Chitosan as an Underrated Polymer in Modern Tissue Engineering
Abstract
:1. Introduction
2. Physical and Chemical Properties
2.1. Obtaining Chitosan
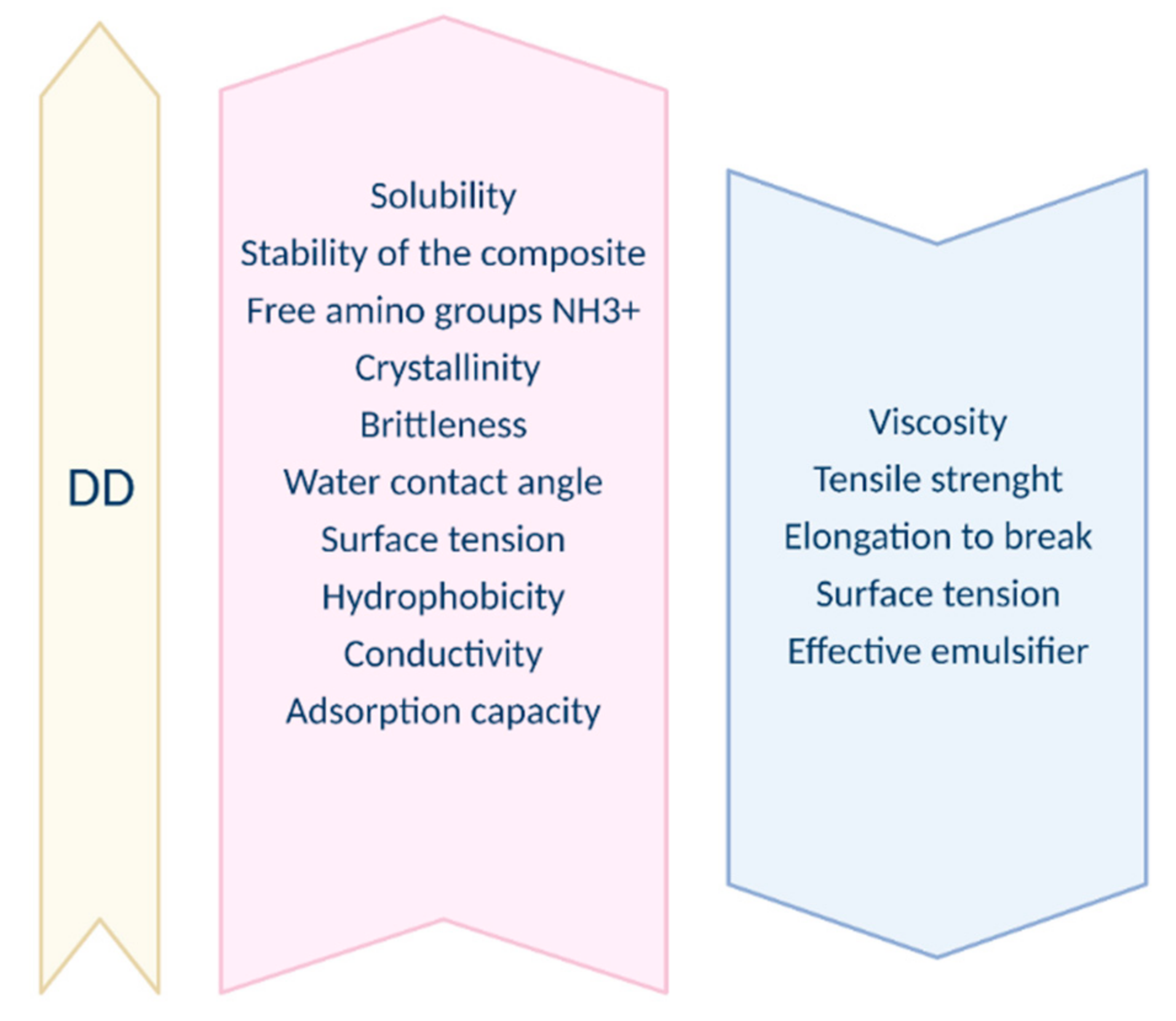
2.2. Degree of Deacetylation
2.3. Molecular Weight
2.4. Solubility
2.5. Viscosity
2.6. Coagulation
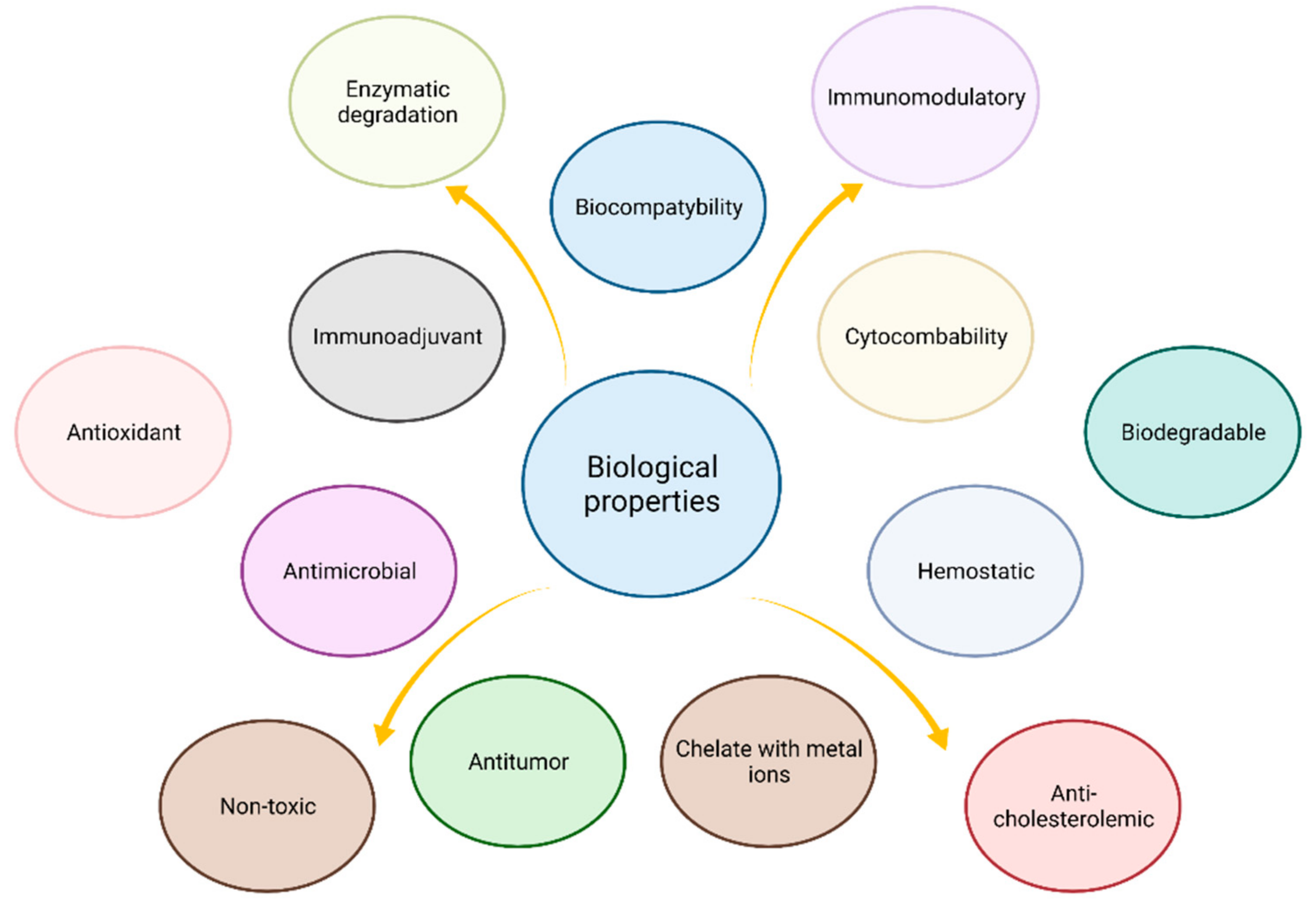
3. Biological Properties
3.1. Biocompatibility
3.2. Biodegradation
3.3. Cytocompability
3.4. Antimicrobial and Antifungal Properties
3.5. Antioxidant Activity
3.6. Enzymatic Degradation
3.7. Immunoadjuvancy
3.8. Absorption-Enhancing Effect
3.9. Immunomodulatory (Anti-Inflammatory Action, Analgesic) Properties
3.10. Hemostatic and Blood Clotting Properties
3.11. Antitumor Activity
3.12. Macrophage Activation
3.13. Pro-Health Properties (Antihyperlipidemic, Mineral Absorption, Bodyweight Reduction)
4. Chitosan Blends with Other Materials
4.1. Natural Polymers
4.1.1. Gelatin
4.1.2. Collagen
4.1.3. Hyaluronic Acid
4.1.4. Alginate
4.1.5. Agarose
4.1.6. κ-Carrageenan
4.1.7. Silk
4.1.8. Starch/Pullulan
4.1.9. Natural Rubber Latex (NR)
4.1.10. Cellulose
4.1.11. Carbon Nanotubes (CNT)
4.2. Synthetic Polymers
4.2.1. PVA Poly(Vinyl Alcohol)
4.2.2. PEO Poly(Ethylene Oxide)
4.2.3. PVP Poly(Vinyl Pyrrolidone)
4.2.4. PNIPPAM Poly(N-Iso-propul-acrylamide)
4.2.5. PCL (Polycaprolactone)
4.2.6. PLA Polylactic Acid, or Polylactide
4.2.7. PLLA Poly(L-Lactide)
4.2.8. PAA Poly(Acrylamide)
4.3. Other Promising Substances
4.3.1. Silica
4.3.2. Montmorillonite (MMt)
5. Chitosan Derivatives
5.1. Carboxymethyl Chitosan CMC
5.2. Acylated Modified Chitosan
5.3. Quaternary Ammonium Chitosan
5.4. Thiolated Chitosan
5.5. Grafting Copolymerization of Chitosan-Polyethylene Glycol PEG
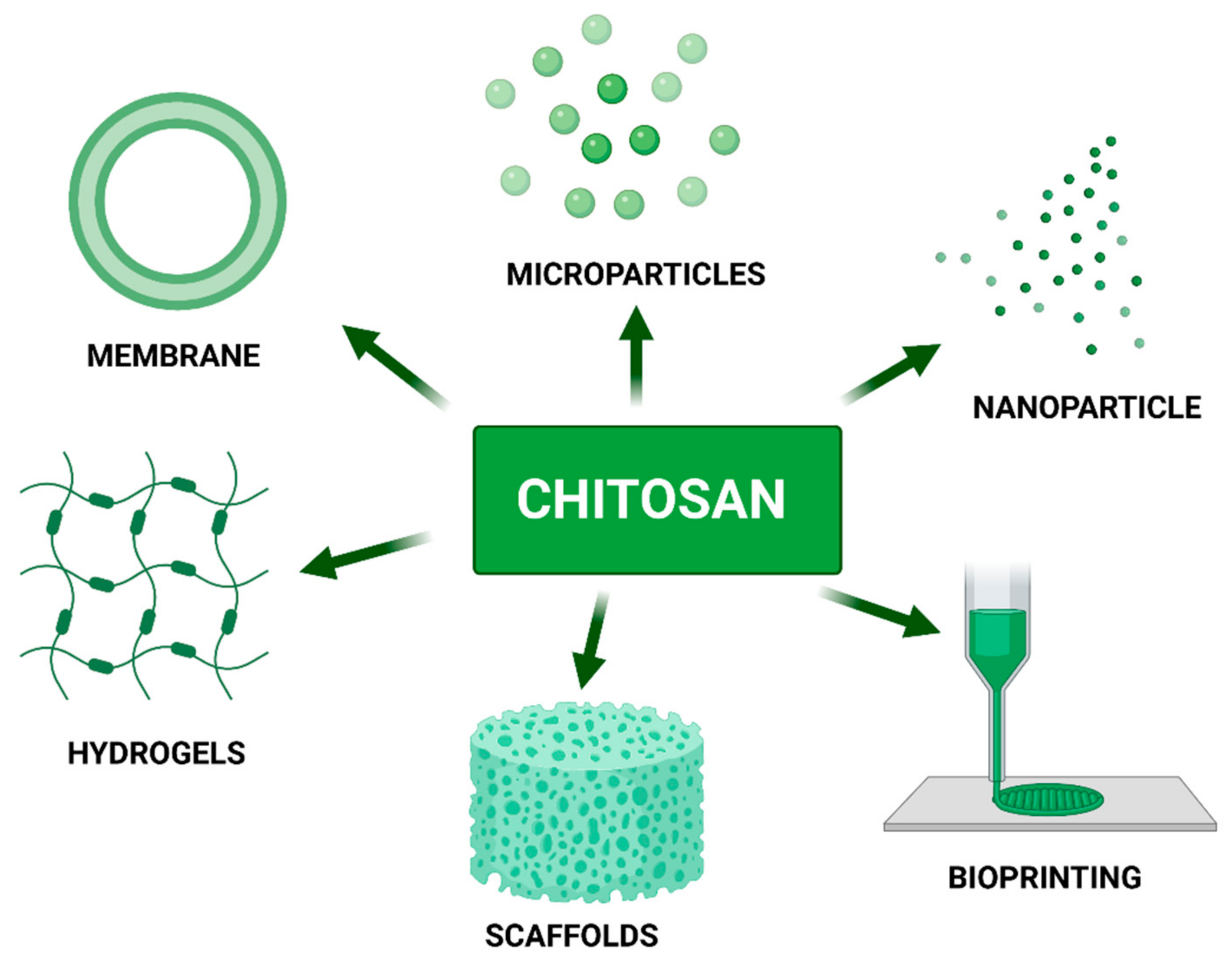
6. Chitosan as a Component of Biomaterials
6.1. Chitosan Membranes
6.2. Chitosan-Based Scaffolds
6.3. Hydrogels
6.4. Chitosan Microparticles and Nanoparticles as Drug Carriers
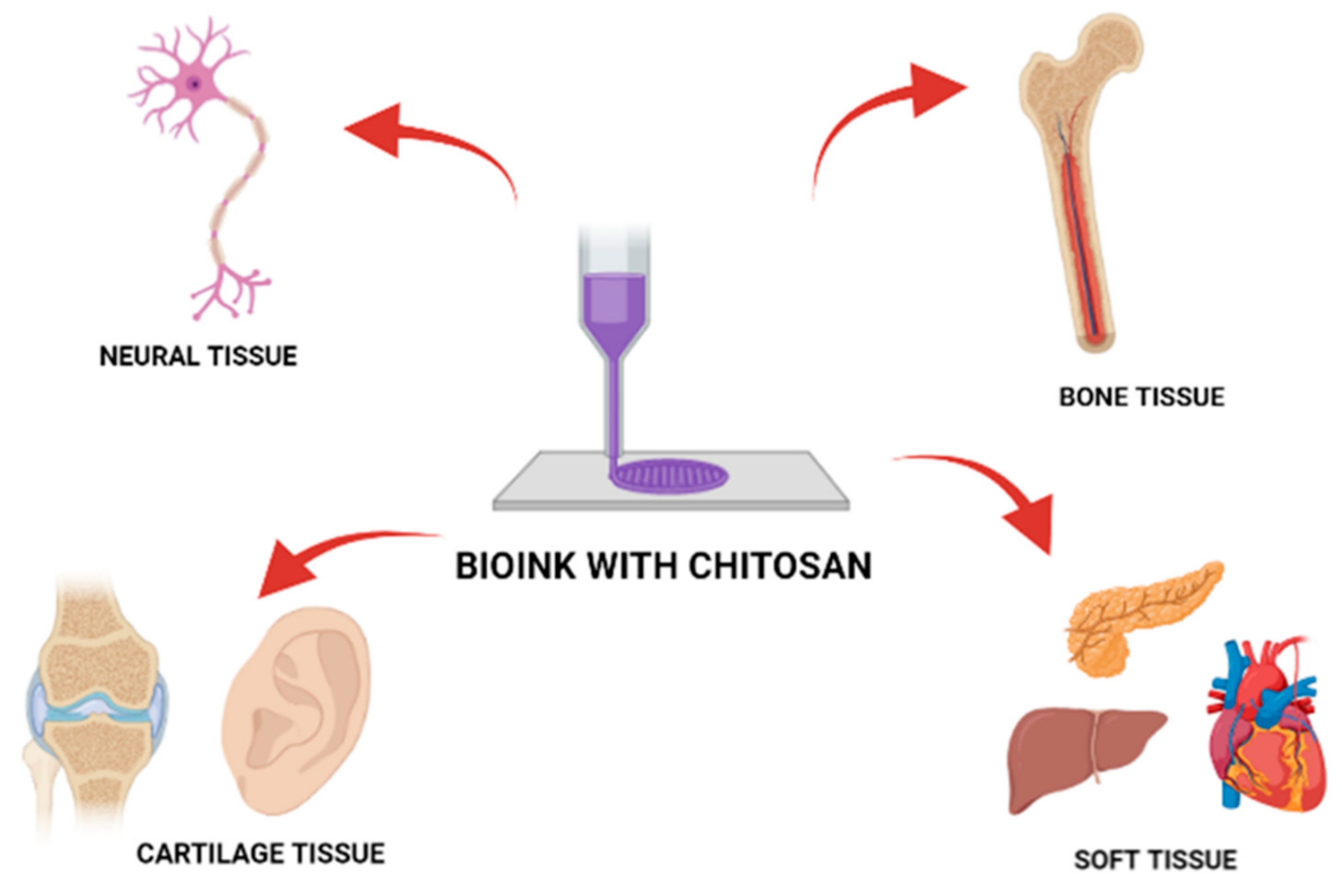
7. Chitosan as a Bio-Ink Composition Material
8. Limitations and Future Prospective in Biomedicine
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.K.; Ramadurai, K.W. 3D Printing and Bio-Based Materials in Global Health; Springer Briefs Materials; Springer: Cham, Switzerland, 2017; Chapter 3; p. 39. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.; Becherán, L.; Bocourt, M.; Peniche, C. Chitosan in biomedicine. From gels to nanoparticles. In Proceedings of the 6th Iberoamerican Chitin Symposium and 12th International Conference on Chitin and Chitosan, Fortaleza, Brazil, 2 September 2012. [Google Scholar]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of chitosan for biomedical applications. Chitosan Based Biomater. 2017, 1, 3–30. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.R.; Mallik, A.K.; Rahman, M.M. Fundamentals of chitosan for biomedical applications. Handb. Chitin Chitosan 2020, 1, 199–230. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marcal, H. Chitosan as a Biomaterial: Influence of Degree of Deacetylation on Its Physiochemical, Material and Biological Properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Huang, J.; Zhang, Z. Hydrogels based on chitosan in tissue regeneration: How do they work? A mini review. J. Appl. Polym. Sci. 2019, 136, 47235. [Google Scholar] [CrossRef] [Green Version]
- Mora Boza, A.; Wlodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasal, B.; San Román, J. Chitosan-based inks: 3D printing and bioprinting strategies to improve shape fidelity, mechanical properties, and biocompatibility of 3D scaffolds. Biomecánica 2019, 27, 1. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.M.; Boeriu, C.G. Chitin and Chitosan: Properties and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2019; Chapter 3; pp. 61–62. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Miralles, B.; Acosta, N.; Calderon, L.; Sanchez, A.; Heras, A. Role of Physicochemical Properties of Chitin and Chitosan on their Functionality. Curr. Chem. Biol. 2014, 8, 27–42. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Budishevska, O.; Popadyuk, N.; Musyanovych, A.; Kohut, A.; Donchak, V.; Voronov, A.; Voronov, S. Formation of three-dimensional polymer structures through radical and ionic reactions of peroxychitosan. Stud. Nat. Prod. Chem. 2020, 64, 365–390. [Google Scholar] [CrossRef]
- Lv, S.H. High-performance superplasticizer based on chitosan. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 7; pp. 131–150. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Functional Properties of Chitosan; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Chapter 1; p. 7. ISBN 9780128027356. [Google Scholar]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- De Moura, C.M.; de Moura, J.M.; Soares, N.M.; de Almeida Pinto, L.A. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. Chem. Eng. Process. Process Intensif. 2011, 50, 351–355. [Google Scholar] [CrossRef]
- Bough, W.A.; Salter, W.L.; Wu, A.C.M.; Perkins, B.E. Influence of manufacturing variables on the characteristics and effectiveness of chitosan products. I. Chemical composition, viscosity, and molecular-weight distribution of chitosan products. Biotechnol. Bioeng. 1978, 20, 1931–1943. [Google Scholar] [CrossRef]
- Kaplan, D.L. Introduction to Biopolymers from Renewable Resources. In Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998; Chapter 4; pp. 104–107. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrie, J.; Tolaimate, A.; Alagui, A.; Vottero, P. Investigation of different natural sources of chitin: Influence of the source and deacetylation process on the physicochemical characteristics of chitosan. Polym. Int. 2000, 49, 337–344. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. Sustain. Agric. Rev. 2019, 35, 49–123. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ding, C.L.; Liu, H.; Liu, L.H.; Zhao, C.Q. Protective effects of ion-imprinted chitooligosaccharides as uranium-specific chelating agents against the cytotoxicity of depleted uranium in human kidney cells. Toxicology 2011, 286, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Aiba, S.I. Studies on chitosan: 3. Evidence for the presence of random and block copolymer structures in partially N-acetylated chitosans. Int. J. Biol. Macromol. 1991, 13, 40–44. [Google Scholar] [CrossRef]
- Kubota, N.; Eguchi, Y. Facile Preparation of Water-Soluble N-Acetylated Chitosan and Molecular Weight Dependence of Its Water-Solubility. Polym. J. 1997, 29, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R. Natural Chelating Polymers; Alginic Acid, Chitin, and Chitosan, 1st ed.; Pergamon Press: Oxford, UK; New York, NY, USA, 1973; ISBN 9780080172354. [Google Scholar]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Horisberger, M.; Clerc, M.-F. Chitosan-colloidal gold complexes as polycationic probes for the detection of anionic sites by transmission and scanning electron microscopy. Histochemistry 1988, 90, 165–175. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S. Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr. Polym. 2010, 81, 931–936. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Saesoo, S. Synthesis and antibacterial activity of methylated N-(4-N,N-dimethylaminocinnamyl) chitosan chloride. Eur. Polym. J. 2009, 45, 2319–2328. [Google Scholar] [CrossRef]
- Kim, S.-K. Anticancer Effects of Chitin and Chitosan Derivatives. In Chitin and Chitosan Derivatives; CRC Press: Boca Raton, FL, USA, 2013; pp. 199–206. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Gallic acid-grafted chitooligosaccharides suppress antigen-induced allergic reactions in RBL-2H3 mast cells. Eur. J. Pharm. Sci. 2012, 47, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Senevirathne, M.; Ahn, C.B.; Kim, S.K.; Je, J.Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cai, J.; Wu, K.; Li, D.; Hu, Y.; Li, G.; Du, Y. Preparation, characterization and anticoagulant activity in vitro of heparin-like 6-carboxylchitin derivative. Int. J. Biol. Macromol. 2012, 50, 1158–1164. [Google Scholar] [CrossRef]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm. J. 2020, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Qian, Z.J.; Vo, T.S.; Ryu, B.; Ngo, D.N.; Kim, S.K. Antioxidant activity of gallate-chitooligosaccharides in mouse macrophage RAW264.7 cells. Carbohydr. Polym. 2011, 84, 1282–1288. [Google Scholar] [CrossRef]
- Nishimura, K.; Nishimura, S.I.; Seo, H.; Nishi, N.; Tokura, S.; Azuma, I. Macrophage activation with multi-porous beads prepared from partially deacetylated chitin. J. Biomed. Mater. Res. 1986, 20, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Koide, S.S. Chitin-chitosan: Properties, benefits and risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K. Potential anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Kim, S.K.; Ahn, C.B.; Je, J.Y. Inhibition of acetylcholinesterase by gallic acid-grafted-chitosans. Carbohydr. Polym. 2011, 84, 690–693. [Google Scholar] [CrossRef]
- Cho, E.J.; Rahman, A.; Kim, S.W.; Baek, Y.M.; Hwang, H.J.; Oh, J.Y.; Hwang, H.-S.; Lee, S.-H.; Yun, J.-W. Chitosan oligosaccharides inhibit adipogenesis in 3T3-L1 adipocytes. J. Microbiol. Biotechnol. 2008, 18, 80–87. [Google Scholar] [PubMed]
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Malette, W.G.; Quigley, H.J., Jr. Method of Altering Growth and Development and Suppressing Contamination Microorganisms in Cell or Tissue Culture. U.S. Patent US-4605623-A, 12 August 1986. [Google Scholar]
- Muzzarelli, R.; Baldassarre, V.; Conto, F.; Ferrara, P.; Biagini, G.; Gazzanelli, G.; Vasi, V. Biological activity of chitosan: Ultrastructural study. Biomaterials 1988, 9, 247–252. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Pêgo, A.P.; Amaral, I.F. 2.213—Chitosan. In Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 221–237. [Google Scholar] [CrossRef]
- Matica, A.; Menghiu, G.; Ostafe, V. Biodegradability of chitosan based products. Former Ann. West Univ. Timis.-Ser. Chem. 2017, 26, 75–86. [Google Scholar]
- Zhang, H.; Neau, S.H. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Su, F.; Wang, Y.; Liu, X.; Shen, X.; Zhang, X.; Xing, Q.; Wang, L.; Chen, Y. Biocompatibility and in vivo degradation of chitosan based hydrogels as potential drug carrier. J. Biomater. Sci. Polym. Ed. 2018, 29, 1515–1528. [Google Scholar] [CrossRef]
- Denuziere, A.; Ferrier, D.; Damour, O.; Domard, A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes: Biological properties. Biomaterials 1998, 19, 1275–1285. [Google Scholar] [CrossRef]
- Amaral, I.F.; Lamghari, M.; Sousa, S.R.; Sampaio, P.; Barbosa, M.A. Rat bone marrow stromal cell osteogenic differentiation and fibronectin adsorption on chitosan membranes: The effect of the degree of acetylation. J. Biomed. Mater. Res. A 2005, 75, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Elçin, M.; Dixit, V.; Gitnic, G. Hepatocyte attachment on biodegradable modified chitosan membranes: In vitro evaluation for the development of liver organoids. Artif. Organs 1998, 22, 837–846. [Google Scholar] [CrossRef]
- Ao, Q.; Wang, A.; Cao, W.; Zhang, L.; Kong, L.; He, Q.; Gong, Y.; Zhang, X. Manufacture of multimicrotubule chitosan nerve conduits with novel molds and characterization in vitro. J. Biomed. Mater. Res. Part A 2006, 77, 11–18. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Nanokompozyty Chitozanowo-Srebrowe—Nowoczesne Materiały Antybakteryjne—Chemik—Tom Vol. 67, nr 8 (2013)—Biblioteka Nauki—Yadda. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztech-fc9db26b-476f-4c7e-9456-752cc209c5d9 (accessed on 28 July 2021).
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Seo, H.; Mitsuhashi, K.; Tanibe, H. Antibacterial and Antifungal Fiber Blended by Chitosan|Aspergillus & Aspergillosis Website. In Advances in Chitin and Chitosan; Elsevier: New York, NY, USA, 1992. [Google Scholar]
- Hussain, I.; Singh, T.; Chittenden, C. Preparation of chitosan oligomers and characterization: Their antifungal activities and decay resistance. Holzforschung 2012, 66, 119–125. [Google Scholar] [CrossRef]
- Don, T.M.; Chen, C.C.; Lee, C.K.; Cheng, W.Y.; Cheng, L.P. Preparation and antibacterial test of chitosan/PAA/PEGDA bi-layer composite membranes. J. Biomater. Sci. Polym. Ed. 2005, 16, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, A.; Passarinha, L.A.; Gaspar, C.; Palmeira-de-Oliveira, R.; Sarmento, B.; Martinez-de-Oliveira, J.; Pina-Vaz, C.; Rodrigues, A.G.; Queiroz, J.A. The relationship between Candida species charge density and chitosan activity evaluated by ion-exchange chromatography. J. Chromatogr. B 2011, 879, 3749–3751. [Google Scholar] [CrossRef] [PubMed]
- García-Rincón, J.; Vega-Pérez, J.; Guerra-Sánchez, M.G.; Hernández-Lauzardo, A.N.; Peña-Díaz, A.; Velázquez-Del Valle, M.G. Effect of chitosan on growth and plasma membrane properties of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Pestic. Biochem. Physiol. 2010, 97, 275–278. [Google Scholar] [CrossRef]
- Hernández-Lauzardo, A.N.; Bautista-Baños, S.; Velázquez-del Valle, M.G.; Méndez-Montealvo, M.G.; Sánchez-Rivera, M.M.; Bello-Pérez, L.A. Antifungal effects of chitosan with different molecular weights on in vitro development of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Carbohydr. Polym. 2008, 73, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yang, Q.; Huang, G.; Ding, C.; Cao, P.; Huang, L.; Xiao, T.; Guo, J.; Su, Z. Hypolipidemic effects of chitosan and its derivatives in hyperlipidemic rats induced by a high-fat diet. Food Nutr. Res. 2016, 60, 31137. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Anraku, M.; Kabashima, M.; Namura, H.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Furutani, N.; Tomida, H. Antioxidant protection of human serum albumin by chitosan. Int. J. Biol. Macromol. 2008, 43, 159–164. [Google Scholar] [CrossRef]
- Tomida, H.; Fujii, T.; Furutani, N.; Michihara, A.; Yasufuku, T.; Akasaki, K.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Anraku, M. Antioxidant properties of some different molecular weight chitosans. Carbohydr. Res. 2009, 344, 1690–1696. [Google Scholar] [CrossRef]
- Feng, T.; Du, Y.; Li, J.; Hu, Y.; Kennedy, J.F. Enhancement of antioxidant activity of chitosan by irradiation. Carbohydr. Polym. 2008, 73, 126–132. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitin; Pergamon Press: New York, NY, USA, 1977; Chapter 4; pp. 155–181. [Google Scholar]
- Han, T.; Nwe, N.; Furuike, T.; Tokura, S.; Tamura, H. Methods of N-acetylated chitosan scaffolds and its In-vitro biodegradation by lysozyme. J. Biomed. Sci. Eng. 2012, 5, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Vårum, K.M.; Myhr, M.M.; Hjerde, R.J.; Smidsrød, O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef]
- Onishi, H.; Machida, Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Matthew, H.W.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafiee-Tehranie, M.; Junginger, H.E. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef]
- Mei, D.; Mao, S.; Sun, W.; Wang, Y.; Kissel, T. Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur. J. Pharm. Biopharm. 2008, 70, 874–881. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Fong, D. Immunological responses to chitosan for biomedical applications. Chitosan Based Biomater. 2017, 1, 45–79. [Google Scholar] [CrossRef]
- Fong, D.; Hoemann, C.D. Chitosan immunomodulatory properties: Perspectives on the impact of structural properties and dosage. Futur. Sci. OA 2018, 4, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Mechanizmy Przewodzenia Bólu: Rola Układu Odpornościowego w Regulacji Odczuwania Bólu—Filipczak-Bryniarska, Iwona—FBC. Available online: https://fbc.pionier.net.pl/details/nnlT0v2 (accessed on 29 July 2021).
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Huang, S.; Han, B.; Shao, K.; Yu, M.; Liu, W. Analgesis and wound healing effect of chitosan and carboxymethyl chitosan on scalded rats. J. Ocean Univ. China 2014, 13, 837–841. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef] [Green Version]
- Klokkevold, P.R.; Fukayama, H.; Sung, E.C.; Bertolami, C.N. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral Maxillofac. Surg. 1999, 57, 49–52. [Google Scholar] [CrossRef]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Tan, G.; Kaya, M.; Tevlek, A.; Sargin, I.; Baran, T. Antitumor activity of chitosan from mayfly with comparison to commercially available low, medium and high molecular weight chitosans. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosic, L.; Calabró, D.; Avallone, B.; Balsamo, G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Mori, T.; Murakami, M.; Okumura, M.; Kadosawa, T.; Uede, T.; Fujinaga, T. Mechanism of macrophage activation by chitin derivatives. J. Vet. Med. Sci. 2005, 67, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maezaki, Y.; Tsuji, K.; Nakagawa, Y.; Kawai, Y.; Akimoto, M.; Tsugita, T.; Takekawa, W.; Terada, A. Hiroyoshi Hara & Tomotari Mitsuoka (1993) Hypocholesterolemic Effect of Chitosan in Adult Males. Biotechnol. Biochem. 2014, 57, 1439–1444. [Google Scholar] [CrossRef]
- Wuolioki, E.; Hirvela, T.; Ylitalo, P. Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Find. Exp. Clin. Pharmacol. 1999, 21, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Bokura, H.; Kobayashi, S. Chitosan decreases total cholesterol in women: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2003, 57, 721–725. [Google Scholar] [CrossRef] [Green Version]
- Gallaher, D. Chitosan, Cholesterol Lowering, and Caloric Loss. Food Sci. Nutr. 2003, 14, 32–35. [Google Scholar]
- Panith, N.; Wichaphon, J.; Lertsiri, S.; Niamsiri, N. Effect of physical and physicochemical characteristics of chitosan on fat-binding capacities under in vitro gastrointestinal conditions. LWT Food Sci. Technol. 2016, 71, 25–32. [Google Scholar] [CrossRef]
- Cornelli, U.; Belcaro, G. Meta-analysis of studies on body weight and cholesterol reduction using the chitosan derivative polyglucosamine L112. Gen. Med. Open 2018, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.Y.; Oh, T.W.; Nakajima, D.; Maeda, A.; Naka, T.; Kim, C.S.; Igawa, S.; Ohta, F. Effects of habitual chitosan intake on bone mass, bone-related metabolic markers and duodenum CaBP D9K mRNA in ovariectomized SHRSP rats. J. Nutr. Sci. Vitaminol. 2002, 48, 371–378. [Google Scholar] [CrossRef]
- Wada, M.; Nishimura, Y.; Watanabe, Y.; Takita, T.; Innami, S. Accelerating Effect of Chitosan Intake on Urinary Calcium Excretion by Rats. Biosci. Biotechnol. Biochem. 2014, 61, 1206–1208. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Correlo, V.M.; Boesel, L.F.; Bhattacharya, M.; Mano, J.F.; Neves, N.M.; Reis, R.L. Properties of melt processed chitosan and aliphatic polyester blends. Mater. Sci. Eng. A 2005, 403, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Elhefian, E.A.; El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Chitosan-Based Polymer Blends: Current Status and Applications. J. Chem. Soc. Pak. 2014, 36, 11–27. [Google Scholar]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.R.; Lee, D.H.; Chung, P.H.; Yang, H.C. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e94–e100. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 264–272. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Macrophage activation through phagocytosis of muramyl dipeptide encapsulated in gelatin microspheres. J. Pharm. Pharmacol. 1987, 39, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative study of chitosan and chitosan–gelatin scaffold for tissue engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Pulieri, E.; Chiono, V.; Ciardelli, G.; Vozzi, G.; Ahluwalia, A.; Domenici, C.; Vozzi, F.; Giusti, P. Chitosan/gelatin blends for biomedical applications. J. Biomed. Mater. Res. Part A 2008, 86, 311–322. [Google Scholar] [CrossRef]
- Shi, Y.; Rittman, L.; Vesely, I. Novel geometries for tissue-engineered tendonous collagen constructs. Tissue Eng. 2006, 12, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Indrani, D.J.; Lukitowati, F.; Yulizar, Y. Preparation of Chitosan/Collagen Blend Membranes for Wound Dressing: A Study on FTIR Spectroscopy and Mechanical Properties. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012020. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.G.; Wang, P.W.; Wei, B.; Mo, X.M.; Cui, F.Z. Electrospun collagen-chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010, 6, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Skwarczyńska, A.; Biniaś, D.; Modrzejewska, Z. Structural research of thermosensitive chitosan-collagen gels containing ALP. Prog. Chem. Appl. Chitin Its Deriv. 2016, 21, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Raftery, R.M.; Woods, B.; Marques, A.L.; Moreira-Silva, J.; Silva, T.H.; Cryan, S.A.; Reis, R.L.; O’Brien, F.J. Multifunctional biomaterials from the sea: Assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater. 2016, 43, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, B.; Sionkowska, A. Chitosan/collagen blends with inorganic and organic additive—A review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska, S. Influence of the intermolecular interaction on physico-chemical properties of chitosan/hyaluronic acid blends. Prog. Chem. Appl. Chitin Its Deriv. 2015, 20, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Michalska, M. Characterisation of chitosan/hyaluronic acid blend films modified by collagen. Prog. Chem. Appl. Chitin Its Deriv. 2017, 22, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Kaczmarek, B. Preparation and characterization of composites based on the blends of collagen, chitosan and hyaluronic acid with nano-hydroxyapatite. Int. J. Biol. Macromol. 2017, 102, 658–666. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Kaczmarek, B. Modification of 3D materials based on chitosan and collagen blends by sodium alginate. Mol. Cryst. Liq. Cryst. 2016, 640, 39–45. [Google Scholar] [CrossRef]
- Iwasaki, N.; Yamane, S.T.; Majima, T.; Kasahara, Y.; Minami, A.; Harada, K.; Nonaka, S.; Maekawa, N.; Tamura, H.; Tokura, S.; et al. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: Evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules 2004, 5, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Stephen, A.M.; Phillips, G.O.; Williams, P. Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 7.2; p. 219. [Google Scholar]
- Cao, Z.; Gilbert, R.J.; He, W. Simple Agarose-Chitosan Gel Composite System for Enhanced Neuronal Growth in Three Dimensions. Biomacromolecules 2009, 10, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Kumar, A. Cell proliferation on three-dimensional chitosan-agarose-gelatin cryogel scaffolds for tissue engineering applications. J. Biosci. Bioeng. 2012, 114, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Du, Y.; Zhang, B.; Yang, J.; Zhou, J.; Kennedy, J.F. Preparation and properties of alginate/carboxymethyl chitosan blend fibers. Carbohydr. Polym. 2006, 65, 447–452. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and characterization of chitosan/agar blended films: Part 1. chemical structure and morphology. E-J. Chem. 2012, 9, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Hong, P.; Liao, M.; Kong, S.; Huang, N.; Ou, C.; Li, S. Preparation and Characterization of Chitosan-Agarose Composite Films. Materials 2016, 9, 816. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Mora, V.; Velasco, D.; Hernández, R.; Mijangos, C.; Kumacheva, E. Chitosan/agarose hydrogels: Cooperative properties and microfluidic preparation. Carbohydr. Polym. 2014, 111, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Lee, B.I.; Jung, S.T.; Park, H.J. Biopolymer composite films based on κ-carrageenan and chitosan. Mater. Res. Bull. 2001, 36, 511–519. [Google Scholar] [CrossRef]
- Yu, H.C.; Zhang, H.; Ren, K.; Ying, Z.; Zhu, F.; Qian, J.; Ji, J.; Wu, Z.L.; Zheng, Q. Ultrathin κ-Carrageenan/Chitosan Hydrogel Films with High Toughness and Antiadhesion Property. ACS Appl. Mater. Interfaces 2018, 10, 9002–9009. [Google Scholar] [CrossRef]
- Al-Zebari, N.; Best, S.M.; Cameron, R.E. Effects of reaction pH on self-crosslinked chitosan-carrageenan polyelectrolyte complex gels and sponges. J. Phys. Mater. 2018, 2, 015003. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Um, I.C.; Park, Y.H. Structural and thermal characteristics of Antheraea pernyi silk fibroin/chitosan blend film. Polymer 2001, 42, 6651–6656. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Chiellini, F.; Wang, X.; Chiellini, E.; Declercq, H.A.; Kaplan, D.L. Silk/chitosan biohybrid hydrogels and scaffolds via green technology. RSC Adv. 2014, 4, 53547–53556. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Ingber, D.E. Unexpected Strength and Toughness in Chitosan-Fibroin Laminates Inspired by Insect Cuticle. Adv. Mater. 2012, 24, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Aliabadi, H.A.M.; Sukhtezari, S.; Tahmasebi, B.; Maleki, A.; Madanchi, H. Chitosan hydrogel/silk fibroin/Mg(OH) 2 nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Pullulan as a Promising Biomaterial for Biomedical Applications: A Perspective. Trends Biomater. Artif. Organs 2007, 20, 116–121. [Google Scholar]
- Lazaridou, A.; Biliaderis, C.G. Thermophysical properties of chitosan, chitosan-starch and chitosan-pullulan films near the glass transition. Carbohydr. Polym. 2002, 48, 179–190. [Google Scholar] [CrossRef]
- Zhai, M.; Zhao, L.; Yoshii, F.; Kume, T. Study on antibacterial starch/chitosan blend film formed under the action of irradiation. Carbohydr. Polym. 2004, 57, 83–88. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Wittaya-areekul, S.; Prahsarn, C. Development and in vitro evaluation of chitosan-polysaccharides composite wound dressings. Int. J. Pharm. 2006, 313, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Boonrasri, S.; Sae–Oui, P.; Rachtanapun, P. Chitosan and Natural Rubber Latex Biocomposite Prepared by Incorporating Negatively Charged Chitosan Dispersion. Molecules 2020, 25, 2777. [Google Scholar] [CrossRef]
- Paoribut, K.; Taweepreda, W.; Boonkerd, K. Preparation and Characterization of Natural Rubber/Chitosan Films. Key Eng. Mater. 2015, 659, 484–489. [Google Scholar] [CrossRef]
- Duan, J.; Han, C.; Liu, L.; Jiang, J.; Li, J.; Li, Y.; Guan, C. Binding cellulose and chitosan via intermolecular inclusion interaction: Synthesis and characterisation of gel. J. Spectrosc. 2015, 2015, 179258. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Wu, T.; Farnood, R.; O’Kelly, K.; Chen, B. Mechanical behavior of transparent nanofibrillar cellulose–chitosan nanocomposite films in dry and wet conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Carbon Nanotubes as Biomaterials. Encycl. Biophys. 2013, 238–240. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. A 2014, 102, 2704–2712. [Google Scholar] [CrossRef]
- Arora, S.; Kaur, H.; Kumar, R.; Kaur, R.; Rana, D.; Rayat, C.S.; Kaur, I.; Arora, S.K.; Bubber, P.; Bharadwaj, L.M. In Vitro Cytotoxicity of Multiwalled and Single-Walled Carbon Nanotubes on Human Cell Lines. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 377–382. [Google Scholar] [CrossRef]
- Muhamad, I.; Selvakumaran, S.; Lazim, N.A.M. Designing Polymeric Nanoparticles for Targeted Drug Delivery System Nanomedicine. Nanomedicine 2015, 287, 287–313. [Google Scholar]
- Kasai, D.; Chougale, R.; Masti, S.; Chalannavar, R.; Malabadi, R.B.; Gani, R.; Gouripur, G. An Investigation into the Influence of Filler Piper nigrum Leaves Extract on Physicochemical and Antimicrobial Properties of Chitosan/Poly (Vinyl Alcohol) Blend Films. J. Polym. Environ. 2019, 27, 472–488. [Google Scholar] [CrossRef]
- Yang, J.M.; Su, W.Y.; Leu, T.L.; Yang, M.C. Evaluation of chitosan/PVA blended hydrogel membranes. J. Memb. Sci. 2004, 236, 39–51. [Google Scholar] [CrossRef]
- Khoo, C.G.; Frantzich, S.; Rosinski, A.; Sjöström, M.; Hoogstraate, J. Oral gingival delivery systems from chitosan blends with hydrophilic polymers. Eur. J. Pharm. Biopharm. 2003, 55, 47–56. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Vicini, S.; Marsano, E. The influence of UV irradiation on the mechanical properties of chitosan/poly(vinyl pyrrolidone) blends. Polym. Degrad. Stab. 2005, 88, 261–267. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan-poly(vinyl pyrrolidone) blends as membranes for direct methanol fuel cell applications. J. Power Sources 2006, 159, 846–854. [Google Scholar] [CrossRef]
- Don, T.M.; King, C.F.; Chiu, W.Y.; Peng, C.A. Preparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carbohydr. Polym. 2006, 63, 331–339. [Google Scholar] [CrossRef]
- Minoura, N.; Koyano, T.; Koshizaki, N.; Umehara, H.; Nagura, M.; Kobayashi, K.I. Preparation, properties, and cell attachment/growth behavior of PVA/chitosan-blended hydrogels. Mater. Sci. Eng. C 1998, 6, 275–280. [Google Scholar] [CrossRef]
- De Souza Costa-Júnior, E.; Pereira, M.M.; Mansur, H.S. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J. Mater. Sci. Mater. Med. 2009, 20, 553–561. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. The preparation and characterization of Chitosan/Poly (Vinyl Alcohol) blended films. E-J. Chem. 2010, 7, 1212–1219. [Google Scholar] [CrossRef]
- Amiji, M.M. Permeability and blood compatibility properties of chitosan-poly(ethylene oxide) blend membranes for haemodialysis. Biomaterials 1995, 16, 593–599. [Google Scholar] [CrossRef]
- Zivanovic, S.; Li, J.; Davidson, P.M.; Kit, K. Physical, mechanical, and antibacterial properties of chitosan/PEO blend films. Biomacromolecules 2007, 8, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zivanovic, S.; Davidson, P.M.; Kit, K. Characterization and comparison of chitosan/PVP and chitosan/PEO blend films. Carbohydr. Polym. 2010, 79, 786–791. [Google Scholar] [CrossRef]
- Kolhe, P.; Kannan, R.M. Improvement in ductility of chitosan through blending and copolymerization with PEG: FTIR investigation of molecular interactions. Biomacromolecules 2003, 4, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, J.-T.; Chen, C.-L.; Huang, K.S.; Nien, Y.H.; Chen, J.L.; Huang, P.Z. Synthesis, characterization, and application of PVP/chitosan blended polymers. J. Appl. Polym. Sci. 2006, 101, 885–891. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Cho, S.M.; Lee, Y.M.; Kim, S.J. Thermo- and pH-Responsive Behaviors of Graft Copolymer and Blend Based on Chitosan and N-Isopropylacrylamide—Hanyang University. J. Appl. Polym. Sci. 2000, 78, 1381–1391. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone microparticles and their biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Processing and Products; William Andrew: Norwich, NY, USA, 2014; pp. 1–601. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chen, F. Preparation and characterization of photocured poly (ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopolymerization-type 3D printing tissue engineering scaffold application. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 81, 66–73. [Google Scholar] [CrossRef]
- Nowak, B.; Pająk, J. Biodegradacja polilaktydu (PLA). Arch. Gospod. Odpad. Ochr. Sr. 2010, 12, 1–10. [Google Scholar]
- Sébastien, F.; Stéphane, G.; Copinet, A.; Coma, V. Novel biodegradable films made from chitosan and poly(lactic acid) with antifungal properties against mycotoxinogen strains. Carbohydr. Polym. 2006, 65, 185–193. [Google Scholar] [CrossRef]
- Chen, C.; Dong, L.; Cheung, M.K. Preparation and characterization of biodegradable poly(l-lactide)/chitosan blends. Eur. Polym. J. 2005, 41, 958–966. [Google Scholar] [CrossRef]
- Rajeswari, A.; Gopi, S.; Jackcina Stobel Christy, E.; Jayaraj, K.; Pius, A. Current research on the blends of chitosan as new biomaterial. In Handbook of Chitin and Chitosan. Volume 1: Preparation and Properties; Elsevier: Amsterdam, The Netherlands, 2020; pp. 247–283. ISBN 9780128179703. [Google Scholar]
- Desai, K.; Kit, K. Effect of spinning temperature and blend ratios on electrospun chitosan/poly(acrylamide) blends fibers. Polymer 2008, 49, 4046–4050. [Google Scholar] [CrossRef]
- López Angulo, D.E.; Ambrosio, C.E.; Lourenço, R.; Nardelli Gonçalves, N.J.; Cury, F.S.; José do Amaral Sobral, P. Fabrication, characterization and in vitro cell study of gelatin-chitosan scaffolds: New perspectives of use of aloe vera and snail mucus for soft tissue engineering. Mater. Chem. Phys. 2019, 234, 268–280. [Google Scholar] [CrossRef]
- Assaad, E.; Maire, M.; Lerouge, S. Injectable thermosensitive chitosan hydrogels with controlled gelation kinetics and enhanced mechanical resistance. Carbohydr. Polym. 2015, 130, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pieklarz, K.; Tylman, M.; Modrzejewska, Z. Preparation and characterization of a new generation of chitosan hydrogels containing pyrimidine ribonucleotides. Prog. Chem. Appl. Chitin Its Deriv. 2020, 25, 192–200. [Google Scholar] [CrossRef]
- Pieklarz, K.; Galita, G.; Tylman, M.; Maniukiewicz, W.; Kucharska, E.; Majsterek, I.; Modrzejewska, Z. Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application. J. Funct. Biomater. 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Budnyak, T.M.; Błachnio, M.; Slabon, A.; Jaworski, A.; Tertykh, V.A.; Deryło-Marczewska, A.; Marczewski, A.W. Chitosan Deposited onto Fumed Silica Surface as Sustainable Hybrid Biosorbent for Acid Orange 8 Dye Capture: Effect of Temperature in Adsorption Equilibrium and Kinetics. J. Phys. Chem. C 2020, 124, 15312–15323. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; El-Baz, N.M. A Review on Bionanocomposites Based on Chitosan and Its Derivatives for Biomedical Applications. Adv. Struct. Mater. 2015, 74, 173–208. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Vlasova, N.N.; Golovkova, L.P.; Markitan, O.; Baryshnikov, G.; Ågren, H.; Slabon, A. Nucleotide Interaction with a Chitosan Layer on a Silica Surface: Establishing the Mechanism at the Molecular Level. Langmuir 2021, 37, 1511–1520. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jafari, H.; Atlasi, Z.; Mahdavinia, G.R.; Hadifar, S.; Sabzi, M. Magnetic κ-carrageenan/chitosan/montmorillonite nanocomposite hydrogels with controlled sunitinib release. Mater. Sci. Eng. C 2021, 124, 112042. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Li, S.; Xing, L.; Du, S.; Yuan, G.; Li, J.; Zhou, T.; Xiong, D.; Tan, H.; et al. Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int. J. Biol. Macromol. 2020, 164, 2204–2214. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.H.; Park, A.; Rim, M.; Youn, J.; Lee, W.; Song, J.E.; Khang, G. Advanced gellan gum-based glycol chitosan hydrogel for cartilage tissue engineering biomaterial. Int. J. Biol. Macromol. 2020, 158, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Carmagnola, I.; Chiappone, A.; Feng, Z.; Ciardelli, G.; Hakkarainen, M.; Sangermano, M. Photocurable chitosan as bioink for cellularized therapies towards personalized scaffold architecture. Bioprinting 2020, 18, e00082. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Tariq, M.; Khan, S.M.; Gull, N.; Sagar Iqbal, S.; Aziz, A.; Nazir, A.; Iqbal, M. Synthesis and characterization of chitosan and guar gum based ternary blends with polyvinyl alcohol. Int. J. Biol. Macromol. 2020, 143, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Bukzem, A.L.; Signini, R.; dos Santos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.Q.; Cheng, L.Y.; Xiao, J.X.; Han, X.N. Preparation and characterization of O-carboxymethyl chitosan–sodium alginate polyelectrolyte complexes. Colloid Polym. Sci. 2015, 293, 401–407. [Google Scholar] [CrossRef]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups. Int. J. Biol. Macromol. 2018, 114, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Wahba, S.M.; Darwish, A.S.; Kamal, S.M. Ceria-containing uncoated and coated hydroxyapatite-based galantamine nanocomposites for formidable treatment of Alzheimer’s disease in ovariectomized albino-rat model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, S.; Mortazavian, E.; Mohammadi, Z.; Samadi, F.Y.; Samadikhah, H.; Taheritarigh, S.; Tehrani, N.R.; Rafiee-Tehrani, M. Thiolated methylated dimethylaminobenzyl chitosan: A novel chitosan derivative as a potential delivery vehicle. Int. J. Biol. Macromol. 2017, 95, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, F.; Wu, Z.; Jin, J.; Li, F.; Wang, Y.; Wang, Z.; Tang, S.; Wu, C.; Wang, Y. O-acylation of chitosan nanofibers by short-chain and long-chain fatty acids. Carbohydr. Polym. 2017, 177, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Mi, Y.; Zhang, J.; Li, Q.; Dong, F.; Guo, Z. Evaluation of quaternary ammonium chitosan derivatives differing in the length of alkyl side-chain: Synthesis and antifungal activity. Int. J. Biol. Macromol. 2019, 129, 1127–1132. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Bao, X.; Xu, G.; Yao, P. Fatty acid and quaternary ammonium modified chitosan nanoparticles for insulin delivery. Colloids Surf. B Biointerfaces 2018, 170, 136–143. [Google Scholar] [CrossRef]
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A. Thiolated Chitosans: A Multi-talented Class of Polymers for Various Applications. Biomacromolecules 2020, 22, 24–56. [Google Scholar] [CrossRef]
- Sreenivas, S.; Pai, K. Thiolated Chitosans: Novel Polymers for Mucoadhesive Drug Delivery—A Review. Trop. J. Pharm. Res. 2008, 7, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.; Lanthaler, M.; Laffleur, F.; Huck, C.W.; Bernkop-Schnürch, A. Thiolated chitosan micelles: Highly mucoadhesive drug carriers. Carbohydr. Polym. 2017, 167, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ayensu, I.; Boateng, J. Development and Evaluation of Lyophilized Thiolated-Chitosan Wafers for Buccal Delivery of Protein. J. Sci. Technol. 2012, 32, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 14, 1189–1204. [Google Scholar] [CrossRef]
- Chang, F.C.; Tsao, C.T.; Lin, A.; Zhang, M.; Levengood, S.L.; Zhang, M. PEG-chitosan hydrogel with tunable stiffness for study of drug response of breast cancer cells. Polymers 2016, 8, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.K.; Halder, M.; Srivastava, U.; Singh, Y. Antibacterial PEG-Chitosan Hydrogels for Controlled Antibiotic/Protein Delivery. ACS Appl. Bio. Mater. 2019, 2, 5313–5322. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, X.; Han, J.; Song, G.; Nie, J. Photo-polymeriable chitosan derivative prepared by Michael reaction of chitosan and polyethylene glycol diacrylate (PEGDA). Int. J. Biol. Macromol. 2009, 45, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Al-Remawi, M. Application of N-hexoyl chitosan derivatives with high degree of substitution in the preparation of super-disintegrating pharmaceutical matrices. J. Drug Deliv. Sci. Technol. 2015, 29, 31–41. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 2004, 25, 4273–4278. [Google Scholar] [CrossRef]
- Sadhasivam, B.; Ravishankar, K.; Desingh, R.; Subramaniyam, R.; Dhamodharan, R. Biocompatible Porous Scaffolds of Chitosan/Poly(EG-ran-PG) Blends with Tailored Pore Size and Nontoxic to Mesenchymal Stem Cells: Preparation by Controlled Evaporationfrom Aqueous Acetic Acid Solution. ACS Omega 2018, 3, 10286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latańska, I.; Kolesińska, B.; Draczyński, Z.; Sujka, W. The use of chitin and chitosan in manufacturing dressing materials. Prog. Chem. Appl. Chitin Its Deriv. 2020, 25, 16–36. [Google Scholar]
- Enumo, A.; Argenta, D.F.; Bazzo, G.C.; Caon, T.; Stulzer, H.K.; Parize, A.L. Development of curcumin-loaded chitosan/pluronic membranes for wound healing applications. Int. J. Biol. Macromol. 2020, 163, 167–179. [Google Scholar] [CrossRef]
- Sari, M.; Tamrin; Kaban, J.; Alfian, Z. A novel composite membrane pectin from Cyclea Barbata Miers blend with chitosan for accelerated wound healing. Polym. Test. 2021, 99, 107207. [Google Scholar] [CrossRef]
- Ho, T.T.P.; Doan, V.K.; Tran, N.M.P.; Nguyen, L.K.K.; Le, A.N.M.; Ho, M.H.; Trinh, N.T.; Van Vo, T.; Tran, L.D.; Nguyen, T.H. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater. Sci. Eng. C 2021, 120, 111724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Yang, R.; Ma, Q. Asymmetric wetting and antibacterial composite membrane obtained by spraying bacterial cellulose grafted with chitosan for sanitary products surface layers. Carbohydr. Polym. 2021, 256, 117602. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.; Yang, X.; Gao, Y.; Ren, Y.; Li, Q.; Qu, Y.; Chen, G.; Zeng, R. Investigation into the physical properties, antioxidant and antibacterial activity of Bletilla striata polysaccharide/chitosan membranes. Int. J. Biol. Macromol. 2021, 182, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Ooi, C.W.; Song, C.P.; Wang, C.Y.; Liu, B.L.; Lin, G.Y.; Chiu, C.Y.; Chang, Y.K. Antibacterial efficacy of quaternized chitosan/poly (vinyl alcohol) nanofiber membrane crosslinked with blocked diisocyanate. Carbohydr. Polym. 2021, 262, 117910. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Ashraf, S.S. Cinnamon extract loaded electrospun chitosan/gelatin membrane with antibacterial activity. Int. J. Biol. Macromol. 2021, 173, 580–590. [Google Scholar] [CrossRef]
- Siahaan, P.; Sasongko, N.A.; Lusiana, R.A.; Prasasty, V.D.; Martoprawiro, M.A. The validation of molecular interaction among dimer chitosan with urea and creatinine using density functional theory: In application for hemodyalisis membrane. Int. J. Biol. Macromol. 2021, 168, 339–349. [Google Scholar] [CrossRef]
- Jaisankar, E.; Pavithra, M.E.; Krishna, S.; Thirumarimurugan, M.; Azarudeen, R.S. Dual property of chitosan blended copolymer membranes: Antidiabetic drug release profile and antimicrobial assay. Int. J. Biol. Macromol. 2020, 145, 42–52. [Google Scholar] [CrossRef]
- İlk, S.; Ramanauskaitė, A.; Koç Bilican, B.; Mulerčikas, P.; Çam, D.; Onses, M.S.; Torun, I.; Kazlauskaitė, S.; Baublys, V.; Aydın, Ö.; et al. Usage of natural chitosan membrane obtained from insect corneal lenses as a drug carrier and its potential for point of care tests. Mater. Sci. Eng. C 2020, 112, 110897. [Google Scholar] [CrossRef] [PubMed]
- Szczepański, R.; Gadomska, L.; Michalak, M.; Bakun, P.; Pawlak, K.; Goslinski, T.; Ziegler-Borowska, M.; Czarczynska-Goslinska, B. Chitosan-derivatives in combinations with selected porphyrinoids as novel hybrid materials for medicine and pharmacy. Prog. Chem. Appl. Chitin Deriv. 2020, 25, 63–78. [Google Scholar] [CrossRef]
- Shen, F.; Cui, Y.L.; Yang, L.F.; Yao, K.D.; Dong, X.H.; Jia, W.Y.; Shi, H.D. A Study on the Fabrication of Porous Chitosan/ Gelatin Network Scaffold for Tissue Engineering. Polym. Int. 2000, 49, 1596–1599. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2005, 75, 485–493. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and Characterization of Macroporous Chitosan/Calcium Phosphate Composite Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Wang, L.Y.; Gu, Y.H.; Zhou, Q.Z.; Ma, G.H.; Wan, Y.H.; Su, Z.G. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf. B Biointerfaces 2006, 50, 126–135. [Google Scholar] [CrossRef]
- Asikainen, A.J.; Hagström, J.; Sorsa, T.; Noponen, J.; Kellomäki, M.; Juuti, H.; Lindqvist, C.; Hietanen, J.; Suuronen, R. Soft tissue reactions to bioactive glass 13-93 combined with chitosan. J. Biomed. Mater. Res. Part A 2007, 83, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Maganti, N.; Venkat Surya, P.K.C.; Thein-Han, W.W.; Pesacreta, T.C.; Misra, R.D.K. Structure-process-property relationship of biomimetic chitosan-based nanocomposite scaffolds for tissue engineering: Biological, physico-chemical, and mechanical functions. Adv. Eng. Mater. 2011, 13, B108–B122. [Google Scholar] [CrossRef]
- Gobin, A.S.; Froude, V.E.; Mathur, A.B. Structural and mechanical characteristics of silk fibroin and chitosan blend scaffolds for tissue regeneration. J. Biomed. Mater. Res. Part A 2005, 74, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ye, F.; Cui, J.; Zhang, F.; Li, X.; Yao, K. Preparation and Characterization of Macroporous Chitosan-Gelatin/-Tricalcium Phosphate Composite Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. A 2003, 67, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Majima, T.; Iwasaki, N.; Yamane, S.; Masuko, T.; Minami, A.; Harada, K.; Tamura, H.; Tokura, S.; Nishimura, S.I. Novel chitosan-based hyaluronan hybrid polymer fibers as a scaffold in ligament tissue engineering. J. Biomed. Mater. Res. Part A 2005, 74, 338–346. [Google Scholar] [CrossRef]
- In, K.S.; Sang, Y.L.; Yoon, J.P.; Myung, C.L.; Sang, H.L.; Jue, Y.L.; Lee, S.J. Homogeneous chitosan-PLGA composite fibrous scaffolds for tissue regeneration. J. Biomed. Mater. Res. Part A 2008, 84, 247–255. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Akrami-Hasan-Kohal, M.; Ghorbani, M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 198–205. [Google Scholar] [CrossRef]
- Ham, T.R.; Pukale, D.D.; Hamrangsekachaee, M.; Leipzig, N.D. Subcutaneous priming of protein-functionalized chitosan scaffolds improves function following spinal cord injury. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110656. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, K.; Du, L.; Cheng, Z.; Zhang, T.; Ding, J.; Li, W.; Xu, B.; Zhu, M. Construction of chitosan scaffolds with controllable microchannel for tissue engineering and regenerative medicine. Mater. Sci. Eng. C 2021, 126, 112178. [Google Scholar] [CrossRef]
- Saeedi Garakani, S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 143, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Bombaldi de Souza, R.F.; Bombaldi de Souza, F.C.; Thorpe, A.; Mantovani, D.; Popat, K.C.; Moraes, Â.M. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int. J. Biol. Macromol. 2020, 143, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Parvizifard, M.; Karbasi, S. Physical, mechanical and biological performance of PHB-Chitosan/MWCNTs nanocomposite coating deposited on bioglass based scaffold: Potential application in bone tissue engineering. Int. J. Biol. Macromol. 2020, 152, 645–662. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Yan, Y.; Zhang, R. Liver tissue responses to gelatin and gelatin/chitosan gels. J. Biomed. Mater. Res. Part A 2008, 87, 62–68. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [Google Scholar] [CrossRef]
- Wu, X.; Black, L.; Santacana-Laffitte, G.; Patrick, C.W. Preparation and assessment of glutaraldehyde-crosslinked collagen-chitosan hydrogels for adipose tissue engineering. J. Biomed. Mater. Res. Part A 2007, 81, 59–65. [Google Scholar] [CrossRef]
- Feng, Z.; Hakkarainen, M.; Grützmacher, H.; Chiappone, A.; Sangermano, M. Photocrosslinked Chitosan Hydrogels Reinforced with Chitosan-Derived Nano-Graphene Oxide. Macromol. Chem. Phys. 2019, 220, 1900174. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, T.; Wang, Y.; Liu, J.; Zhang, J.; Yao, R.; Wu, F. Modulating cationicity of chitosan hydrogel to prevent hypertrophic scar formation during wound healing. Int. J. Biol. Macromol. 2020, 154, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, P.; Liu, Y.; Zhu, C.; Fan, D. Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. Int. J. Biol. Macromol. 2020, 155, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, A.; Gómez-Gil, V.; Ortega, M.A.; Asúnsolo, Á.; Coca, S.; Román, J.S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Chitosan hydrogels functionalized with either unfractionated heparin or bemiparin improve diabetic wound healing. Biomed. Pharmacother. 2020, 129, 110498. [Google Scholar] [CrossRef]
- Zhang, D.; Ouyang, Q.; Hu, Z.; Lu, S.; Quan, W.; Li, P.; Chen, Y.; Li, S. Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int. J. Biol. Macromol. 2021, 173, 591–606. [Google Scholar] [CrossRef]
- Qianqian, O.; Songzhi, K.; Yongmei, H.; Xianghong, J.; Sidong, L.; Puwang, L.; Hui, L. Preparation of nano-hydroxyapatite/chitosan/tilapia skin peptides hydrogels and its burn wound treatment. Int. J. Biol. Macromol. 2021, 181, 369–377. [Google Scholar] [CrossRef]
- Arafa, A.A.; Nada, A.A.; Ibrahim, A.Y.; Sajkiewicz, P.; Zahran, M.K.; Hakeim, O.A. Preparation and characterization of smart therapeutic pH-sensitive wound dressing from red cabbage extract and chitosan hydrogel. Int. J. Biol. Macromol. 2021, 182, 1820–1831. [Google Scholar] [CrossRef]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel chitosan-ulvan hydrogel reinforcement by cellulose nanocrystals with epidermal growth factor for enhanced wound healing: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, X.; Tian, D.; Yu, A.; Wan, Y. Thermo-responsive chitosan/silk fibroin/amino-functionalized mesoporous silica hydrogels with strong and elastic characteristics for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Mahya, S.; Ai, J.; Shojae, S.; Khonakdar, H.A.; Darbemamieh, G.; Shirian, S. Berberine loaded chitosan nanoparticles encapsulated in polysaccharide-based hydrogel for the repair of spinal cord. Int. J. Biol. Macromol. 2021, 182, 82–90. [Google Scholar] [CrossRef]
- Duceac, I.A.; Vere, L.; Coroaba, A.; Coseri, S. All-polysaccharide hydrogels for drug delivery applications: Tunable chitosan beads surfaces via physical or chemical interactions, using oxidized pullulan. Int. J. Biol. Macromol. 2021, 181, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Vijaya, A.; Mariadoss, A.; Wang, M. pH-controlled nucleolin targeted release of dual drug from chitosan-gold based aptamer functionalized nano drug delivery system for improved glioblastoma treatment. Carbohydr. Polym. 2021, 262, 117907. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E. Gamma radiation synthesis of a novel amphiphilic terpolymer hydrogel pH-responsive based chitosan for colon cancer drug delivery. Carbohydr. Polym. 2021, 263, 117975. [Google Scholar] [CrossRef]
- Yang, D.; Gao, K.; Bai, Y.; Lei, L.; Jia, T.; Yang, K.; Xue, C. Microfluidic synthesis of chitosan-coated magnetic alginate microparticles for controlled and sustained drug delivery. Int. J. Biol. Macromol. 2021, 182, 639–647. [Google Scholar] [CrossRef]
- Lemos, T.S.A.; De Souza, J.F.; Fajardo, R. Magnetic microspheres based on pectin coated by chitosan towards smart drug release. Carbohydr. Polym. 2021, 265, 118013. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Ai, Y.; Liu, F.; Chen, M.; Liu, D. Lactobionic acid-modified thymine-chitosan nanoparticles as potential carriers for methotrexate delivery. Carbohydr. Res. 2021, 501, 108275. [Google Scholar] [CrossRef] [PubMed]
- Tadjarodi, A.; Zare-dorabei, R. Low molecular weight chitosan-cyanocobalamin nanoparticles for controlled delivery of ciprofloxacin: Preparation and evaluation. Int. J. Biol. Macromol. 2021, 176, 459–467. [Google Scholar] [CrossRef]
- Li, C.; Fang, K.; He, W.; Li, K.; Jiang, Y.; Li, J. Evaluation of chitosan-ferulic acid microcapsules for sustained drug delivery: Synthesis, characterizations, and release kinetics in vitro. J. Mol. Struct. 2021, 1227, 129353. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.G.; Jiao, W.; Yang, H.; Liu, J.; Liu, D. Phenylboronic acid-conjugated chitosan nanoparticles for high loading and efficient delivery of curcumin. Carbohydr. Polym. 2021, 256, 117497. [Google Scholar] [CrossRef]
- Horo, H.; Bhattacharyya, S.; Mandal, B.; Mohan, L. Synthesis of functionalized silk-coated chitosan-gold nanoparticles and microparticles for target-directed delivery of antitumor agents. Carbohydr. Polym. 2021, 258, 117659. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2, 014110. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Derakhshanfar, S.; Zhong, W.; Li, B.; Lu, F.; Xing, M.; Li, X. Characterization and Application of Carboxymethyl Chitosan-Based Bioink in Cartilage Tissue Engineering. J. Nanomater. 2020, 2020, 2057097. [Google Scholar] [CrossRef]
- Ku, J.; Seonwoo, H.; Park, S.; Jang, K.J.; Lee, J.; Lee, M.; Lim, J.W.; Kim, J.; Chung, J.H. Cell-laden thermosensitive chitosan hydrogel bioinks for 3d bioprinting applications. Appl. Sci. 2020, 10, 2455. [Google Scholar] [CrossRef] [Green Version]
- Butler, H.M.; Naseri, E.; MacDonald, D.S.; Andrew Tasker, R.; Ahmadi, A. Optimization of starch- and chitosan-based bio-inks for 3D bioprinting of scaffolds for neural cell growth. Materialia 2020, 12, 100737. [Google Scholar] [CrossRef]
- Huang, J.; Fu, H.; Wang, Z.; Meng, Q.; Liu, S.; Wang, H.; Zheng, X.; Dai, J.; Zhang, Z. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016, 6, 108423–108430. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C.; et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2019, 14, 025006. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Liu, W.G.; Xu, Z.Y.; Li, J.G.; Huang, T.T.; Lu, Y.J.; Huang, H.G.; Lin, J.X. Chitosan ducts fabricated by extrusion-based 3D printing for soft-tissue engineering. Carbohydr. Polym. 2020, 236, 116058. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Caballero, R.; Saiz, E.; Montembault, A.; Tadier, S.; David, L.; Delair, T.; Grémillard, L.; Maire, E. 3-D printing of chitosan-calcium phosphate inks: Rheology, interactions and characterization. J. Mater. Sci. Mater. Med. 2019, 30, 1–15. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Scocozza, F.; Mariotti, C.; Chiesa, E.; Bruni, G.; Genta, I.; Auricchio, F.; Conti, M.; Conti, B. Preliminary investigation on a new natural based poly(gamma-glutamic acid)/Chitosan bioink. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2718–2732. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.P.; Koh, M.Y.; Kim, P.; Lee, J.; Shin, M.; Lee, H. Chitosan-catechol: A writable bioink under serum culture media. Biomater. Sci. 2018, 6, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurakula, M.; Naveen, N.R. Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef]
- Ramya, R.; Venkatesan, J.; Kim, S.K.; Sudha, P.N. Biomedical applications of chitosan: An overview. J. Biomater. Tissue Eng. 2012, 2, 100–111. [Google Scholar] [CrossRef]

| Component | Aim/System Dedication | Cytotoxity/Cell Proliferation | Biodegradability/ Swelling Ratio | Drug Loading Efficiency (DLE)/Drug Encapsulation Efficiency (DEE) | Drug Release | Comments | Bibliography |
|---|---|---|---|---|---|---|---|
| Pullulan oxidation with TEMPO/NaBr/NaClO (PT) Pullulan oxidation using sodium periodate (PP) Chitosan (C) | Drug delivery | Lack of information | 37 °C in PBS, 24 h–168 h, after 7 cycles of drying-rehydration swelling degree was over 500% for each beads type (CPT, CPP, C) | As a test compound was used ibuprofen, neomycin, and bacitracin: 95–85% drug release efficiency (from loaded) | Ibuprofen release profile: 4–6 h to reach plateau | Tunable release behaviour, antibacterial activity | [265] |
| Chitosan-gold based aptamer consisting of bio-synthesized gold nanoparticles (Au NPs), chitosan (CS) with aptamer (Apt) | Drug delivery system of 5-fluorouracil (5FU) and doxorubicin (Dox) to improve glioblastoma treatment | Glioblastoma cell line (LN229); Fluorescent microscope, cytometry | Lack of information | DEF: 75–96% DLE: 1.3–11% for various formulations of nanoparticle | pH 5.4 for 5FU 90% in 120 h, for DOX 55% in 120 h: pH 7.4 for 5FU 40% in 120 h, for DOX 15% in 120 h | Average particle size: 67 nm–196 nm | [266] |
| (Acrylic acid)-co-(2-acrylamido-2-methylpropane-sulfonic acid) (AAc/AMPS) | Colon cancer drug delivery | Lack of information | At pH 7:8 g/g in 300 min; at pH 1:2.5 g/g in 300 min | Lack of information | 96% of 5-FU drug at pH 7 after 7 h | Incorporation of Cs with AAc and AMPS forming terpolymer hydrogel | [267] |
| Chitosan-coated magnetic alginate (CMAM) | Drug release under mild condition | Lack of information | Lack of information | Lack of information | 77% in 420 min at pH 7.4 and 10% at pH 2.1 | The smaller particles (220 μm) show a faster release rate than the bigger materials (1000 μm) | [268] |
| Magnetic microspheres based on pectin coated by chitosan (mag-Pec-Cs) Metamimzole (MTZ) as a drug model | Multi-responsive drug delivery system | Lack of information | pH 1.2: 275% and 200% for magnetic and pectin covered particles; pH 6.8: 110% and 60% for pectin covered particles; | Pec-Cs/MTZ, DEF% and DLE% 85 ± 1% and 0.14 ± 0.02%, while mag-Pec-Cs/MTZ DEF% and DLE% equal to 88 ± 2% and 0.15 ± 0.04%, | At pH 6.8, the 75% MTZ released after 12 h. | The release process can be adjusted by varying the pH of the medium | [269] |
| Lactobionic acid-modified thymine-chitosan nanoparticles (Thy-Cs NPs) | Carriers for methotrexate delivery (MTX) | HepG2 cells, MTT test; cell viability close to 90% for particle concentration rage 31–1000 µg/mL non-loaded particles | Lack of information | DLE (Drug Loading Efficiency) around 63–90% | Thy-Cs/MTX NP at pH 7.4, 6.5 and 5.5 release in 120 h incubation 90%, 60% and 20% incorporated MTT respectively | Size about 190–250 nm; growth inhibition in three-dimensional multicellular tumour spheroids. | [270] |
| Low molecular weight chitosan-cyanocobalamin nanoparticles (LMCSCNbl) | Oral delivery of ciprofloxacin (CIP) | HEK 293 cell lines, 24 h MTT test 18, 9, and 1% cytotoxicity for LMCS-CNCbl at concentrations of 100, 10, and 1 μg/mL, | Lack of information | DLE 57% | Drug release close to 80% in 24 h, pH 7.4 and 0.1 M HCl | Average diameter 85 nm | [271] |
| Chitosan-ferulic acid microcapsules (CF) loaded with BSA | Oral carrier in functional foods and drug delivery systems. | Lack of information | Swelling ratio: CS and CS-FA (Chitosan-Feluric Acid) after 400 min: 120% and 160% respectively | Encapsulation efficiency: 64–78%; | Drug accumulated release of CS-FA microspheres: after 14 h: 55%, 48% and 46% was noticed for 0.15 g, 0.2 g and 0.1g, of drug | The chitosan-ferulic acid conjugates exhibited low crystallinity but high thermal stability compared with that of chitosan. | [272] |
| Phenylboronic acid-conjugated chitosan nanoparticles | Curcumin delivery for tumor treatment | HepG2 cells, MTT 24 h test: 90%, for phenylboronic acid-conjugated chitosan nanoparticles | Lack of information | DLE in range: 40–90% for curcumin | Cumulative release: pH 7.4, 5.5, and 5.5 and mM H2O2 | Spherical shape, size 200–230 nm; NPs exhibited efficient antitumor efficiency against cancer cells | [273] |
| Silk-coated chitosan-gold nanoparticles (CAu) | Target-directed delivery of antitumor agents | HeLa cell line: 100%, CAu-DOX was 40% and 30% for 0.25, 0.5 and 1.5 µM concentration | Lack of information | The DLE in NPs was 70.83%, and the drug encapsulation efficiency of the beads was calculated to be 87.45%. | Coated and uncoated nanoparticles: 60% and 90% during 32 h, respectively | Nanoparticle size 8 + 3 nm; beads size: 900–1000 µm | [274] |
| Chitosan Blends | Material Dedication | Rheology | Toxicity/ Cell Proliferation | Biodegradability Swelling Ratio | Comments | Bibliography |
|---|---|---|---|---|---|---|
| Carboxymethyl Chitosan-Based Bioink: Ethylenediaminetetraacetic acid (EDTA) stabilized with 0.5 M calcium chloride | Cartilage tissue | Storage modulus G′ at 23 °C: 112 kPa | Rabbit chondrocytes; flow cytometry; 95:9 ± 1:3% After 36 h seeded on mesh; similar proliferation rate between the control group (9:9 ± 0:7%) | Swelling ratio: 14–22% weight increase after 22 days in water | Bioprinting of scaffolds for cells | [277] |
| Cell-Laden Thermosensitive Chitosan Hydrogel Bioink: β-glycerophosphate Potassium phosphate Sodium bicarbonate | Development of chitosan-based bioink | Storage modulus G′ at 36 °C: around 1000 Pa | Human periodontal ligament stem cells; WST (Colorimetric assay for the nonradioactive quantification of cell proliferation, cell viability, and cytotoxicity )assay showed that there was no significant difference in cell viability until day five | Lack of information | Cell encapsulation is associated with minimal cytotoxicity | [278] |
| Cell-laden hydrogels, bioink: Potato starch | 3D bioprinting scaffolds for neural cell growth | Neuro-2a, mouse neuroblastoma cells LDH (Lactate Dehydrogenase)assay kit and fluorescent microscopy: viability after 10 days-10% and lower | Degradation time is decreasing with the addition of the potato starch component | Chitosan dissolution and crosslinking must be optimized | [279] | |
| BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel: Gelatin Sodium alginate Carboxymethyl chitosan | 3D bioink for tissue scaffolds | Young modulus: 80–120 mPa | Bone mesenchymal stem cells (BMSC); Live/Dead cells staining: 85% of the printed cells were viable at 0 and 2 days of culturing | Biodegradation in 60 days in the physiological environment: 35–50% mass loss | Bioink showing antimicrobial properties towards E.coli | [280] |
| Fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels: Hydroxybutyl chitosan (HBC) Oxidized chondroitin sulfate via Shift base reaction | Cell delivery system for cartilage tissue engineering | Turn into stabile hydrogel at 35 °C–40 °C, Storage modulus G′: −150–300 Pa for 50 mg/mL HBC concentration | Mesenchymal stem cells, Live/ Dead assay after via fluorescent microscopy; Cell viability was verified inside the hydrogels in 14 days, showing gradually spreading in the hydrogel with the appearance of pseudopodia | Lack of information | Injectable hydrogel with a porous structure of average 100 µm pore size was developed to form a microporous hydrogel | [281] |
| DLP printing photocurable chitosan: Methacrylic anhydride Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) | Photocurable bioink for digital light processing (DLP) technology for tissue engineering | Stress-strain for CHIMA (methacrylated derivative of chitosan) 33.6% 80 kPa; CHIMA 44.6% 33.6% −150 kPa | Human umbilical vein endothelial cells (HUVECs); LIVE/DEAD Viability/Cytotoxicity kit: Viability for 4 examined samples oscillated around 90% after 3 days from incubation | The swelling ratios of hydrogels 11.7–33.6% DS exhibit a decreased trend from 500% to 150% 2 during the incubation time | The CHI-MA (1 wt%) with 33.6% DS was selected as the photocuring bioink for DLP | [282] |
| Photocurable chitosan as bioink Methacrylated chitosan Β-glycerol phosphate salt (β-GP) | Bioink for cellularized therapies towards personalized scaffold architecture | 40 s of exposition at 37 °C initiate crosslinking bioink: Storage modulus G’: −90–100 Pa | NIH, 3T3, Saos-2, SH-SY5Y cell lines; LIVE/DEAD Viability/Cytotoxicity kit, fluorescence microscopy: viability: around 95–115% compering to control, after 24 h | Decreased mass of 55% after 14 days of incubation in the cultured medium at 37 °C; thermogravimetric analysis | Bioink did not adversely affect the hosting cells and allowed cell proliferation and organization towards tissue formation. | [196] |
| Chitosan ducts fabricated by extrusion-based 3D printing Formic acid Acetic acid Glycolic acid Lactic acid | Soft tissue restoration | Young modulus: 12.38 ± 1.19 MPa | MTT test on L929 mouse fibroblast cell line for 24 h cell viability of CS ducts prepared by 30% GA close to 90% | Stable after soaking in two weeks in Tris-HCl with the addition of lysozyme | The 30 wt.% GA was optimal based on tensile properties and preliminary cytotoxicity | [283] |
| Chitosan-calcium phosphate inks: Calcium Phosphate Acetic acid orthophosphate solutions | Bioinks as potential bone substitute | For all other inks, Loss modulus G″ were higher than G′ from the start, thus the inks were liquid-like | Not tested | Lack of information | More printable inks are obtained with higher chitosan concentration (0.19 mol·L−1). | [284] |
| Cell-Laden Nanocellulose/Chitosan-Based Bioinks: Glycerophosphate Hydroxyethyl cellulose Cellulose nanocrystals | Bioprinting and enhancing cell differentiation for bone tissue | Viscosity in the range of 30 Pa·s–6 × 104 Pa·s; Yield stress 412.35 ± 45.35 pa | MC3T3, a pre-osteoblast cell line; Live/Dead cell staining kit; after 7 days incubation in media at 37 °C there is neither significant proliferation nor cell toxicity | The shrinkage of scaffolds after 24 h incubation in DMEM (Dulbecco’s Modified Eagle Medium) at 37 °C ranges between 30–34% | [285] | |
| Natural based poly(gamma-glutamic acid)/Chitosan bioink: Poly(gamma-glutamic acid) | Alternative to other materials used in 3D bioprinting | Storage modulus G′ around 50 Pa and 30 Pa for 4.5% and 6% Chitosan hydrogels | Human adult fibroblast: Cell viability after 14 days incubation of DMEM on bioink around 80% | 35% Mass loss after 35 days incubation in cell medium | FTIR analysis demonstrated Gamma-PGA/Cs interpolyelectrolyte complex formation | [286] |
| A writable bioink under serum culture media: Catechol Vanadyl ions | Polymer for 3D printing | Storge modulus G′ value of the V-Chi-C gels at 1 Hz was gradually enhanced up to 6 ± 0.5 × 106 Pa at 168 hrs from 69 ± 18 Pa at 0 hr | LIVE⁄DEAD® Viability/Cytotoxicity Kit; 90% L929 cells viability after 5 days incubation on scaffolds | Weight loss down to 50% of initial mass at 12 h of incubation in PBS, and remained constant (40%) for next 7 days | [281] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. https://doi.org/10.3390/nano11113019
Kołodziejska M, Jankowska K, Klak M, Wszoła M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials. 2021; 11(11):3019. https://doi.org/10.3390/nano11113019
Chicago/Turabian StyleKołodziejska, Marta, Kamila Jankowska, Marta Klak, and Michał Wszoła. 2021. "Chitosan as an Underrated Polymer in Modern Tissue Engineering" Nanomaterials 11, no. 11: 3019. https://doi.org/10.3390/nano11113019
APA StyleKołodziejska, M., Jankowska, K., Klak, M., & Wszoła, M. (2021). Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials, 11(11), 3019. https://doi.org/10.3390/nano11113019








