Eliciting Specific Electrochemical Reaction Behavior by Rational Design of a Red Phosphorus Electrode for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results
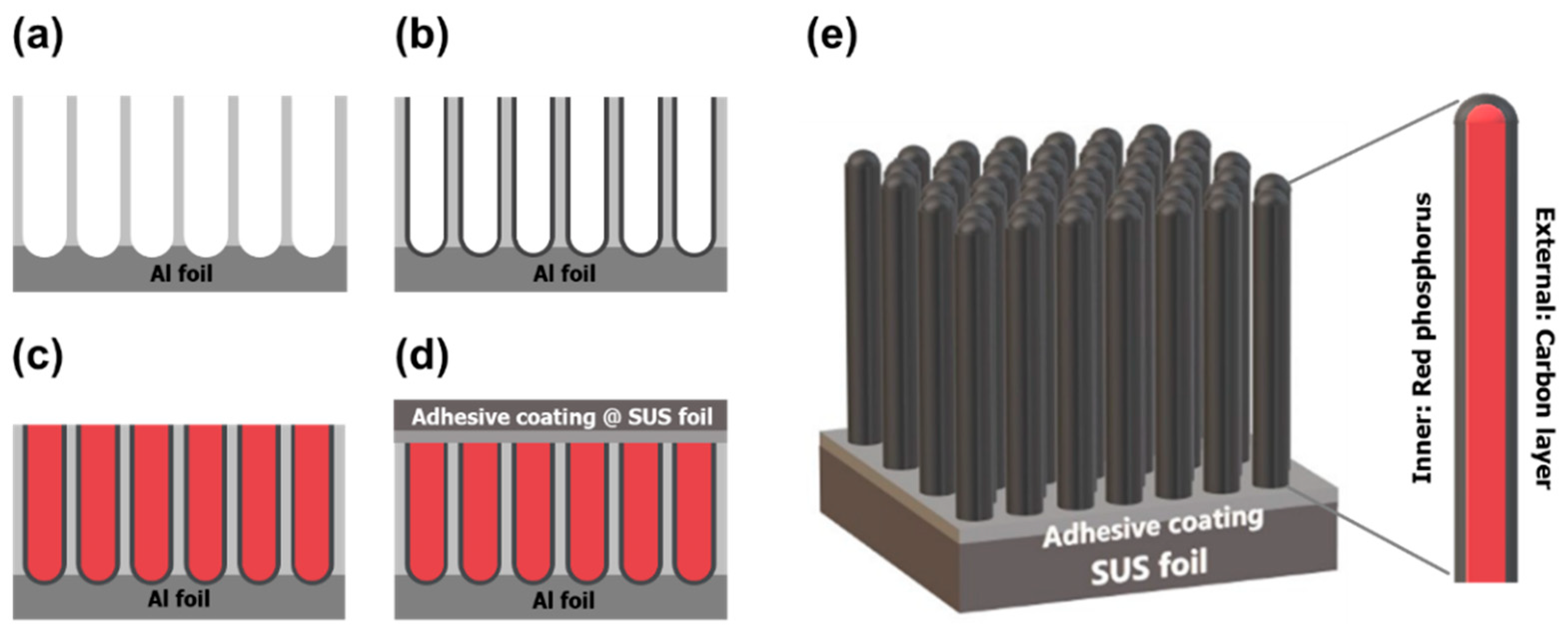
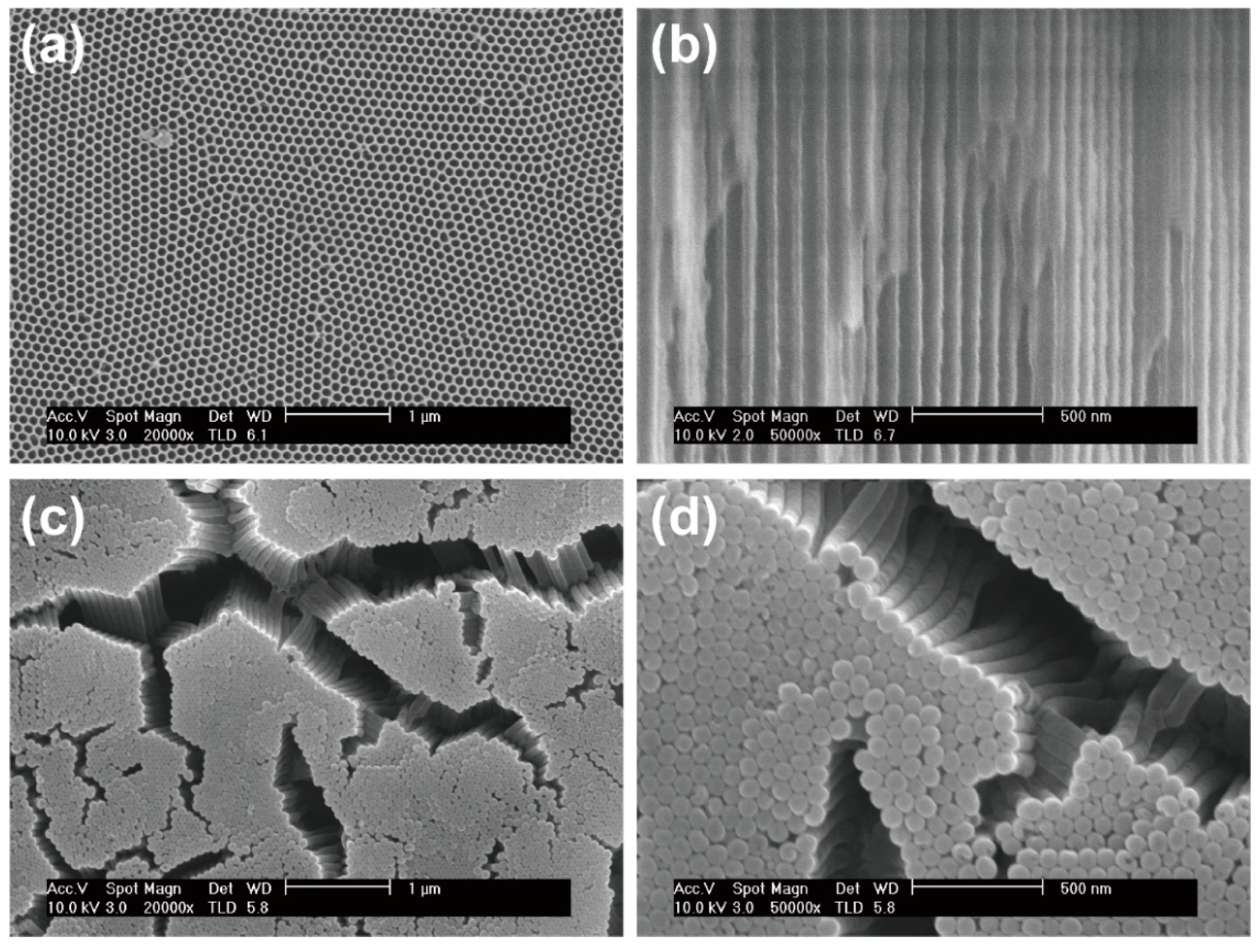
3.1. Fabrication of Electrodes
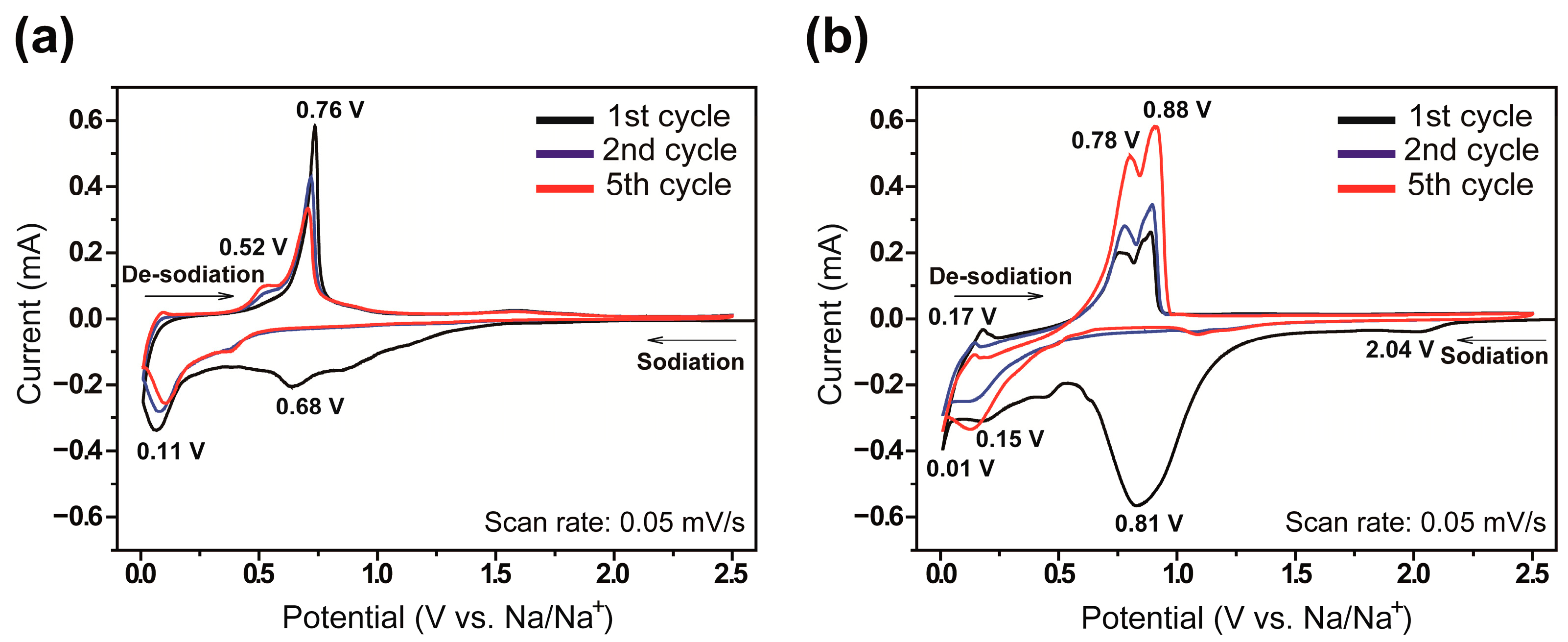
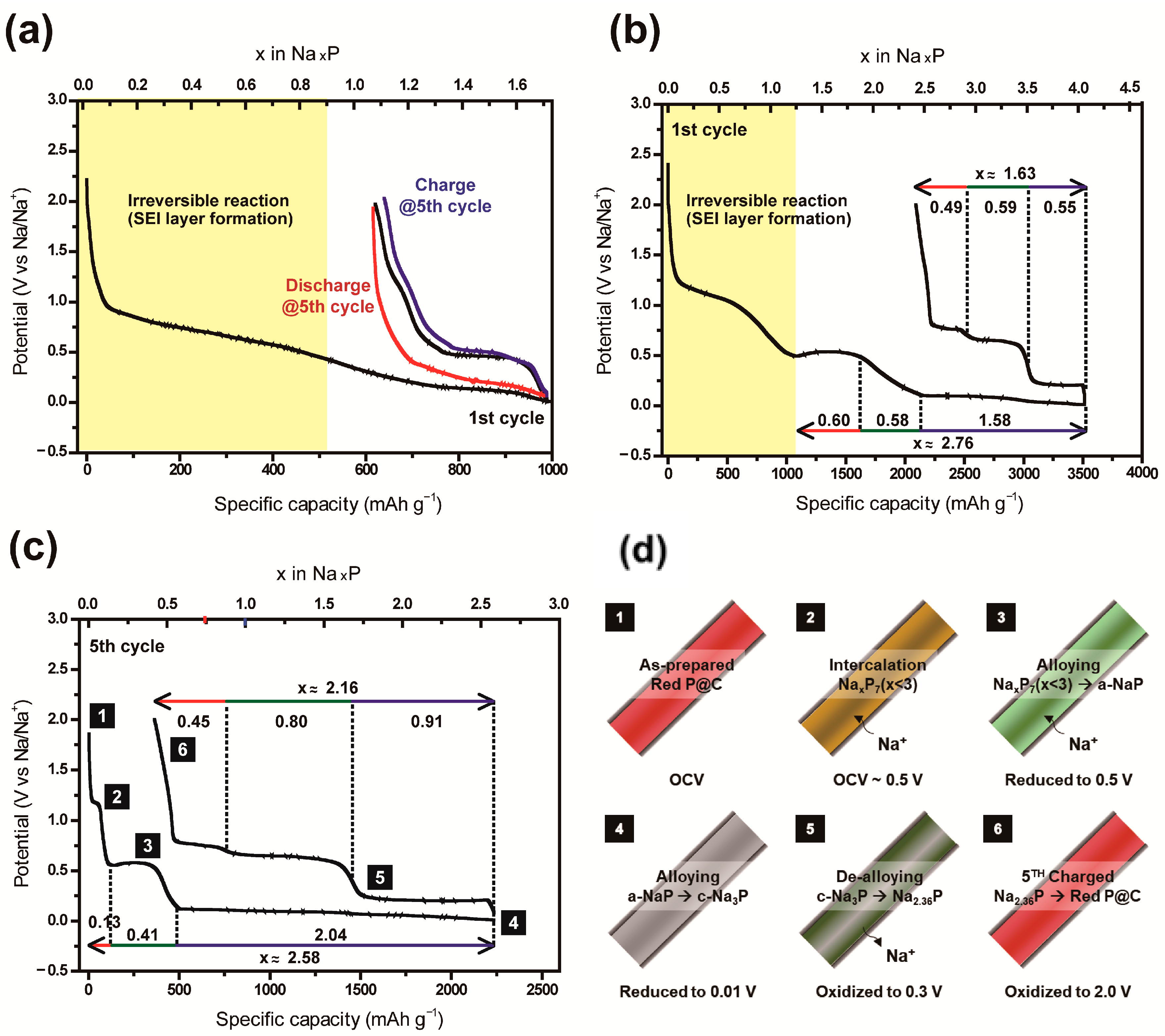
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem. Int. Ed. 2015, 54, 3432–3448. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Kim, J.-H.; Kim, D.K.; Lee, H.-W. Suppressing Polysulfide Dissolution via Cohesive Forces by Interwoven Carbon Nanofibers for High-Areal-Capacity Lithium–Sulfur Batteries. Nano Lett. 2018, 18, 475–481. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Ceder, G. Challenges for Na-ion Negative Electrodes. J. Electrochem. Soc. 2011, 158, A1011. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on anode materials for Na-ion batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Mehtab, T.; Shakeel, M.; Mushtaq, M.A.; Kumar, A.; Nguyen, T.A.; Slimani, Y.; Nazir, M.T.; Song, H. A novel strategy for the synthesis of hard carbon spheres encapsulated with graphene networks as a low-cost and large-scalable anode material for fast sodium storage with an ultralong cycle life. Inorg. Chem. Front. 2020, 7, 402–410. [Google Scholar] [CrossRef]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-organic frameworks for energy storage devices: Batteries and supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Zhou, Z.; Liu, X.; Zhang, H.; Wei, M. Hierarchical Porous Anatase TiO2 Microspheres with High-Rate and Long-Term Cycling Stability for Sodium Storage in Ether-Based Electrolyte. ACS Appl. Energy Mater. 2020, 3, 3619–3627. [Google Scholar] [CrossRef]
- Wei, P.; Xu, Y.; Sun, X.; Fang, C.; Han, J.; Huang, Y. Porous NaTi2(PO4)3/C Hierarchical Nanofibers for Ultrafast Electrochemical Energy Storage. ACS Appl. Mater. Interfaces 2018, 10, 27039–27046. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, L.; Zhang, Y.; Zhao, H.; Kong, L.; Gao, S. Confined formation of monoclinic Na4Ti5O12 nanoparticles embedded into porous CNTs: Towards enhanced electrochemical performances for sodium ion batteries. New J. Chem. 2018, 42, 19340–19343. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, Y.H.; Yun, J.H.; Ragupathy, P.; Kim, D.K. Enhancing the Sequential Conversion-Alloying Reaction of Mixed Sn–S Hybrid Anode for Efficient Sodium Storage by a Carbon Healed Graphene Oxide. Small 2018, 14, 1702605. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yun, J.H.; Kim, D.K. A Robust Approach for Efficient Sodium Storage of GeS2 Hybrid Anode by Electrochemically Driven Amorphization. Adv. Energy Mater. 2018, 8, 1703499. [Google Scholar] [CrossRef]
- Wang, L.; Swiatowska, J.; Dai, S.; Cao, M.; Zhong, Z.; Shen, Y.; Wang, M. Promises and challenges of alloy-type and conversion-type anode materials for sodium–ion batteries. Mater. Today Energy 2019, 11, 46–60. [Google Scholar] [CrossRef]
- Puthusseri, D.; Wahid, M.; Ogale, S. Conversion-type Anode Materials for Alkali-Ion Batteries: State of the Art and Possible Research Directions. ACS Omega 2018, 3, 4591–4601. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Zeng, Q.; Long, Y.; Liu, Z. Black Phosphorus Nanosheets: Synthesis, Characterization and Applications. Small 2016, 12, 3480–3502. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M. The Use of Phosphorus in Sodium-Ion Batteries (A Review). Russ. J. Electrochem. 2020, 56, 3–19. [Google Scholar] [CrossRef]
- Ni, J.; Li, L.; Lu, J. Phosphorus: An Anode of Choice for Sodium-Ion Batteries. ACS Energy Lett. 2018, 3, 1137–1144. [Google Scholar] [CrossRef]
- Fang, K.; Liu, D.; Xiang, X.; Zhu, X.; Tang, H.; Qu, D.; Xie, Z.; Li, J.; Qu, D. Air-stable red phosphorus anode for potassium/sodium-ion batteries enabled through dual-protection design. Nano Energy 2020, 69, 104451. [Google Scholar] [CrossRef]
- Li, W.J.; Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Simply mixed commercial red phosphorus and carbon nanotube composite with exceptionally reversible sodium-ion storage. Nano Lett. 2013, 13, 5480–5484. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Z.; Li, M.; Jiang, Y.; Wei, X.; Zhong, X.; Gu, L.; Yu, Y. Amorphous Red Phosphorus Embedded in Highly Ordered Mesoporous Carbon with Superior Lithium and Sodium Storage Capacity. Nano Lett. 2016, 16, 1546–1553. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Shen, C.; Liu, Q.; Cao, X.; Ma, Y.; Chen, L.; Lau, C.; Chen, T.-C.; Wei, F.; et al. Red Phosphorus Nanodots on Reduced Graphene Oxide as a Flexible and Ultra-Fast Anode for Sodium-Ion Batteries. ACS Nano 2017, 11, 5530–5537. [Google Scholar] [CrossRef]
- Capone, I.; Hurlbutt, K.; Naylor, A.J.; Xiao, A.W.; Pasta, M. Effect of the Particle-Size Distribution on the Electrochemical Performance of a Red Phosphorus-Carbon Composite Anode for Sodium-Ion Batteries. Energy Fuels 2019, 33, 4651–4658. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, S.; Luo, X.; Li, Z.; Sun, X.; Li, M.; Liu, F.; Yu, Y. Confined Amorphous Red Phosphorus in MOF-Derived N-Doped Microporous Carbon as a Superior Anode for Sodium-Ion Battery. Adv. Mater. 2017, 29, 1605820. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, G.; Wang, P.; Wang, C.-Y.; Yin, Y.-X.; Li, Y.-K.; Cao, F.-F.; Guo, Y.-G. Confined Red Phosphorus in Edible Fungus Slag-Derived Porous Carbon as an Improved Anode Materials in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 47948–47955. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Jung, Y.H.; Jung, W.K.; Jung, D.S.; Choi, J.W.; Kim, D.K. Encapsulated Monoclinic Sulfur for Stable Cycling of Li–S Rechargeable Batteries. Adv. Mater. 2013, 25, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Kim, J.-H.; Ragupathy, P.; Kim, D.J.; Kim, D.K. Functional and structural insight into lignocellulosic fibers for high-areal-capacity lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 18260–18271. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in nano-engineered anodic aluminum oxide membrane development. Materials 2010, 4, 487–526. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Black phosphorus and its composite for lithium rechargeable batteries. Adv. Mater. 2007, 19, 2465–2468. [Google Scholar] [CrossRef]
- Dahbi, M.; Yabuuchi, N.; Fukunishi, M.; Kubota, K.; Chihara, K.; Tokiwa, K.; Yu, X.F.; Ushiyama, H.; Yamashita, K.; Son, J.Y.; et al. Black Phosphorus as a High-Capacity, High-Capability Negative Electrode for Sodium-Ion Batteries: Investigation of the Electrode/Electrolyte Interface. Chem. Mater. 2016, 28, 1625–1635. [Google Scholar] [CrossRef]
- Mayo, M.; Griffith, K.J.; Pickard, C.J.; Morris, A.J. Ab Initio Study of Phosphorus Anodes for Lithium- and Sodium-Ion Batteries. Chem. Mater. 2016, 28, 2011–2021. [Google Scholar] [CrossRef]
- Sottmann, J.; Michiel, M.D.; Fjellvåg, H.; Malavasi, L.; Margadonna, S.; Vajeeston, P.; Vaughan, G.B.M.; Wragg, D.S. Chemical Structures of Specific Sodium Ion Battery Components Determined by Operando Pair Distribution Function and X-ray Diffraction Computed Tomography. Angew. Chem. Int. Ed. 2017, 56, 11385–11389. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Li, Y.; Li, Y.; Lee, H.R.; Pei, A.; He, X.; Cui, Y. Design of Red Phosphorus Nanostructured Electrode for Fast-Charging Lithium-Ion Batteries with High Energy Density. Joule 2019, 3, 1080–1093. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Choi, A.; Choi, N.S.; Kim, J.; Lee, J.; Ryu, J.H.; Oh, S.M.; Lee, K.T. An amorphous red phosphorus/carbon composite as a promising anode material for sodium ion batteries. Adv. Mater. 2013, 25, 3045–3049. [Google Scholar] [CrossRef]
- Ma, X.; Chen, L.; Ren, X.; Hou, G.; Chen, L.; Zhang, L.; Liu, B.; Ai, Q.; Zhang, L.; Si, P.; et al. High-performance red phosphorus/carbon nanofibers/graphene free-standing paper anode for sodium ion batteries. J. Mater. Chem. A 2018, 6, 1574–1581. [Google Scholar] [CrossRef]
- Liu, S.; Xu, H.; Bian, X.; Feng, J.; Liu, J.; Yang, Y.; Yuan, C.; An, Y.; Fan, R.; Ci, L. Nanoporous Red Phosphorus on Reduced Graphene Oxide as Superior Anode for Sodium-Ion Batteries. ACS Nano 2018, 12, 7380–7387. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Hayashi, A.; Tatsumisago, M. All-solid-state lithium secondary batteries with high capacity using black phosphorus negative electrode. J. Power Sources 2011, 196, 6902–6905. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.H.; Moon, S.; Kim, D.K.; Kim, J.-H. Eliciting Specific Electrochemical Reaction Behavior by Rational Design of a Red Phosphorus Electrode for Sodium-Ion Batteries. Nanomaterials 2021, 11, 3053. https://doi.org/10.3390/nano11113053
Yun JH, Moon S, Kim DK, Kim J-H. Eliciting Specific Electrochemical Reaction Behavior by Rational Design of a Red Phosphorus Electrode for Sodium-Ion Batteries. Nanomaterials. 2021; 11(11):3053. https://doi.org/10.3390/nano11113053
Chicago/Turabian StyleYun, Jong Hyuk, San Moon, Do Kyung Kim, and Joo-Hyung Kim. 2021. "Eliciting Specific Electrochemical Reaction Behavior by Rational Design of a Red Phosphorus Electrode for Sodium-Ion Batteries" Nanomaterials 11, no. 11: 3053. https://doi.org/10.3390/nano11113053
APA StyleYun, J. H., Moon, S., Kim, D. K., & Kim, J.-H. (2021). Eliciting Specific Electrochemical Reaction Behavior by Rational Design of a Red Phosphorus Electrode for Sodium-Ion Batteries. Nanomaterials, 11(11), 3053. https://doi.org/10.3390/nano11113053





