary dyes from textile effluent. J. Environ. Chem. Eng. 2021, 9, 104924. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Dragne, M.; Stanica, N.; Munteanu, C.; Preda, S.; Oprea, O. Effect of surfactant concentration on textural, morphological and magnetic properties of CoFe2O4 nanoparticles and evaluation of their adsorptive capacity for Pb(II) ions. Ceram. Int. 2015, 41, 13553–13560. [Google Scholar] [CrossRef]
- Amirthavalli, C.; Thomas, J.M.; Nagaraj, K.; Prince, A.A.M. Facile room temperature CTAB-assisted synthesis of mesoporous nano-cobalt ferrites for enhanced magnetic behavior. Mater. Res. Bull. 2018, 100, 289–294. [Google Scholar] [CrossRef]
- De Medeiros, F.; Madigou, V.; Lopes-Moriyama, A.L.; Pereira de Souza, C.; Leroux, C. Synthesis of CoFe2O4 nanocubes. Nano-Struct. Nano-Objects 2020, 21, 100422. [Google Scholar] [CrossRef]
- Rana, S.; Philip, J.; Raj, B. Micelle based synthesis of cobalt ferrite nanoparticles and its characterization using Fourier Transform Infrared Transmission Spectrometry and Thermogravimetry. Mater. Chem. Phys. 2010, 124, 264–269. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Dosanjh, H.S.; Singh, H. Surface modified spinel cobalt ferrite nanoparticles for cationic dye removal: Kinetics and thermodynamics studies. J. Water Process Eng. 2016, 11, 152–161. [Google Scholar] [CrossRef]
- Tătăruş, A.; Simonescu, C.M.; Nechifor, G.; Scutariu, R.; Iancu, V.I.; Chiriac, L.F. Development of a new high-performance liquid chromatography method with diode array detector (HPLC-DAD) for the detection of Congo Red and Methyl Orange dyes from synthetic water solutions. UPB Sci. Bull. Ser. B 2021, 83, 4, in press. [Google Scholar]
- Simonescu, C.M.; Tătăruş, A.; Culiţă, D.C.; Stănică, N.; Ionescu, I.A.; Butoi, B.; Banici, A.-M. Comparative Study of CoFe2O4 Nanoparticles and CoFe2O4-Chitosan Composite for Congo Red and Methyl Orange Removal by Adsorption. Nanomaterials 2021, 11, 711. [Google Scholar] [CrossRef]
- Das, S.; Bououdina, M.; Manoharan, C. The influence of cationic surfactant CTAB on optical, dielectric and magnetic properties of cobalt ferrite nanoparticles. Ceram. Int. 2020, 46, 11705–11716. [Google Scholar] [CrossRef]
- Mahdikhah, V.; Ataie, A.; Babaei, A.; Sheibani, S.; Ow-Yang, C.W.; Khabbaz Abkenar, S. Control of structural and magnetic characteristics of cobalt ferrite by post-calcination mechanical milling. J. Phys. Chem. Solids 2019, 134, 286–294. [Google Scholar] [CrossRef]
- Shafi, V.P.M.K.; Gedanken, A.; Prozorov, R.; Balogh, J. Sonochemical Preparation and Size-Dependent Properties of Nanostructured CoFe2O4 Particles. Chem. Mater. 1998, 10, 3445–3450. [Google Scholar] [CrossRef]
- Emadi, H.; Kharat, A.N. Synthesis and characterization of ultrafine and mesoporous structure of cobalt ferrite. J. Ind. Eng. Chem. 2015, 21, 951–956. [Google Scholar] [CrossRef]
- Baraka, A. Investigation of temperature effect on surface-interaction and diffusion of aqueous-solution/porous-solid adsorption systems using diffusion—binding model. J. Environ. Chem. Eng. 2015, 3, 129–139. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbas, O.; Dogan, M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater. 2007, 101, 388–396. [Google Scholar] [CrossRef]
- Kumar, P.A.; Ray, M.; Chakraborty, S. Adsorption behaviour of trivalent chromium on amine-based polymer aniline formaldehyde condensate. Chem. Eng. J. 2009, 149, 340–347. [Google Scholar] [CrossRef]
- Malash, G.F.; El-Khaiary, M.I. Piecewise linear regression: A statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem Eng. J. 2010, 163, 256–263. [Google Scholar] [CrossRef]
- Zhang, P.; Lo, I.; O’Connor, D.; Pehkonen, S.; Cheng, H.; Hou, D. High efficiency removal of methylene blue using SDS surface-modified ZnFe2O4 nanoparticles. J. Colloid Interface Sci. 2017, 508, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Manjunath, S.V.; Kumar, M. Evaluation of single-component and multi-component adsorption of metronidazole, phosphate and nitrate on activated carbon from Prosopıs julıflora. Chem. Eng. J. 2018, 346, 525–534. [Google Scholar] [CrossRef]
- Mahamadi, C.; Nharingo, T. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresour. Technol. 2010, 101, 859–864. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, H.; Li, M.; Liu, B.; Xu, Z. Hierarchically porous carbon membranes derived from PAN and their selective adsorption of organic dyes. Chin. J. Polym. Sci. 2016, 34, 23–33. [Google Scholar] [CrossRef]
- Amar, I.A.; Asser, J.O.; Mady, A.S. Adsorptive removal of congo red dye from aqueous solutions using Mo-doped CoFe2O4 magnetic nanoparticles. Pigment. Resin Technol. 2021, 50, 6, 563–573. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Wang, L.; Jiang, J.; Duan, Z.; Zhao, L. Markedly enhanced coercive field and Congo red adsorption capability of cobalt ferrite induced by the doping of non-magnetic metal ions. Chem. Eng. J. 2014, 241, 384–392. [Google Scholar] [CrossRef]
- Rahimi, R.; Kerdari, H.; Rabbani, M.; Shafiee, M. Synthesis, characterization and adsorbing properties of hollow Zn–Fe2O4 nanospheres on removal of Congo red from aqueous solution. Desalination 2011, 280, 412–418. [Google Scholar] [CrossRef]
- Chen, R.; Wang, W.; Zhao, X.; Zhang, Y.; Wu, S.; Li, F. Rapid hydrothermal synthesis of magnetic CoxNi1-xFe2O4 nanoparticles and their application on removal of Congo red. Chem. Eng. J. 2014, 242, 226–233. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cheng, R.M.; Wen, Z.; Zhao, L. Investigation on the room temperature preparation and application of chain-like iron flower and its ramifications in wastewater purification. Chem. Eng. J. 2012, 203, 277–284. [Google Scholar] [CrossRef]
- Li, N.; Zheng, M.; Chang, X.; Ji, G.; Lu, H.; Xue, L.; Pan, L.; Cao, J. Preparation of magnetic CoFe2O4-functionalized graphene sheets via a facile hydrothermal method and their adsorption properties. J. Solid State Chem. 2011, 184, 953–958. [Google Scholar] [CrossRef]
- Zou, L.; Wang, Q.; Shen, X.; Wang, Z.; Jing, M.; Luo, Z. Fabrication and dye removal performance of magnetic CuFe2O4@CeO2 nanofibers. Appl. Surf. Sci. 2015, 332, 674–681. [Google Scholar] [CrossRef]
- Uddin, M.K.; Baig, U. Synthesis of Co3O4 nanoparticles and their performance towards methyl orange dye removal: Characterisation, adsorption and response surface methodology. J. Clean. Prod. 2019, 211, 1141–1153. [Google Scholar] [CrossRef]
- Thomas, B.; Alexander, L.K. Enhanced synergetic effect of Cr(VI) ion removal and anionic dye degradation with superparamagnetic cobalt ferrite meso–macroporous nanospheres. Appl. Nanosci. 2018, 8, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 2021, 323, 115034. [Google Scholar] [CrossRef]





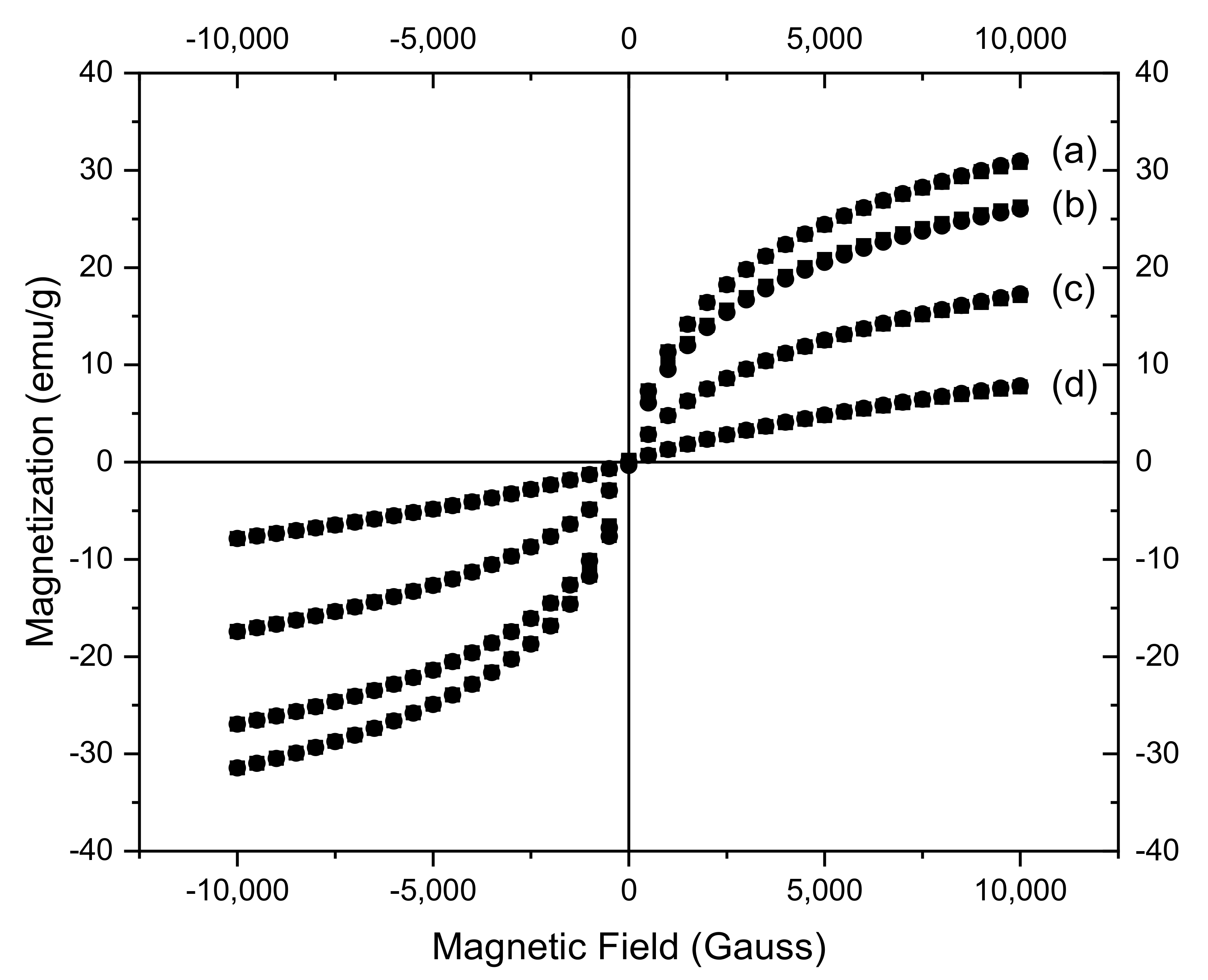








| Sample | SBET (m2g−1) | Pore Volume (cm3g−1) | Average Pore Size (nm) | MS (emu/g) | a (Å) | D (nm) |
|---|---|---|---|---|---|---|
| CoFe2O4–0 | 186.0 | 0.160 | 3.50 | 33.5 | 8.329 | 5 |
| CoFe2O4–0.1 | 171.3 | 0.170 | 3.66 | 28.5 | 8.329 | 5 |
| CoFe2O4–0.25 | 175.3 | 0.163 | 3.41 | 20.1 | 8.329 | 4 |
| CoFe2O4–0.5 | 201.7 | 0.216 | 3.35 | 11.7 | 8.392 | 3 |
| Kinetic Model | Nonlinear Form of the Kinetic Equation | Defining the Parameters of Mathematical Equations | |
|---|---|---|---|
| Pseudo-first-order | (4) | k1—the pseudo-first-order rate constant (min−1), Qe—the adsorption capacity at equilibrium (mg·g−1), Qt—the amount of CR/MO adsorbed at time t (mg·g−1), | |
| Pseudo-second-order | (5) | k2—the rate constant of the pseudo-second-order adsorption process (g·mg−1 min−1), Qe—the adsorption capacity at equilibrium (mg·g−1), Qt—the amount of CR/MO adsorbed at time t (mg·g−1), | |
| Intraparticle diffusion | (6) | kid—the intraparticle diffusion rate constant (mg·g−1⸳ min−1), C—the boundary-layer thickness (mg·g−1). | |
| Sample | CoFe2O4-0 | CoFe2O4-0.1 | CoFe2O4-0.25 | CoFe2O4-0.5 |
| CR | ||||
| Qe exp (mg·g−1) | 130.51 | 148.72 | 166.10 | 182.71 |
| Pseudo-first-order model | ||||
| k1 (min−1) | 0.0160 ± 0.0009 | 0.0145 ± 0.0006 | 0.0131 ± 0.0007 | 0.0124 ± 0.0009 |
| Qe cal (mg·g−1) | 131.73 ± 2.65 | 153.29 ± 2.24 | 173.94 ± 3.56 | 195.87 ± 5.54 |
| R2 adjusted | 0.9918 | 0.9965 | 0.9945 | 0.9907 |
| Pseudo-second-order model | ||||
| k2 (10−4 g·mg−1·min−1) | 0.8610 ± 0.0910 | 0.6419 ± 0.0466 | 0.4869 ± 0.0413 | 0.4023 ± 0.0577 |
| Qe cal (mg·g−1) | 170.50 ± 4.85 | 201.73 ± 4.08 | 232.87 ± 5.71 | 264.43 ± 11.17 |
| R2 adjusted | 0.9935 | 0.9973 | 0.9967 | 0.9912 |
| MO | ||||
| Qe exp (mg·g−1) | 92.19 | 153.29 | 183.29 | 256.32 |
| Pseudo-first-order model | ||||
| k1 (min−1) | 0.0409 ± 0.0037 | 0.0243 ± 0.0023 | 0.0235 ± 0.0016 | 0.0166 ± 0.0015 |
| Qe cal (mg·g−1) | 87.50 ± 2.00 | 147.44 ± 4.15 | 179.20 ± 3.57 | 259.83 ± 7.89 |
| R2 adjusted | 0.9471 | 0.9604 | 0.9816 | 0.9776 |
| Pseudo-second-order model | ||||
| k2 (10−3 g·mg−1·min−1) | 0.5522 ± 0.0522 | 0.1560 ± 0.0146 | 0.1201 ± 0.0062 | 0.4885 ± 0.0067 |
| Qe cal (mg·g−1) | 98.10 ± 1.71 | 175.32 ± 3.72 | 214.72 ± 2.59 | 329.58 ± 11.88 |
| R2 adjusted | 0.9834 | 0.9900 | 0.9970 | 0.9869 |
| Sample | CoFe2O4-0 | CoFe2O4-0.1 | CoFe2O4-0.25 | CoFe2O4-0.5 |
| CR | ||||
| Qe exp (mg·g−1) | 88.87 | 98.46 | 101.32 | 115.64 |
| Pseudo-first-order model | ||||
| k1 (min−1) | 0.0109 ± 0.0007 | 0.0108 ± 0.0074 | 0.0122 ± 0.0087 | 0.0103 ± 0.0015 |
| Qe cal (mg·g−1) | 95.76 ± 2.56 | 105.66 ± 2.99 | 105.85 ± 3.56 | 127.23 ± 8.06 |
| R2 adjusted | 0.9954 | 0.9948 | 0.9933 | 0.9769 |
| Pseudo-second-order model | ||||
| k2 (10−4 g·mg−1·min−1) | 0.6613 ± 0.0740 | 0.6098 ± 0.0116 | 0.7253 ± 0.0710 | 0.4636 ± 0.0084 |
| Qe cal (mg·g−1) | 133.25 ± 4.62 | 146.60 ± 0.8644 | 143.24 ± 4.15 | 178.58 ± 102 |
| R2 adjusted | 0.9966 | 0.9985 | 0.9968 | 0.9982 |
| MO | ||||
| Qe exp (mg·g−1) | 85.55 | 117.34 | 131.65 | 158.22 |
| Pseudo-first-order model | ||||
| k1 (min−1) | 0.0396 ± 0.0037 | 0.0252 ± 0.0019 | 0.0261 ± 0.0025 | 0.0212 ± 0.0026 |
| Qe cal (mg·g−1) | 81.63 ± 1.94 | 113.02 ± 2.44 | 125.02 ± 3.45 | 154.17 ± 5.77 |
| R2 adjusted | 0.9744 | 0.9848 | 0.9742 | 0.9617 |
| Pseudo-second-order model | ||||
| k2 (10−3 g·mg−1·min−1) | 0.5590 ± 0.0690 | 0.2130 ± 0.0119 | 0.2034 ± 0.0019 | 0.1256 ± 0.0208 |
| Qe cal (mg·g−1) | 91.94 ± 2.13 | 133.91 ± 1.68 | 147.33 ± 3.07 | 185.88 ± 7.32 |
| R2 adjusted | 0.9871 | 0.9977 | 0.9933 | 0.9814 |
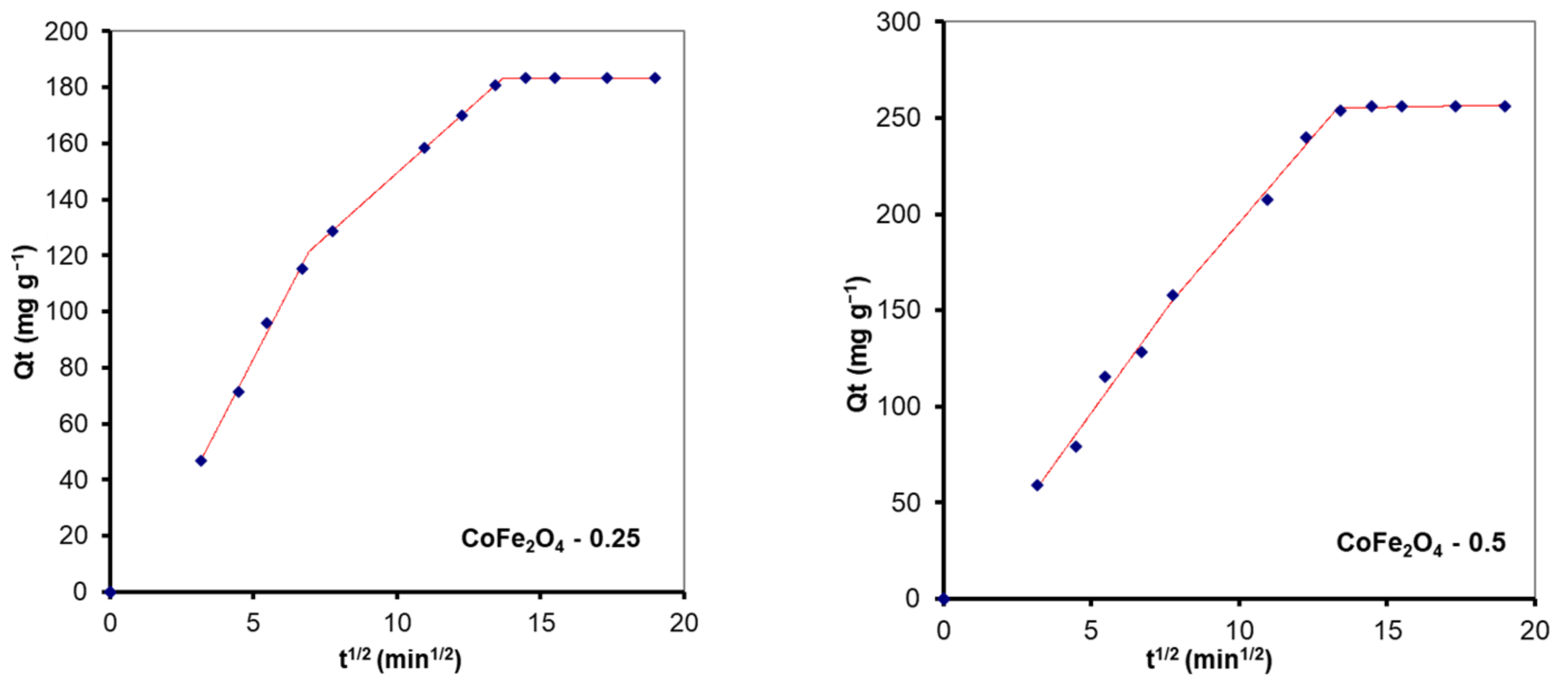
| Sample | Breakpoint (min1/2) | kid (mg·g−1·min−1/2) | C (mg·g−1) | R2 |
| RC Adsorption | ||||
| CoFe2O4–0 | 7.3 | 15.09 | −30.67 | 0.9890 |
| 14.2 | 7.44 | 25.15 | 0.9968 | |
| CoFe2O4–0.1 | 7.8 | 15.15 | −27.20 | 0.9978 |
| 13.8 | 9.50 | 17.11 | 0.9906 | |
| CoFe2O4–0.25 | 7.4 | 15.32 | −24.41 | 0.9875 |
| 13.8 | 11.92 | 0.87 | 0.9991 | |
| CoFe2O4–0.5 | 6.7 | −14.52 | 14.34 | 0.9949 |
| 13.2 | −2.18 | 15.48 | 0.9948 | |
| MO Adsorption | ||||
| CoFe2O4–0 | 5.72 | 13.68 | −10.70 | 0.9669 |
| 14.49 | 2.79 | 51.64 | 0.9866 | |
| CoFe2O4–0.1 | 5.26 | 20.59 | −26.21 | 0.9999 |
| 13.94 | 8.16 | 39.08 | 0.9985 | |
| CoFe2O4–0.25 | 6.92 | 19.78 | −15.73 | 0.9942 |
| 13.70 | 9.15 | 57.92 | 0.9999 | |
| CoFe2O4–0.5 | 7.74 | 21.28 | −9.68 | 0.9547 |
| 13.31 | 17.94 | 16.13 | 0.9856 | |
| Isotherm Model | Nonlinear Form of the Isotherm Equation | Defining the Parameters of Mathematical Equations | |
|---|---|---|---|
| Langmuir | (7) | Ce = equilibrium concentration of the solute in the solution (mg·L−1), KL = the equilibrium constant of the Langmuir model related to the adsorption energy (L·mg−1), Qe = the adsorption capacity at equilibrium (mg·g−1), Qmax = the maximum adsorption capacity (mg·g−1). | |
| Freundlich | (8) | Kf and 1/n = Freundlich adsorption isotherm parameters (adsorption capacity (mg·g−1) and intensity), Ce = the equilibrium concentration of the solute in the solution (mg·L−1). | |
| Dye | CR | |||
| Sample | CoFe2O4-0 | CoFe2O4-0.1 | CoFe2O4-0.25 | CoFe2O4-0.5 |
| Langmuir Parameters | ||||
| Qmax (mg·g−1) | 162.1564 ± 5.6842 | 147.2104 ± 3.7878 | 165.8074 ± 8.4989 | 178.0762 ± 10.3876 |
| KL (L·mg−1) | 0.0927 ± 0.0118 | 3.7115 ± 0.5291 | 1.3116 ± 0.3681 | 1.1944 ± 0.3614 |
| R2adjusted | 0.9966 | 0.9866 | 0.9616 | 0.9546 |
| Freundlich Parameters | ||||
| KF (mg·g−1) | 23.3615 ± 3.7796 | 86.1721 ± 15.1046 | 85.0676 ± 14.1775 | 88.0141 ± 13.3722 |
| 1/n | 0.4629 ± 0.0454 | 0.1563 ± 0.0550 | 0.1920 ± 0.0538 | 0.2079 ± 0.0502 |
| R2 adjusted | 0.9876 | 0.7372 | 0.8310 | 0.8741 |
| Dye | MO | |||
| Sample | CoFe2O4-0 | CoFe2O4-0.1 | CoFe2O4-0.25 | CoFe2O4-0.5 |
| Langmuir Parameters | ||||
| Qmax (mg·g−1) | 94.9929 ± 5.1054 | 154.8871 ± 6.4913 | 178.0100 ± 8.5675 | 257.2528 ± 37.9185 |
| KL (L·mg−1) | 0.3666 ± 0.1108 | 1.7089 ± 0.3616 | 1.6430 ± 0.4021 | 0.9819 ± 0.6989 |
| R2 adjusted | 0.9533 | 0.9702 | 0.9674 | 0.8802 |
| Freundlich Parameters | ||||
| KF (mg·g−1) | 36.8004 ± 6.1289 | 82.7225 ± 17.6008 | 91.8601 ± 15.7835 | 119.1167 ± 9.1690 |
| 1/n | 0.2354 ± 0.0472 | 0.1740 ± 0.0678 | 0.1970 ± 0.0570 | 0.2988 ± 0.0351 |
| R2 adjusted | 0.9107 | 0.6786 | 0.8150 | 0.9718 |
| Adsorbent | Dye | Parameters | Single Component Solution (mg·g−1) | Binary Solution (mg·g−1) | |
|---|---|---|---|---|---|
| CoFe2O4-0 | CR | Qmax,CR | 163.89 | 118.06 | 0.72 |
| MO | Qmax,MO | 95.36 | 120.91 | 1.26 | |
| CoFe2O4-0.1 | CR | Qmax,CR | 149.99 | 111.23 | 0.74 |
| MO | Qmax,MO | 161.09 | 170.94 | 1.06 | |
| CoFe2O4-0.25 | CR | Qmax,CR | 170.40 | 214.59 | 1.25 |
| MO | Qmax,MO | 187.98 | 135.31 | 0.71 | |
| CoFe2O4-0.5 | CR | Qmax,CR | 187.10 | 147.05 | 0.78 |
| MO | Qmax,MO | 262.03 | 116.27 | 0.44 |
| Dye | Adsorbent | Adsorption Capacity (mg·g−1) | Reference |
|---|---|---|---|
| CR | Mo-doped CoFe2O4 | 135.14 | [67] |
| CoFe2O4 | 185.4 | [68] | |
| MnFe2O4 | 92.4 | [40] | |
| NiFe2O4 | 97.1 | [40] | |
| Fe3O4 | 68.5 | [40] | |
| ZnFe2O4 nanospheres | 16.1 | [69] | |
| Co0.3Ni0.7Fe2O4 | 131.75 | [70] | |
| FeFe2O4 | 97.42 | [71] | |
| CoFe2O4-0 | 162.15 | This study | |
| CoFe2O4-0.1 | 147.21 | This study | |
| CoFe2O4-0.25 | 165.80 | This study | |
| CoFe2O4-0.5 | 178.07 | This study | |
| MO | CoFe2O4–FGS nanocomposites | 71.54 | [72] |
| CuFe2O4@CeO2 nanofibers | 100.0 | [73] | |
| Co3O4 nanoparticles | 46.08 | [74] | |
| CoFe2O4 | 94.33 | [75] | |
| ZnFe2O4 | 49.43 | [75] | |
| Co0.5Zn0.5Fe2O4 | 67.1 | [75] | |
| CoFe2O4-0 | 94.99 | This study | |
| CoFe2O4-0.1 | 154.88 | This study | |
| CoFe2O4-0.25 | 178.01 | This study | |
| CoFe2O4-0.5 | 257.25 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonescu, C.M.; Tătăruş, A.; Culiţă, D.C.; Stănică, N.; Butoi, B.; Kuncser, A. Facile Synthesis of Cobalt Ferrite (CoFe2O4) Nanoparticles in the Presence of Sodium Bis (2-ethyl-hexyl) Sulfosuccinate and Their Application in Dyes Removal from Single and Binary Aqueous Solutions. Nanomaterials 2021, 11, 3128. https://doi.org/10.3390/nano11113128
Simonescu CM, Tătăruş A, Culiţă DC, Stănică N, Butoi B, Kuncser A. Facile Synthesis of Cobalt Ferrite (CoFe2O4) Nanoparticles in the Presence of Sodium Bis (2-ethyl-hexyl) Sulfosuccinate and Their Application in Dyes Removal from Single and Binary Aqueous Solutions. Nanomaterials. 2021; 11(11):3128. https://doi.org/10.3390/nano11113128
Chicago/Turabian StyleSimonescu, Claudia Maria, Alina Tătăruş, Daniela Cristina Culiţă, Nicolae Stănică, Bogdan Butoi, and Andrei Kuncser. 2021. "Facile Synthesis of Cobalt Ferrite (CoFe2O4) Nanoparticles in the Presence of Sodium Bis (2-ethyl-hexyl) Sulfosuccinate and Their Application in Dyes Removal from Single and Binary Aqueous Solutions" Nanomaterials 11, no. 11: 3128. https://doi.org/10.3390/nano11113128
APA StyleSimonescu, C. M., Tătăruş, A., Culiţă, D. C., Stănică, N., Butoi, B., & Kuncser, A. (2021). Facile Synthesis of Cobalt Ferrite (CoFe2O4) Nanoparticles in the Presence of Sodium Bis (2-ethyl-hexyl) Sulfosuccinate and Their Application in Dyes Removal from Single and Binary Aqueous Solutions. Nanomaterials, 11(11), 3128. https://doi.org/10.3390/nano11113128