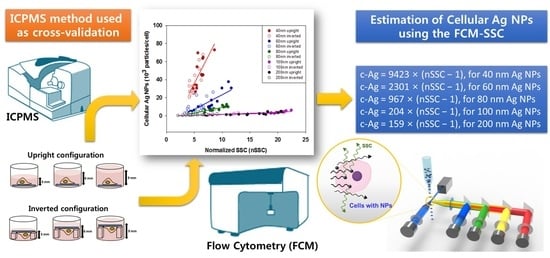
Quantitative Estimation of Cell-Associated Silver Nanoparticles using the Normalized Side Scattering Intensities of Flow Cytometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles
2.2. Physicochemical Characterization of Ag NPs
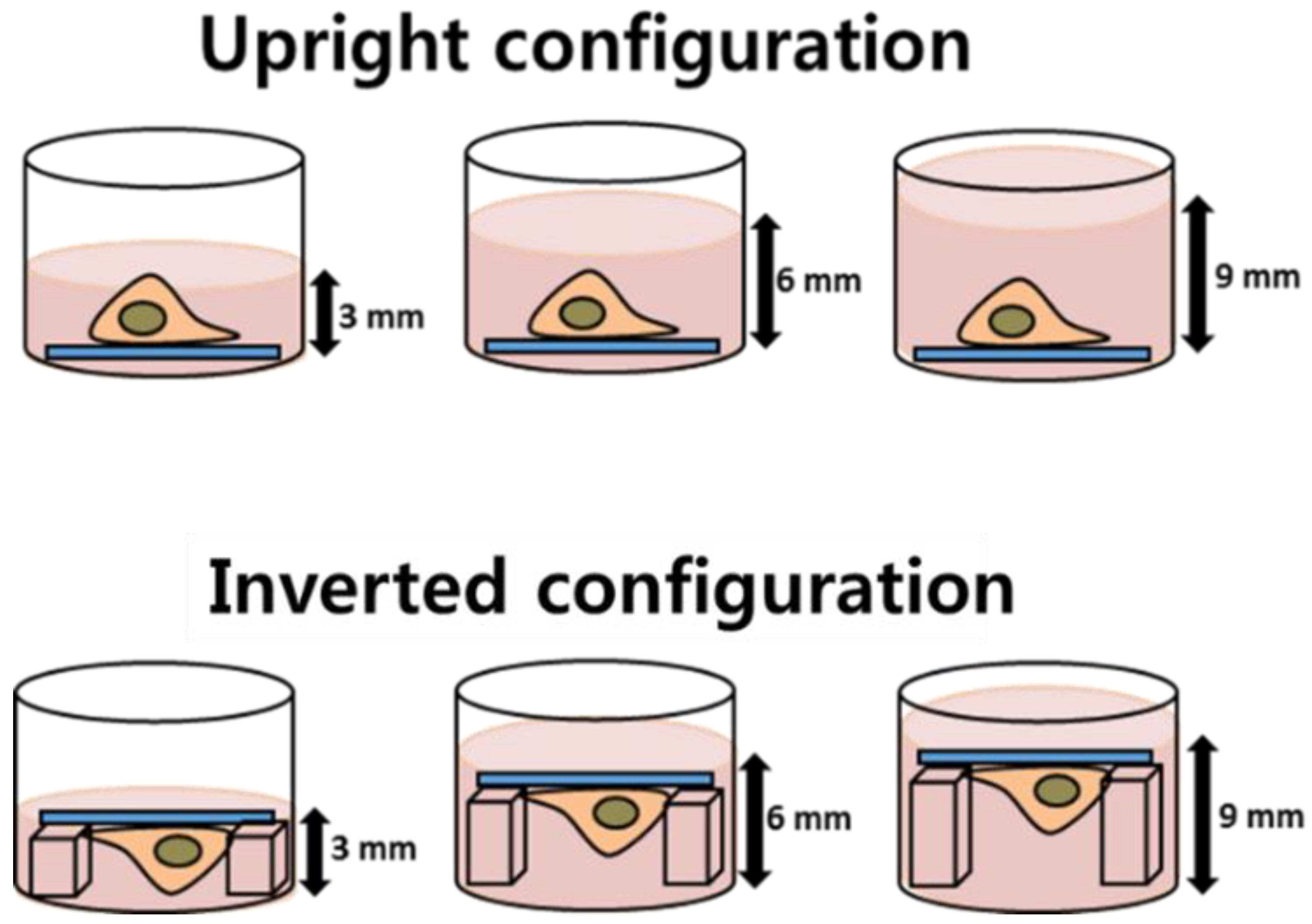
2.3. Cell Culture and Ag NP Exposure
2.4. Cell Culture and Ag NP Exposure
2.5. ICPMS Measurement
2.6. Data Analysis
3. Results
3.1. Physicochemical Characteristics of Ag NPs
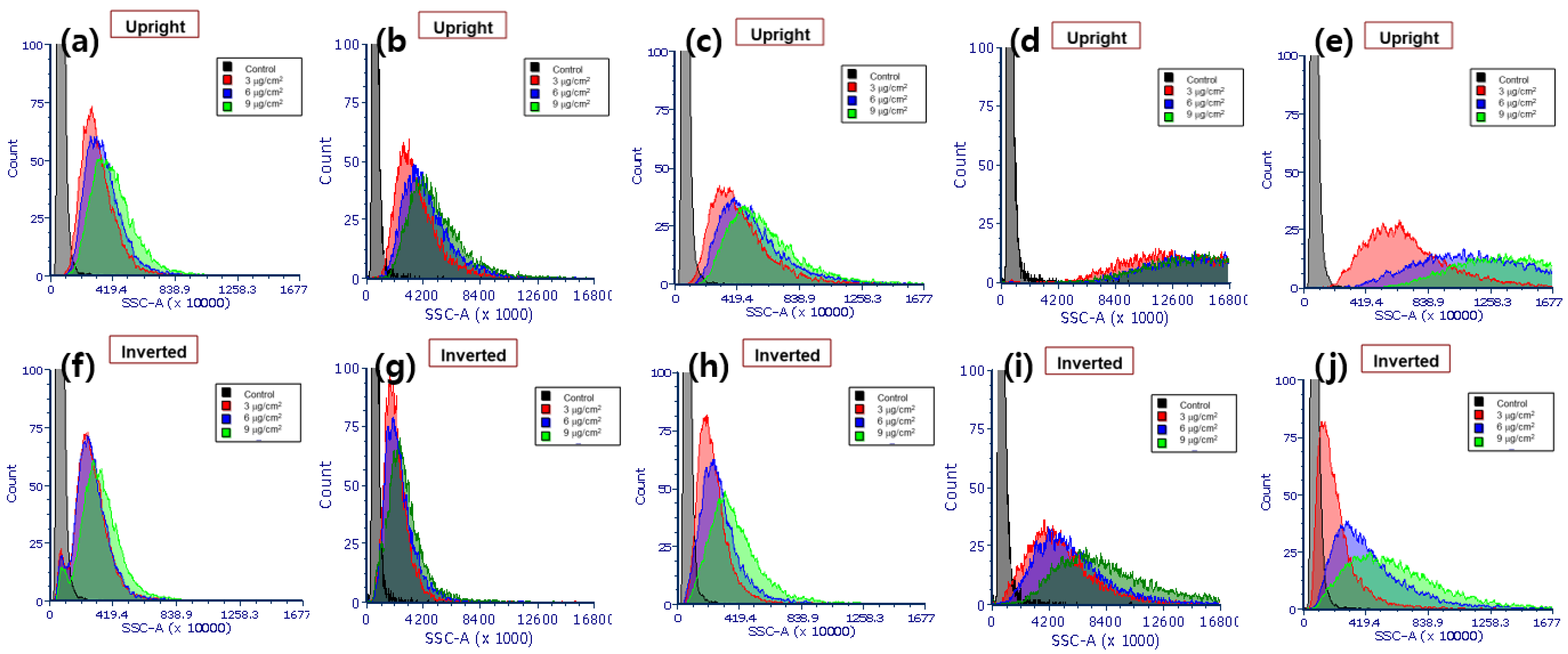
3.2. Cellular Ag NPs Measured by FCM and ICPMS
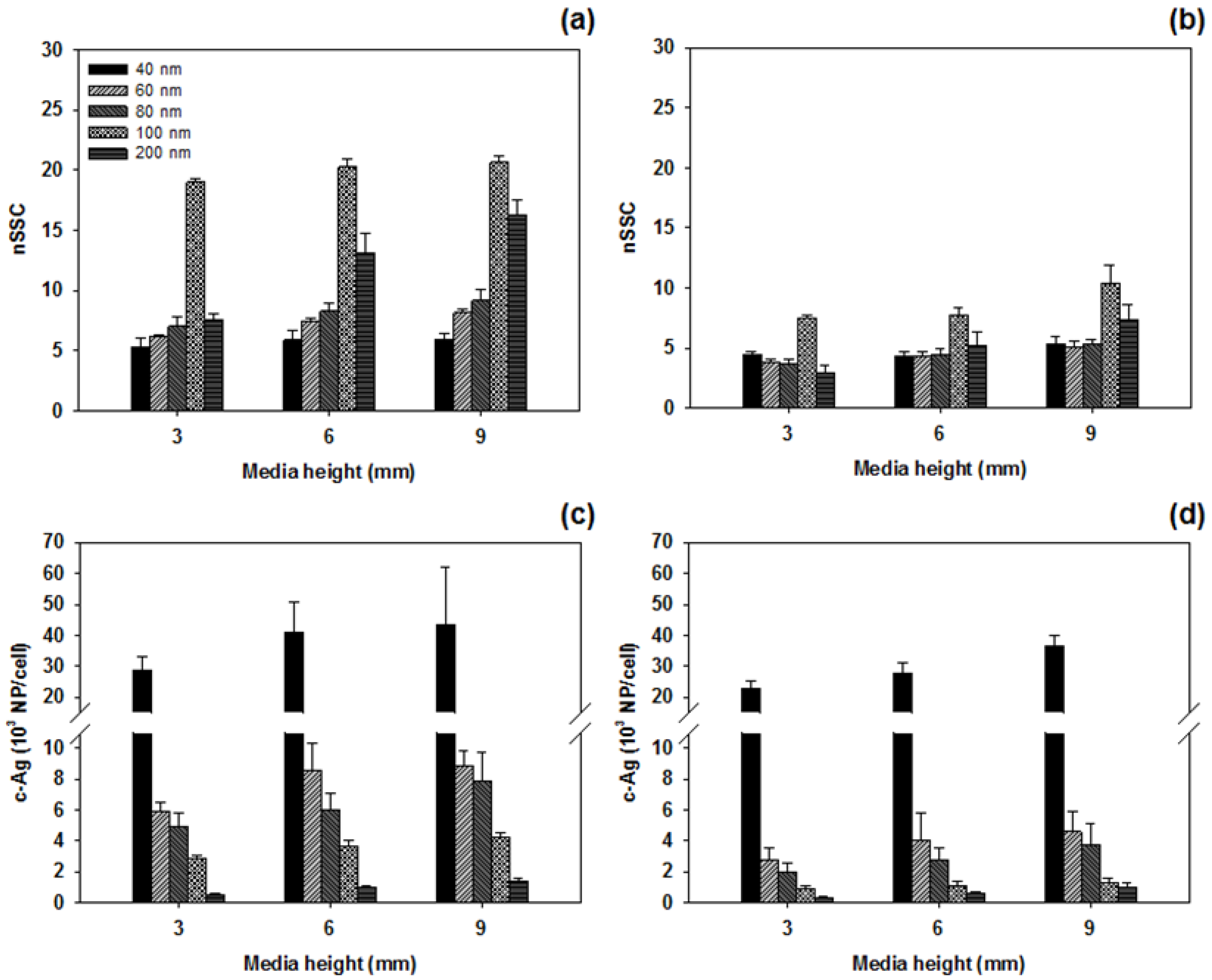
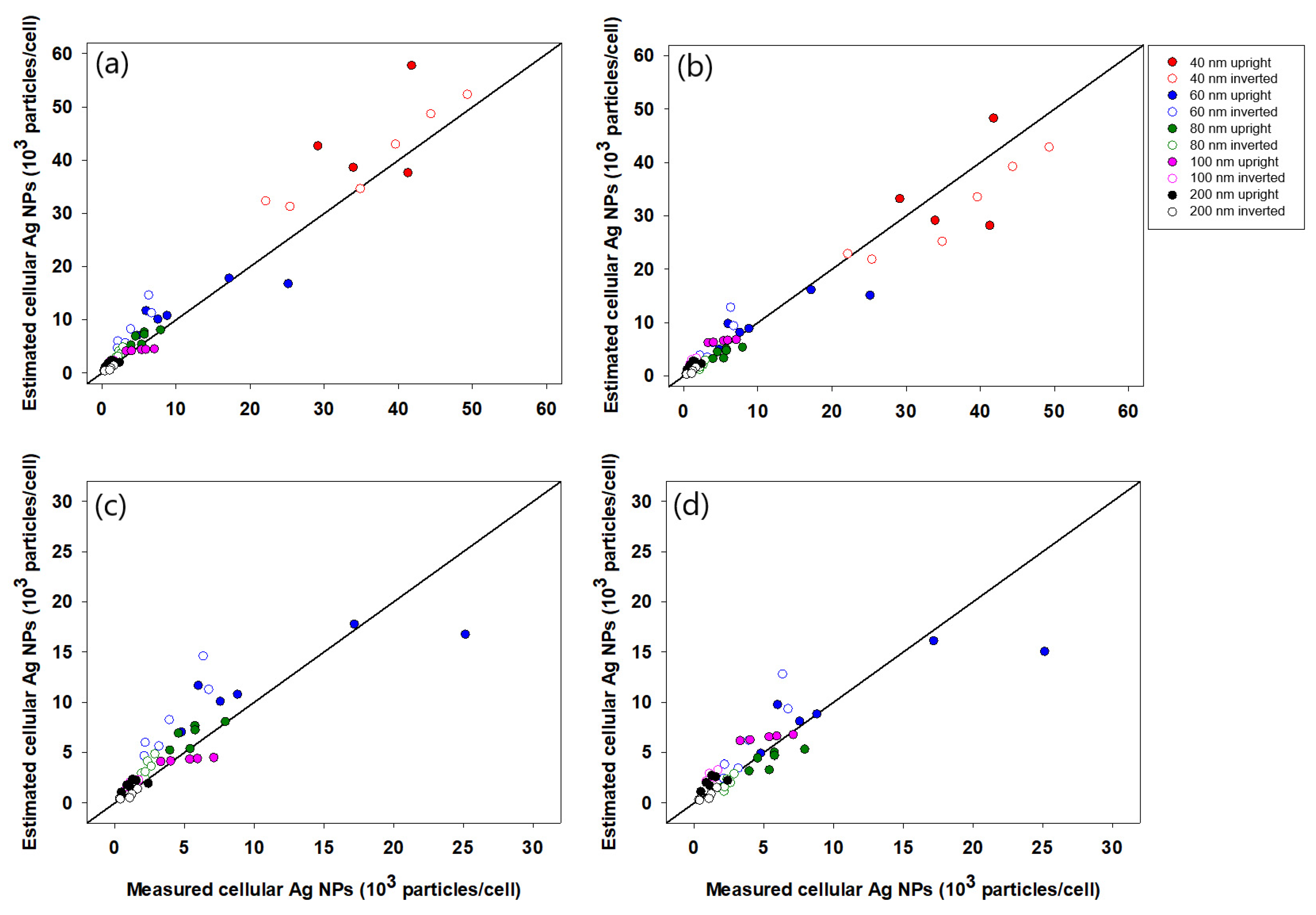
3.3. nSSC-Based Estimation of Cell-Associated Ag NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kwon, D.; Jeon, S.K.; Yoon, T.H. Impact of agglomeration on the bioaccumulation of sub-100nm sized TiO2. Colloids Surf. B Biointerfaces 2014, 116, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Colloid Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Cui, J.; Faria, M.; Björnmalm, M.; Ju, Y.; Suma, T.; Gunawan, S.T.; Richardson, J.J.; Heidari, H.; Bals, S.; Crampin, E.J.; et al. A framework to account for sedimentation and diffusion in particle–cell interactions. Langmuir 2016, 32, 12394–12402. [Google Scholar] [CrossRef] [PubMed]
- Clift, M.J.D.; Rothen-Rutishauser, B.; Brown, D.M.; Duffin, R.; Donaldson, K.; Proudfoot, L.; Guy, K.; Stone, V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol. Appl. Pharmacol. 2008, 232, 418–427. [Google Scholar] [CrossRef]
- Limbach, L.K.; Li, Y.; Grass, R.N.; Brunner, T.J.; Hintermann, M.A.; Muller, M.; Gunther, D.; Stark, W.J. Oxide nanoparticle uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Technol. 2005, 39, 9370–9376. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Giorgio, T. Quantitative measurement of multifunctional quantum dot binding to cellular targets using flow cytometry. Cytom. Part A 2009, 75, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Ha, M.K.; Yang, N.; Yoon, T.H. Flow cytometry-based quantification of cellular Au nanoparticles. Anal. Chem. 2017, 89, 2449–2456. [Google Scholar] [CrossRef]
- Suzuki, H.; Toyooka, T.; Ibuki, Y. Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef]
- Zucker, R.; Massaro, E.; Sanders, K.; Degn, L.; Boyes, W. Detection of TiO2 nanoparticles in cells by flow cytometry. Cytom. Part A 2010, 77, 677–685. [Google Scholar] [CrossRef]
- Zucker, R.; Daniel, K.; Massaro, E.; Karafas, S.; Degn, L.; Boyes, W. Detection of silver nanoparticles in cells by flow cytometry using light scatter and far-red fluorescence. Cytom. Part A 2013, 83, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Yoon, T.H. Flow cytometric assessment of reactive oxygen species generations that are directly related to cellular ZnO nanoparticle uptake. J. Nanosci. Nanotechnol. 2014, 14, 5395–5401. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Tailang, M.; Vijayaraghavan, R. In vitro cytotoxicity of nanoparticles: A comparison between particle size and cell type. J. Nanosci. 2016, 2016, 4023852. [Google Scholar] [CrossRef] [Green Version]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics In Vitro: Dosimetry Considerations for In Vitro Nanoparticle Toxicity Assessments. Toxicol. Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J.G. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.M.; DeLoid, G.M.; Pyrgiotakis, G.; Demokritou, P. Interactions of engineered nanomaterials in physiological media and implications for in vitro dosimetry. Nanotoxicology 2013, 7, 417–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Pirela, S.V.; Pal, A.; Liu, J.; Srebric, J.; Demokritou, P.P. Advanced computational modeling for in vitro nanomaterial dosimetry. Part. Fibre Toxicol. 2015, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Prot. 2017, 12, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.-H.; Park, K.; Yi, J.; Ryu, D.-Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. Vitr. 2009, 23, 1076–1084. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef]
- Xin, L.; Wang, J.; Fan, G.; Che, B.; Wu, Y.; Guo, S.; Tong, J. Oxidative stress and mitochondrial injury-mediated cytotoxicity induced by silver nanoparticles in human A549 and HepG2 cells. Environ. Toxicol. 2016, 31, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, C.C.; Payne, K.C. Nanoparticle-cell interaction: Molecular structure of protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007, 134–135, 167–174. [Google Scholar] [CrossRef]
- Zhao, C.-M.; Wang, W.-X. Size-dependent uptake of silver nanoparticles in Daphnia magna. Environ. Sci. Technol. 2012, 46, 11345–11351. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Zhang, Q.; Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011, 6, 385–391. [Google Scholar] [CrossRef]
- Choi, S.Y.; Yang, N.; Jeon, S.K.; Yoon, T.H. Semi-quantitative estimation of cellular SiO2 nanoparticles using flow cytometry combined with X-ray fluorescence measurements. Cytom. Part A 2014, 85, 771–780. [Google Scholar] [CrossRef]
- Kuang, Y.; He, X.; Zhang, Z.; Li, Y.; Zhang, H.; Ma, Y.; Wu, Z.; Chai, Z. Comparison study on the antibacterial activity of nano-or bulk-cerium oxide. J. Nanosci. Nanotechno. 2011, 11, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef]

| NPs | Hydrodynamic Size (nm) | Surface Charge (mV) | Dispersion Stability | |||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | DI Water | RPMI-1640 | DI Water | RPMI-1640 | |
| DI Water | DI Water | RPMI-1640 | RPMI-1640 | |||||
| (+)bPEIAg40 | 49.5 ± 0.02 | 49.0 ± 0.3 | 132.3 ± 1.0 | 165.5 ± 6.1 | 45.0 ± 0.7 | −6.9 ± 0.4 | 0.998 | 0.769 |
| (+)bPEIAg60 | 68.4 ± 0.4 | 71.2 ± 0.2 | 118.2 ± 1.3 | 138.0 ± 0.7 | 33.8 ± 0.4 | −11.3 ± 1.1 | 0.955 | 0.931 |
| (+)bPEIAg80 | 102.9 ± 0.7 | 102.1 ± 1.4 | 170.5 ± 1.8 | 180.3 ± 3.0 | 42.1 ± 0.7 | −9.8 ± 0.7 | 0.973 | 0.808 |
| (+)bPEIAg100 | 113.1 ± 2.5 | 115.3 ± 1.3 | 213.6 ± 2.2 | 224.3 ± 3.3 | 63.8 ± 0.9 | −11.7 ± 1.8 | 0.935 | 0.788 |
| (+)bPEIAg200 | 223.3 ± 4.7 | 225.5 ± 2.8 | 346.6 ± 3.8 | 342.7 ± 6.7 | 22.9 ± 1.6 | −11.0 ± 1.1 | 0.897 | 0.684 |
| Nanoparticle [Ref.] | Particle Size (nm) | Applicability Domain of Regression Equation | Regression Equation Type | |
|---|---|---|---|---|
| nSSC Intensity Range | Cellular Uptake Range | |||
| AgbPEI | 40, 60, 80, 100, 200 | ~23 | ~80,000 NPs/cell | Linear |
| AubPEI and AuCit. [8] | 40, 60, 80, 100 | ~1.6 | ~3500 NPs/cell | Linear |
| SiO2 [27] | 30, 200, 300 | ~2.88 | ~14.5 ng/cell | Sigmoidal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, Y.J.; Ha, M.K.; Yoon, T.H. Quantitative Estimation of Cell-Associated Silver Nanoparticles using the Normalized Side Scattering Intensities of Flow Cytometry. Nanomaterials 2021, 11, 3079. https://doi.org/10.3390/nano11113079
Shim YJ, Ha MK, Yoon TH. Quantitative Estimation of Cell-Associated Silver Nanoparticles using the Normalized Side Scattering Intensities of Flow Cytometry. Nanomaterials. 2021; 11(11):3079. https://doi.org/10.3390/nano11113079
Chicago/Turabian StyleShim, Yoo Jin, My Kieu Ha, and Tae Hyun Yoon. 2021. "Quantitative Estimation of Cell-Associated Silver Nanoparticles using the Normalized Side Scattering Intensities of Flow Cytometry" Nanomaterials 11, no. 11: 3079. https://doi.org/10.3390/nano11113079
APA StyleShim, Y. J., Ha, M. K., & Yoon, T. H. (2021). Quantitative Estimation of Cell-Associated Silver Nanoparticles using the Normalized Side Scattering Intensities of Flow Cytometry. Nanomaterials, 11(11), 3079. https://doi.org/10.3390/nano11113079